Spectroscopic Methods Used in Implant Material Studies
Abstract
:1. Introduction
2. Metal-Based Implants
2.1. Steel, Titanium, and Titanium Alloy-Based Implants
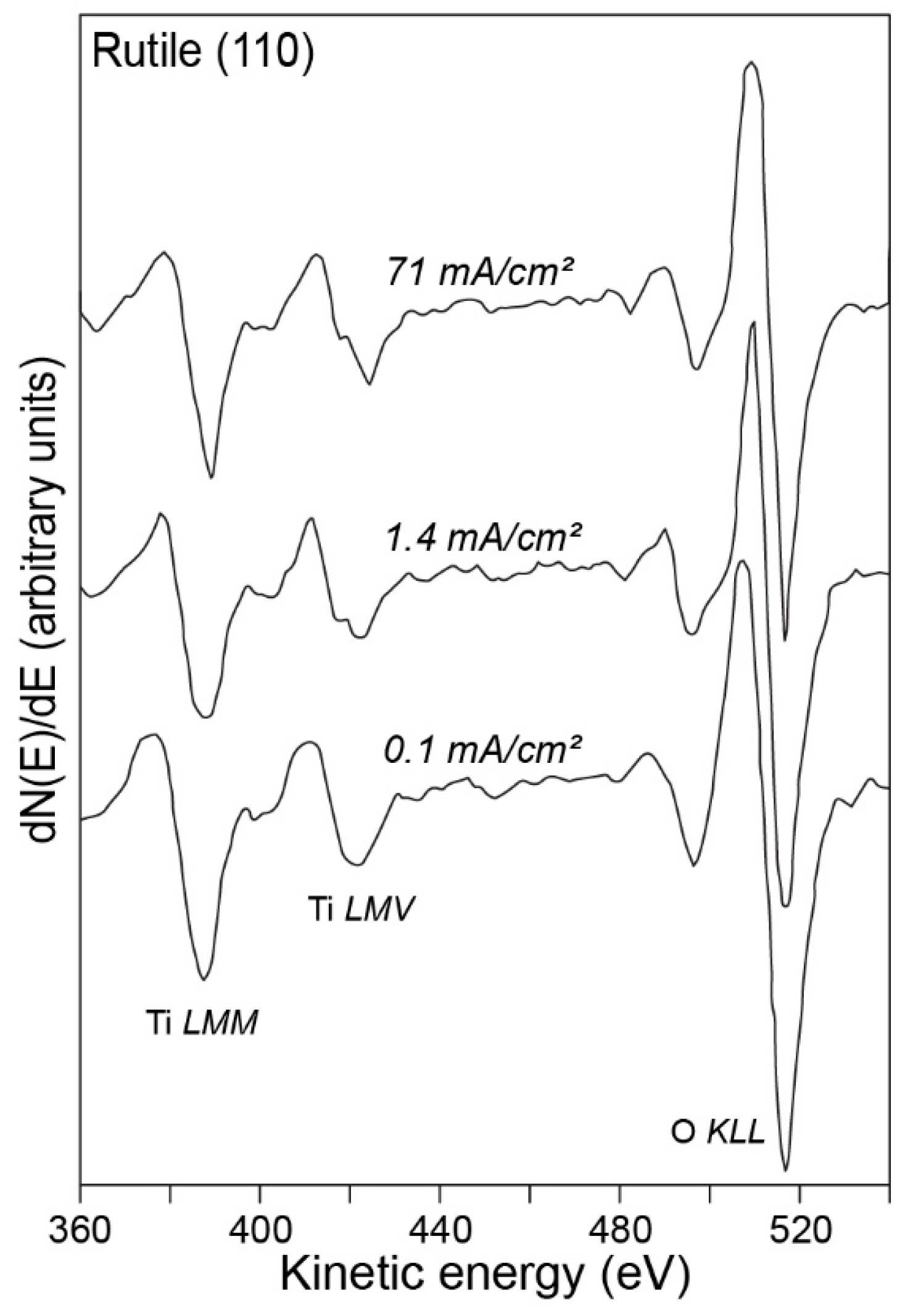
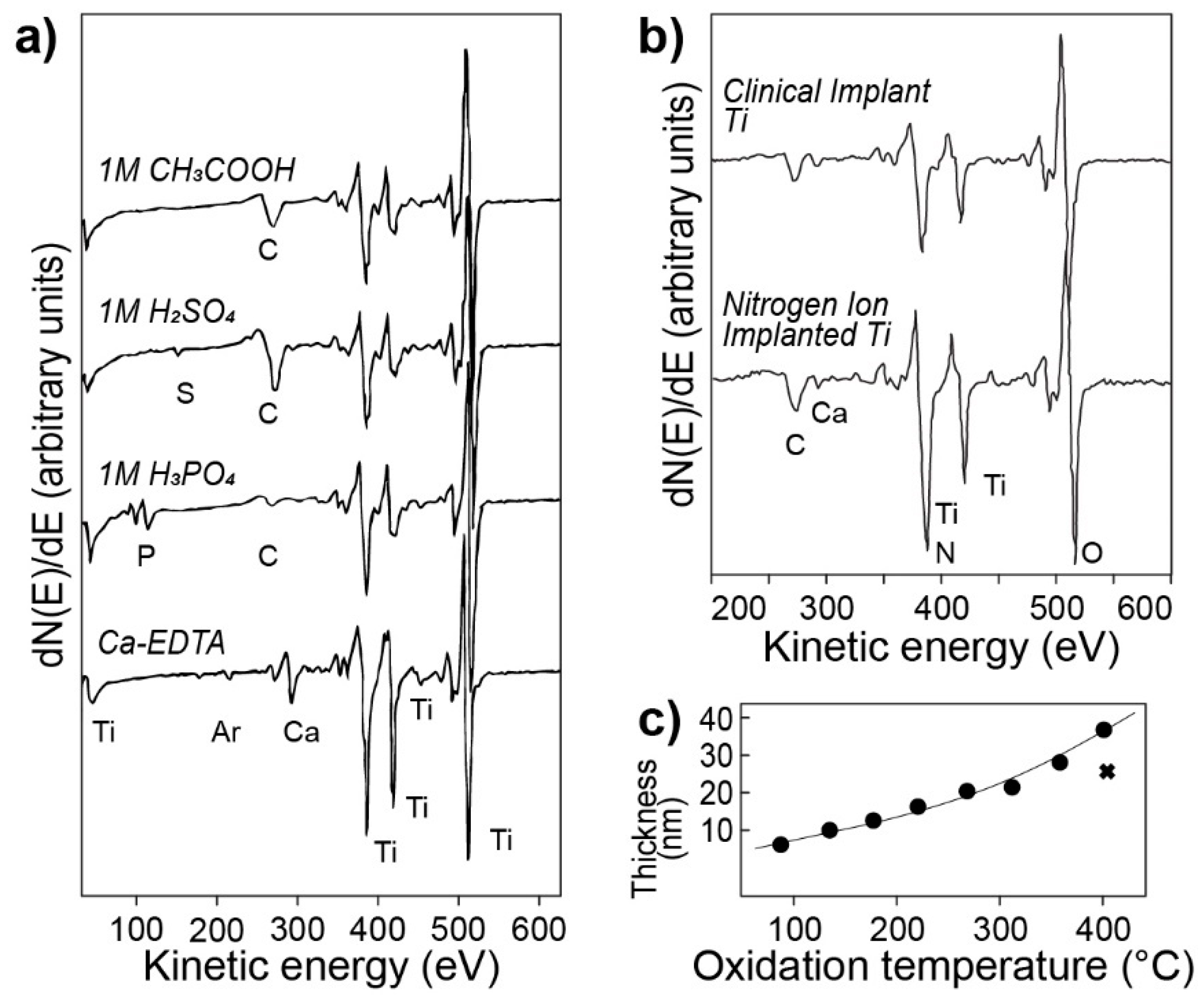
2.1.1. Auger Electron Spectroscopy
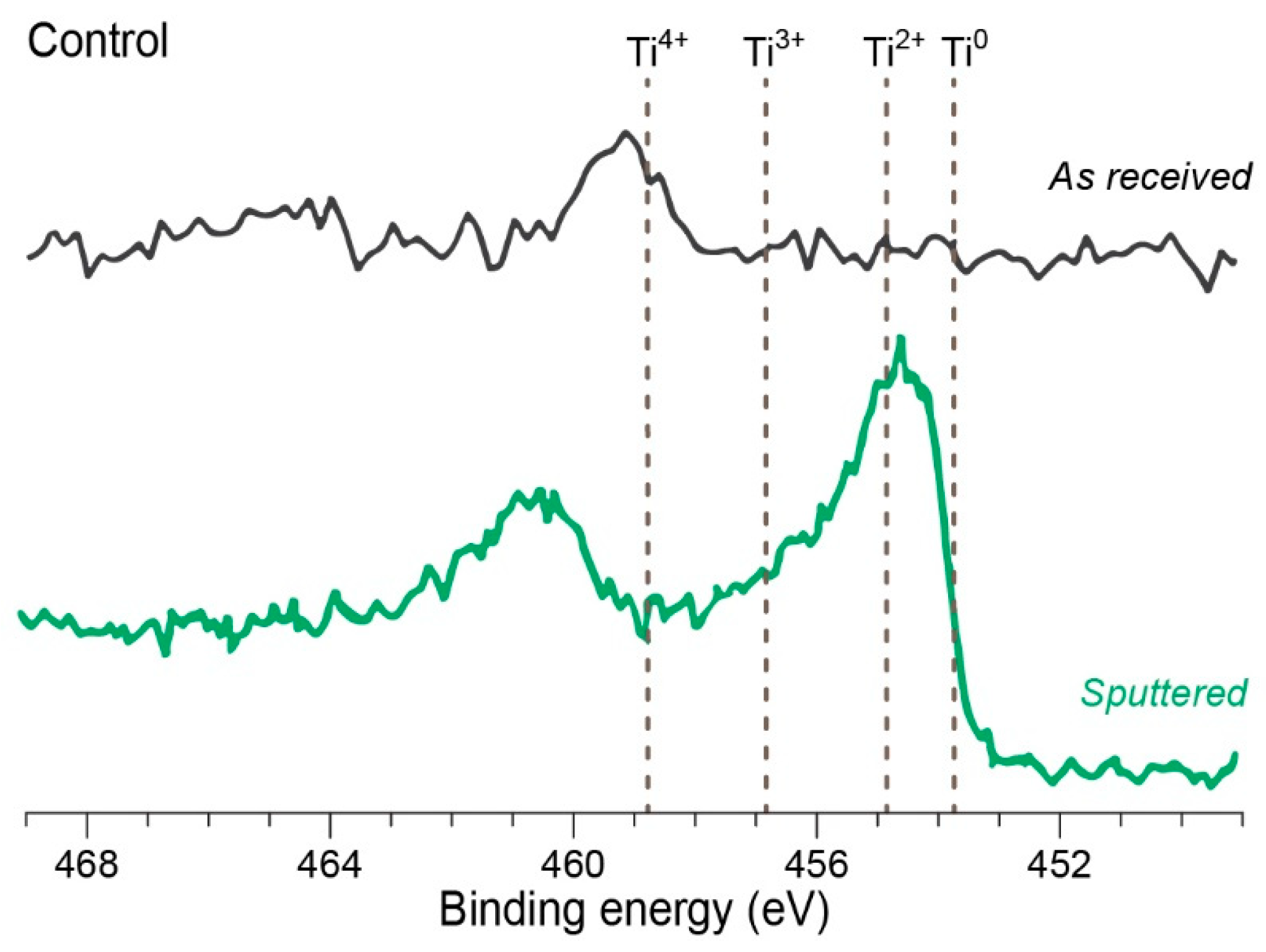
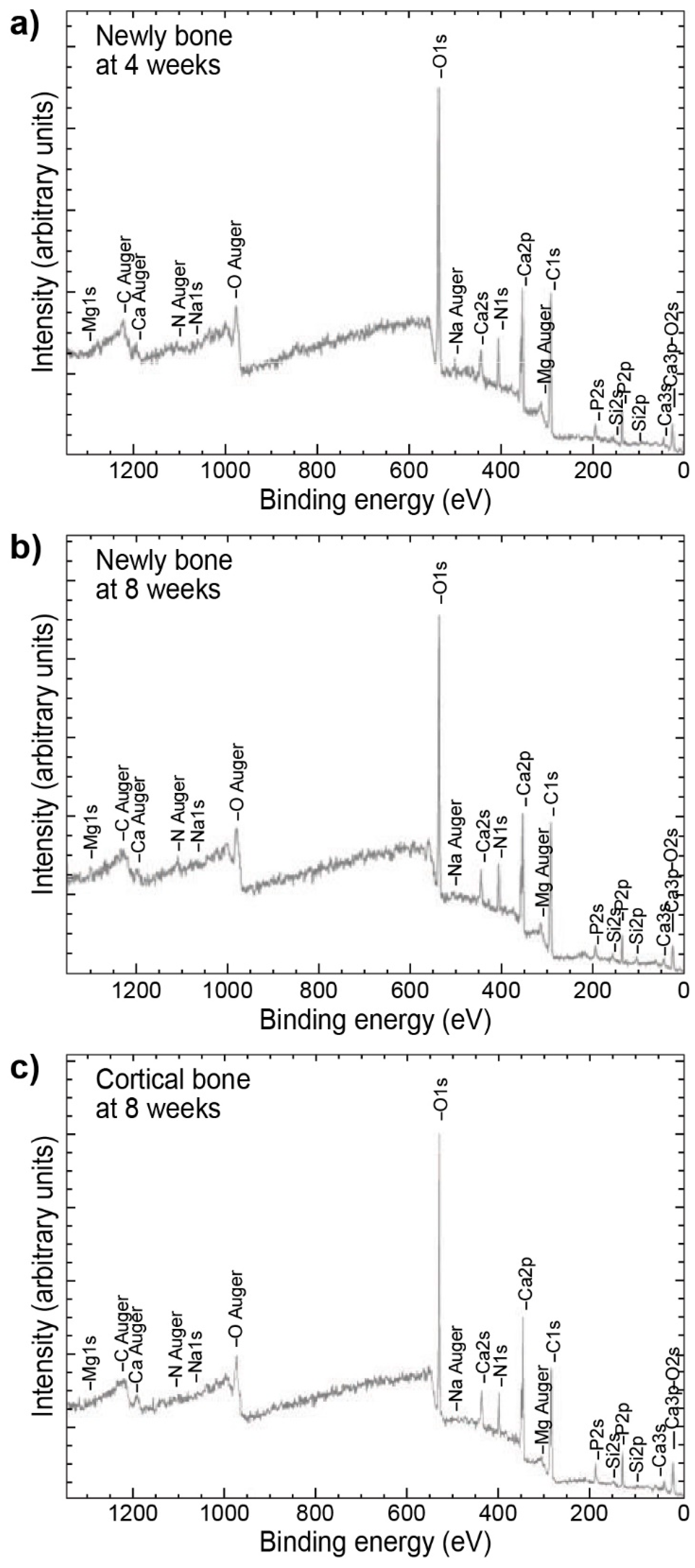
2.1.2. X-ray Photoelectron Spectroscopy also known as Electron Spectroscopy for Chemical Analysis
2.1.3. Raman Spectroscopy
3. Bioceramic Materials
3.1. Photoluminescence Piezospectroscopy
3.2. Raman Spectroscopy
3.3. Diffuse Reflectance Infrared Fourier Transform Spectroscopy
4. Applications of Fluorescence Techniques for the Analysis of Implants and Implant-Surrounding Tissues
4.1. Fluorescence Labeling Methods
4.2. Fluorescence Microscopy Techniques
4.2.1. Fluorescence Microscopy
4.2.2. X-ray Fluorescence Microscopy
4.2.3. Confocal Microscopy
4.2.4. Total Internal Reflection Fluorescence Microscopy (TIRF)
4.2.5. Imaging Systems
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Yang, D.; Ying, Y. Applications of Raman Spectroscopy in Agricultural Products and Food Analysis: A Review. Appl. Spectrosc. Rev. 2011, 46, 539–560. [Google Scholar] [CrossRef]
- Mahesar, S.A.; Lucarini, M.; Durazzo, A.; Santini, A.; Lampe, A.I.; Kiefer, J. Application of Infrared Spectroscopy for Functional Compounds Evaluation in Olive Oil: A Current Snapshot. J. Spectro. 2019, 2019, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Tanner, P.A. Application of Spectroscopic Methods to Environmental Problems. Spectrosc. Lett. 2005, 38, 211–212. [Google Scholar] [CrossRef]
- Nawar, S.; Cipullo, S.; Douglas, R.K.; Coulon, F.; Mouazen, A.M. The applicability of spectroscopy methods for estimating potentially toxic elements in soils: State-of-the-art and future trends. Appl. Spectrosc. Rev. 2019, 154, 1–33. [Google Scholar] [CrossRef]
- Van Schrojenstein Lantman, E.M.; Deckert-Gaudig, T.; Mank, A.J.G.; Deckert, V.; Weckhuysen, B.M. Catalytic processes monitored at the nanoscale with tip-enhanced Raman spectroscopy. Nat. Nanotechnol. 2012, 7, 583–586. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.J.; Elgrishi, N.; Kandemir, B.; Dempsey, J.L. Electrochemical and spectroscopic methods for evaluating molecular electrocatalysts. Nat. Rev. Chem. 2017, 1, 0039EP. [Google Scholar] [CrossRef]
- Piñon, V.; Mateo, M.P.; Nicolas, G. Laser-Induced Breakdown Spectroscopy for Chemical Mapping of Materials. Appl. Spectrosc. Rev. 2013, 48, 357–383. [Google Scholar] [CrossRef]
- Bokobza, L. Some Applications of Vibrational Spectroscopy for the Analysis of Polymers and Polymer Composites. Polymers 2019, 11. [Google Scholar] [CrossRef] [Green Version]
- Chakrapani, S.B.; Minkler, M.J.; Beckingham, B.S. Low-field 1H-NMR spectroscopy for compositional analysis of multicomponent polymer systems. Analyst 2019, 144, 1679–1686. [Google Scholar] [CrossRef]
- Aller, A.J. The Challenge of Spectroscopy in the Microanalysis of Biological Surfaces. Appl. Spectrosc. Rev. 1992, 27, 223–244. [Google Scholar] [CrossRef]
- Clemens, G.; Hands, J.R.; Dorling, K.M.; Baker, M.J. Vibrational spectroscopic methods for cytology and cellular research. Analyst 2014, 139, 4411–4444. [Google Scholar] [CrossRef] [PubMed]
- Austin, L.A.; Osseiran, S.; Evans, C.L. Raman technologies in cancer diagnostics. Analyst 2016, 141, 476–503. [Google Scholar] [CrossRef]
- Smith, R.; Wright, K.L.; Ashton, L. Raman spectroscopy: An evolving technique for live cell studies. Analyst 2016, 141, 3590–3600. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Movasaghi, Z.; Rehman, S.; Rehman, I.U. Raman Spectroscopy of Biological Tissues. Appl. Spectrosc. Rev. 2007, 42, 493–541. [Google Scholar] [CrossRef]
- Movasaghi, Z.; Rehman, S.; ur Rehman, I. Fourier Transform Infrared (FTIR) Spectroscopy of Biological Tissues. Appl. Spectrosc. Rev. 2008, 43, 134–179. [Google Scholar] [CrossRef]
- Talari, A.C.S.; Martinez, M.A.G.; Movasaghi, Z.; Rehman, S.; Rehman, I.U. Advances in Fourier transform infrared (FTIR) spectroscopy of biological tissues. Appl. Spectrosc. Rev. 2017, 52, 456–506. [Google Scholar] [CrossRef]
- Querido, W.; Falcon, J.M.; Kandel, S.; Pleshko, N. Vibrational spectroscopy and imaging: Applications for tissue engineering. Analyst 2017, 142, 4005–4017. [Google Scholar] [CrossRef]
- Larmour, I.A.; Graham, D. Surface enhanced optical spectroscopies for bioanalysis. Analyst 2011, 136, 3831–3853. [Google Scholar] [CrossRef]
- Garrote, B.L.; Santos, A.; Bueno, P.R. Perspectives on and Precautions for the Uses of Electric Spectroscopic Methods in Label-free Biosensing Applications. Acs Sens. 2019, 4, 2216–2227. [Google Scholar] [CrossRef] [Green Version]
- Kendall, C.; Isabelle, M.; Bazant-Hegemark, F.; Hutchings, J.; Orr, L.; Babrah, J.; Baker, R.; Stone, N. Vibrational spectroscopy: A clinical tool for cancer diagnostics. Analyst 2009, 134, 1029–1045. [Google Scholar] [CrossRef]
- Baker, M.J.; Byrne, H.J.; Chalmers, J.; Gardner, P.; Goodacre, R.; Henderson, A.; Kazarian, S.G.; Martin, F.L.; Moger, J.; Stone, N.; et al. Clinical applications of infrared and Raman spectroscopy: State of play and future challenges. Analyst 2018, 143, 1735–1757. [Google Scholar] [CrossRef]
- E Silva de Carvalho, L.F.d.C.; Nogueira, M.S. New insights of Raman spectroscopy for oral clinical applications. Analyst 2018, 143, 6037–6048. [Google Scholar] [CrossRef]
- Nič, M.; Jirát, J.; Košata, B.; Jenkins, A.; McNaught, A. IUPAC Compendium of Chemical Terminology; IUPAC: Research Triagle Park, NC, USA, 2009; ISBN 0-9678550-9-8. [Google Scholar]
- Shayesteh Moghaddam, N.; Taheri Andani, M.; Amerinatanzi, A.; Haberland, C.; Huff, S.; Miller, M.; Elahinia, M.; Dean, D. Metals for bone implants: Safety, design, and efficacy. Biomanuf. Rev. 2016, 1, 4058. [Google Scholar] [CrossRef]
- Prakasam, M.; Locs, J.; Salma-Ancane, K.; Loca, D.; Largeteau, A.; Berzina-Cimdina, L. Biodegradable Materials and Metallic Implants-A Review. J. Funct. Biomater. 2017, 8, 44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prasad, K.; Bazaka, O.; Chua, M.; Rochford, M.; Fedrick, L.; Spoor, J.; Symes, R.; Tieppo, M.; Collins, C.; Cao, A.; et al. Metallic Biomaterials: Current Challenges and Opportunities. Materials 2017, 10, 884. [Google Scholar] [CrossRef] [PubMed]
- Handbook of Auger Electron Spectroscopy: A Book of Reference Data for Identification and Interpretation in Auger Electron Spectroscopy, 3rd ed.; Childs, K.D.; Hedberg, C.L. (Eds.) Physical Electronics: Eden Prairie, MN, USA, 1995; ISBN 0964812401. [Google Scholar]
- Gunawardane, R.P.; Arumainayagam, C.R. Auger Electron Spectroscopy. In Handbook of Applied Solid State Spectroscopy; Vij, D.R., Ed.; Springer: New York, NY, USA, 2006; pp. 451–483. ISBN 978-0-387-32497-5. [Google Scholar]
- Argile, C.; Rhead, G.E. Adsorbed layer and thin film growth modes monitored by Auger electron spectroscopy. Surf. Sci. Rep. 1989, 10, 277–356. [Google Scholar] [CrossRef]
- Powell, C.J. Growth and trends in Auger-electron spectroscopy and x-ray photoelectron spectroscopy for surface analysis. J. Vac. Sci. Technol. A 2003, 21, S42–S53. [Google Scholar] [CrossRef]
- Grant, J.T. Analysis of Surfaces and Thin Films by Using Auger Electron Spectroscopy and X-ray Photoelectron Spectroscopy. J. Korean Phys. Soc. 2007, 51, 925. [Google Scholar] [CrossRef]
- Xu, M.; Takano, Y.; Hatano, T.; Kimura, T.; Fujita, D. Auger electron spectroscopy study of MgB2 surface. Appl. Surf. Sci. 2003, 205, 225–230. [Google Scholar] [CrossRef]
- Orvis, T.; Surendran, M.; Liu, Y.; Cunniff, A.; Ravichandran, J. In situ Auger electron spectroscopy of complex oxide surfaces grown by pulsed laser deposition. J. Vac. Sci. Technol. A 2019, 37, 61401. [Google Scholar] [CrossRef]
- Flewitt, P.E.J.; Moskovic, R.; Wild, R.K. Measurement of segregation to grain boundaries in ferritic steel using Auger electron spectroscopy. Surf. Interface Anal. 2002, 33, 729–734. [Google Scholar] [CrossRef]
- Ott, N.; Yan, Y.; Ramamurthy, S.; Kairy, S.; Birbilis, N. Auger electron spectroscopy analysis of grain boundary microchemistry in an Al–Cu–Li alloy. Scr. Mater. 2016, 119, 17–20. [Google Scholar] [CrossRef] [Green Version]
- Lowry, R.K. Auger Electron Spectroscopy. In Failure Analysis of Integrated Circuits: Tools and Techniques; Wagner, L.C., Ed.; Springer: Boston, MA, USA, 1999; pp. 217–227. ISBN 978-1-4613-7231-8. [Google Scholar]
- Sundgren, J.E.; Bodö, P.; Lundström, I.; Berggren, A.; Hellem, S. Auger electron spectroscopic studies of stainless-steel implants. J. Biomed. Mater. Res. 1985, 19, 663–671. [Google Scholar] [CrossRef] [PubMed]
- Sundgren, J.-E.; Bodö, P.; Lundström, I. Auger electron spectroscopic studies of the interface between human tissue and implants of titanium and stainless steel. J. Colloid Interface Sci. 1986, 110, 9–20. [Google Scholar] [CrossRef]
- Lausmaa, J. Surface spectroscopic characterization of titanium implant materials. J. Electron. Spectrosc. Relat. Phenom. 1996, 81, 343–361. [Google Scholar] [CrossRef]
- Hofmann, S. Auger- and X-ray Photoelectron Spectroscopy in Materials Science: A User-oriented Guide/Siegfried Hofmann; Springer: Heidelberg, Germany, 2013; ISBN 9783642273810. [Google Scholar]
- Moulder, J.F.; Chastain, J. Handbook of X-ray Photoelectron Spectroscopy: A Reference Book of Standard Spectra for Identification and Interpretation of XPS Data; Perkin-Elmer Corporation: Eden Prairie, MN, USA, 1992; ISBN 0962702625. [Google Scholar]
- Van der Heide, P. X-Ray Photoelectron Spectroscopy; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2011; ISBN 9781118162897. [Google Scholar]
- Baer, D.R.; Wang, Y.-C.; Castner, D.G. Use of XPS to Quantify Thickness of Coatings on Nanoparticles. Micros. Today 2016, 24, 40–45. [Google Scholar] [CrossRef] [Green Version]
- Baer, D.R.; Engelhard, M.H.; Johnson, G.E.; Laskin, J.; Lai, J.; Mueller, K.; Munusamy, P.; Thevuthasan, S.; Wang, H.; Washton, N.; et al. Surface characterization of nanomaterials and nanoparticles: Important needs and challenging opportunities. J. Vac. Sci. Technol. A 2013, 31, 50820. [Google Scholar] [CrossRef]
- Ariza, M.J.; Martín, F.; Leinen, D. XPS surface analysis of monocrystalline silicon solar cells for manufacturing control. Appl. Phys. A Mater. Sci. Process. 2001, 73, 579–584. [Google Scholar] [CrossRef]
- Wei, J.; Bai, D.; Yang, L. Polymer Photovoltaic Cells with Rhenium Oxide as Anode Interlayer. PLoS ONE 2015, 10, e0133725. [Google Scholar] [CrossRef] [Green Version]
- Venezia, A.M. X-ray photoelectron spectroscopy (XPS) for catalysts characterization. Catal. Today 2003, 77, 359–370. [Google Scholar] [CrossRef]
- Anbarasan, R.; Palanikumar, S.; Devi, A.A.; Chen, P.-H.; Tung, K.L. Characterization and application of Cu based superhydrophobic catalyst. Prog. Nat. Sci. Mater. Int. 2019. [Google Scholar] [CrossRef]
- Duchoslav, J.; Arndt, M.; Keppert, T.; Luckeneder, G.; Stifter, D. XPS investigation on the surface chemistry of corrosion products on ZnMgAl-coated steel. Anal. Bioanal. Chem. 2013, 405, 7133–7144. [Google Scholar] [CrossRef] [PubMed]
- Stickle, W.F.; Young, C.N. Applying XPS to support industrial research and manufacturing. J. Electron. Spectrosc. Relat. Phenom. 2019, 231, 50–56. [Google Scholar] [CrossRef]
- Rodrigues, D.C.; Sridhar, S.; Gindri, I.M.; Siddiqui, D.A.; Valderrama, P.; Wilson, T.G.; Chung, K.-H.; Wadhwani, C. Spectroscopic and microscopic investigation of the effects of bacteria on dental implant surfaces. RSC Adv. 2016, 6, 48283–48293. [Google Scholar] [CrossRef]
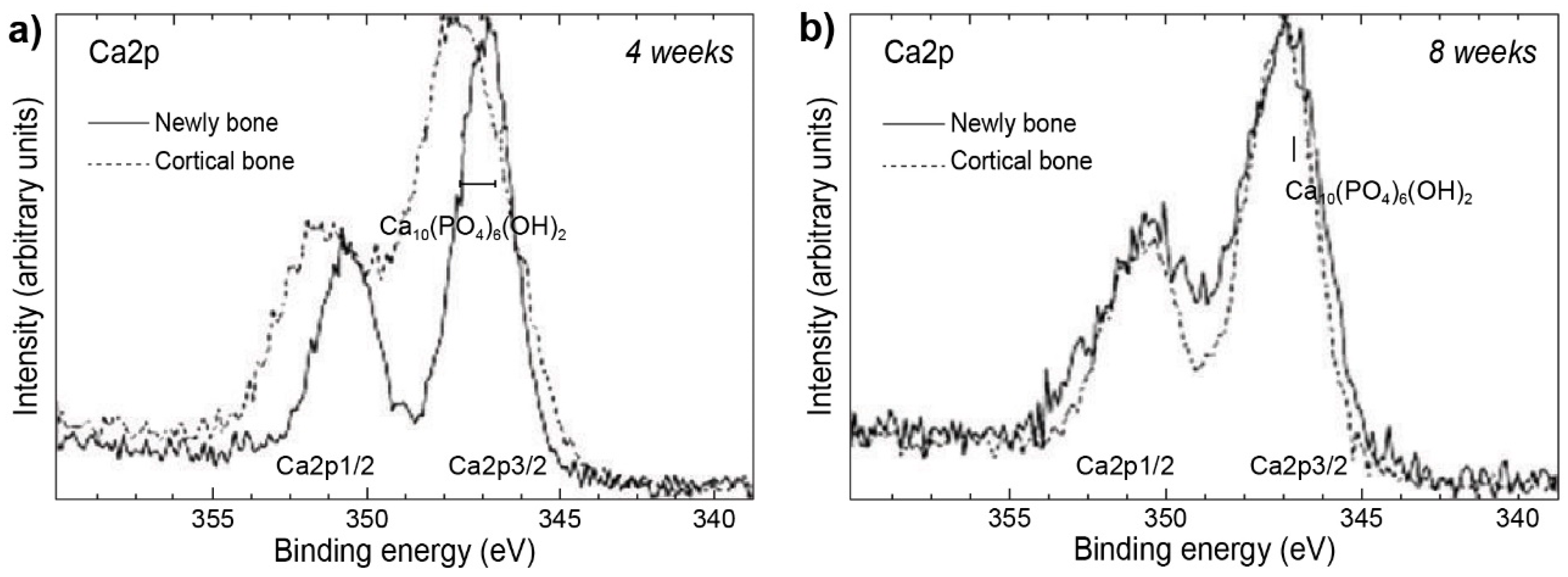
- Nakada, H.; Sakae, T.; Tanimoto, Y.; Teranishi, M.; Kato, T.; Watanabe, T.; Saeki, H.; Kawai, Y.; Legeros, R.Z. Assessment of the Quality of Newly Formed Bone around Titanium Alloy Implants by Using X-Ray Photoelectron Spectroscopy. Int. J. Biomater. 2012, 2012, 615018. [Google Scholar] [CrossRef] [PubMed]
- Schlösser, M. Theory of Quantitative Raman Spectroscopy. In Accurate Calibration of Raman Systems; Schlösser, M., Ed.; Springer: New York, NY, USA, 2014; pp. 53–74. ISBN 978-3-319-06220-4. [Google Scholar]
- Cialla-May, D.; Schmitt, M.; Popp, J. Theoretical principles of Raman spectroscopy. Phys. Sci. Rev. 2019, 4, 557. [Google Scholar] [CrossRef]
- Butler, H.J.; Ashton, L.; Bird, B.; Cinque, G.; Curtis, K.; Dorney, J.; Esmonde-White, K.; Fullwood, N.J.; Gardner, B.; Martin-Hirsch, P.L.; et al. Using Raman spectroscopy to characterize biological materials. Nat. Protoc. 2016, 11, 664EP. [Google Scholar] [CrossRef] [Green Version]
- Kuhar, N.; Sil, S.; Verma, T.; Umapathy, S. Challenges in application of Raman spectroscopy to biology and materials. Rsc Adv. 2018, 8, 25888–25908. [Google Scholar] [CrossRef] [Green Version]
- Zrimsek, A.B.; Chiang, N.; Mattei, M.; Zaleski, S.; McAnally, M.O.; Chapman, C.T.; Henry, A.-I.; Schatz, G.C.; van Duyne, R.P. Single-Molecule Chemistry with Surface- and Tip-Enhanced Raman Spectroscopy. Chem. Rev. 2017, 117, 7583–7613. [Google Scholar] [CrossRef]
- Nguyen, A.H.; Peters, E.A.; Schultz, Z.D. Bioanalytical applications of surface-enhanced Raman spectroscopy: De novo molecular identification. Rev. Anal. Chem 2017, 36. [Google Scholar] [CrossRef]
- Lee, J.; Crampton, K.T.; Tallarida, N.; Apkarian, V.A. Visualizing vibrational normal modes of a single molecule with atomically confined light. Nature 2019, 568, 78–82. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Weckhuysen, B.M.; Wain, A.J.; Pollard, A.J. Nanoscale chemical imaging using tip-enhanced Raman spectroscopy. Nat. Protoc. 2019, 14, 1169–1193. [Google Scholar] [CrossRef]
- Neuville, D.R.; de Ligny, D.; Henderson, G.S. Advances in Raman Spectroscopy Applied to Earth and Material Sciences. Rev. Mineral. Geochem. 2014, 78, 509–541. [Google Scholar] [CrossRef]
- Cantarero, A. Raman Scattering Applied to Materials Science. Procedia Mater. Sci. 2015, 9, 113–122. [Google Scholar] [CrossRef] [Green Version]
- Gunter, G.; Hoffmann, G.G. Raman Spectroscopy, Volume I//Raman Spectroscopy: Principles and Applications in Chemistry, Physics, Materials Science, and Biology//Principles and Applications in Chemistry, Physics, Materials Science, and Biology; Momentum Press: New York, NY, USA, 2019; ISBN 9781945612008. [Google Scholar]
- Paudel, A.; Raijada, D.; Rantanen, J. Raman spectroscopy in pharmaceutical product design. Adv. Drug Deliv. Rev. 2015, 89, 3–20. [Google Scholar] [CrossRef] [Green Version]
- Jentzsch, P.; Ramos, L.; Ciobotă, V. Handheld Raman Spectroscopy for the Distinction of Essential Oils Used in the Cosmetics Industry. Cosmetics 2015, 2, 162–176. [Google Scholar] [CrossRef] [Green Version]
- Fluegel, B.; Mialitsin, A.V.; Beaton, D.A.; Reno, J.L.; Mascarenhas, A. Electronic Raman scattering as an ultra-sensitive probe of strain effects in semiconductors. Nat. Commun. 2015, 6, 7136. [Google Scholar] [CrossRef] [Green Version]
- Wheelis, S.E.; Wilson, T.G.; Valderrama, P.; Rodrigues, D.C. Surface characterization of titanium implant healing abutments before and after placement. Clin. Implant Dent. Relat. Res. 2018, 20, 180–190. [Google Scholar] [CrossRef]
- Daood, U.; Banday, N.; Akram, Z.; Tsoi, J.; Neelakantan, P.; Omar, H.; Abduljabbar, T.; Vohra, F.; Al-Hamoudi, N.; Fawzy, A. Mechanical and Spectroscopic Analysis of Retrieved/Failed Dental Implants. Coatings 2017, 7, 201. [Google Scholar] [CrossRef] [Green Version]
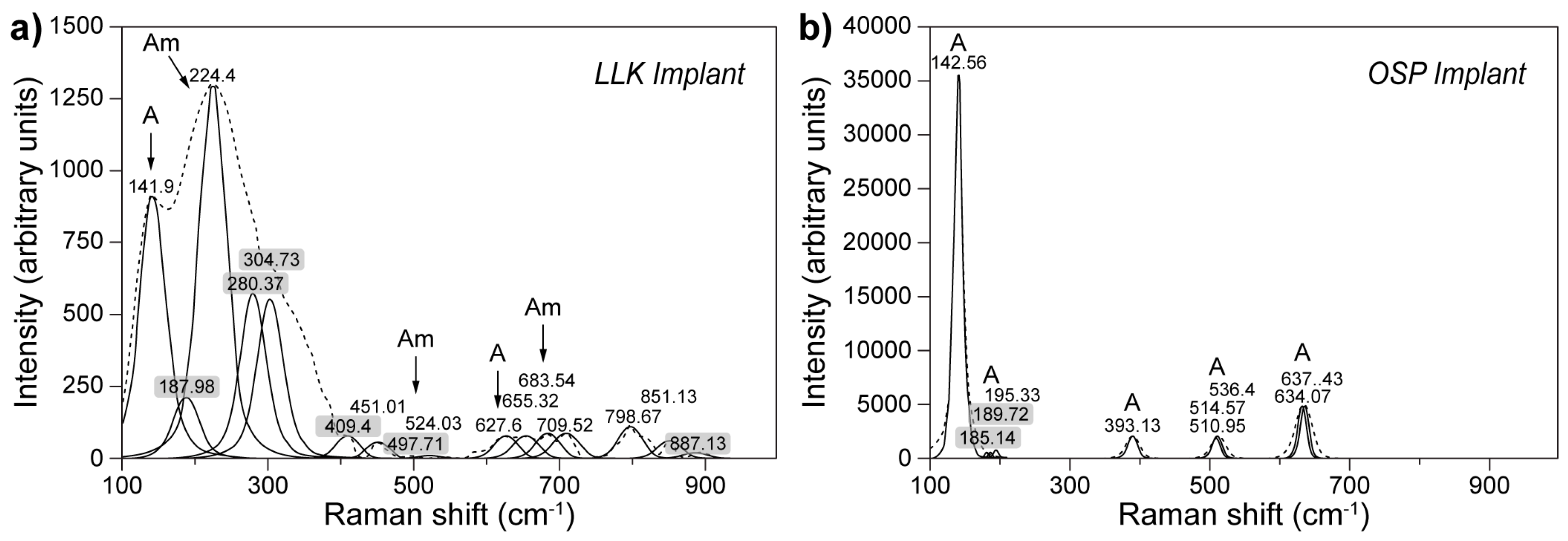
- Gaintantzopoulou, M.; Zinelis, S.; Silikas, N.; Eliades, G. Micro-Raman spectroscopic analysis of TiO2 phases on the root surfaces of commercial dental implants. Dent. Mater. 2014, 30, 861–867. [Google Scholar] [CrossRef]
- Xia, W.; Lindahl, C.; Lausmaa, J.; Engqvist, H. Biomimetic Hydroxyapatite Deposition on Titanium Oxide Surfaces for Biomedical Application. In Advances in Biomimetics; George, A., Ed.; InTech: Rijeka, Croatia; CA, USA, 2011; ISBN 978-953-307-191-6. [Google Scholar]
- Kokubo, T. Bioceramics and Their Clinical Applications; CRC Press: Cambridge, UK, 2008; ISBN 978-1-4200-7207-5. [Google Scholar]
- Rahaman, M.N.; Day, D.E.; Bal, B.S.; Fu, Q.; Jung, S.B.; Bonewald, L.F.; Tomsia, A.P. Bioactive glass in tissue engineering. Acta Biomater. 2011, 7, 2355–2373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baino, F.; Hamzehlou, S.; Kargozar, S. Bioactive Glasses: Where Are We and Where Are We Going? J. Funct. Biomater. 2018, 9, 25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baino, F.; Verné, E.; Fiume, E.; Peitl, O.; Zanotto, E.D.; Brandão, S.M.; Schellini, S.A. Bioactive glass and glass-ceramic orbital implants. Int. J. Appl. Ceram. Technol. 2019, 16, 1850–1863. [Google Scholar] [CrossRef]
- Vallittu, P.K. Bioactive glass-containing cranial implants: An overview. J. Mater. Sci. 2017, 52, 8772–8784. [Google Scholar] [CrossRef] [Green Version]
- Han, J.; Zhao, J.; Shen, Z. Zirconia ceramics in metal-free implant dentistry. Adv. Appl. Ceram. 2017, 116, 138–150. [Google Scholar] [CrossRef] [Green Version]
- Osman, R.B.; Swain, M.V. A Critical Review of Dental Implant Materials with an Emphasis on Titanium versus Zirconia. Materials 2015, 8, 932–958. [Google Scholar] [CrossRef] [Green Version]
- Vallet-Regí, M. Evolution of bioceramics within the field of biomaterials. C.R. Chim. 2010, 13, 174–185. [Google Scholar] [CrossRef]
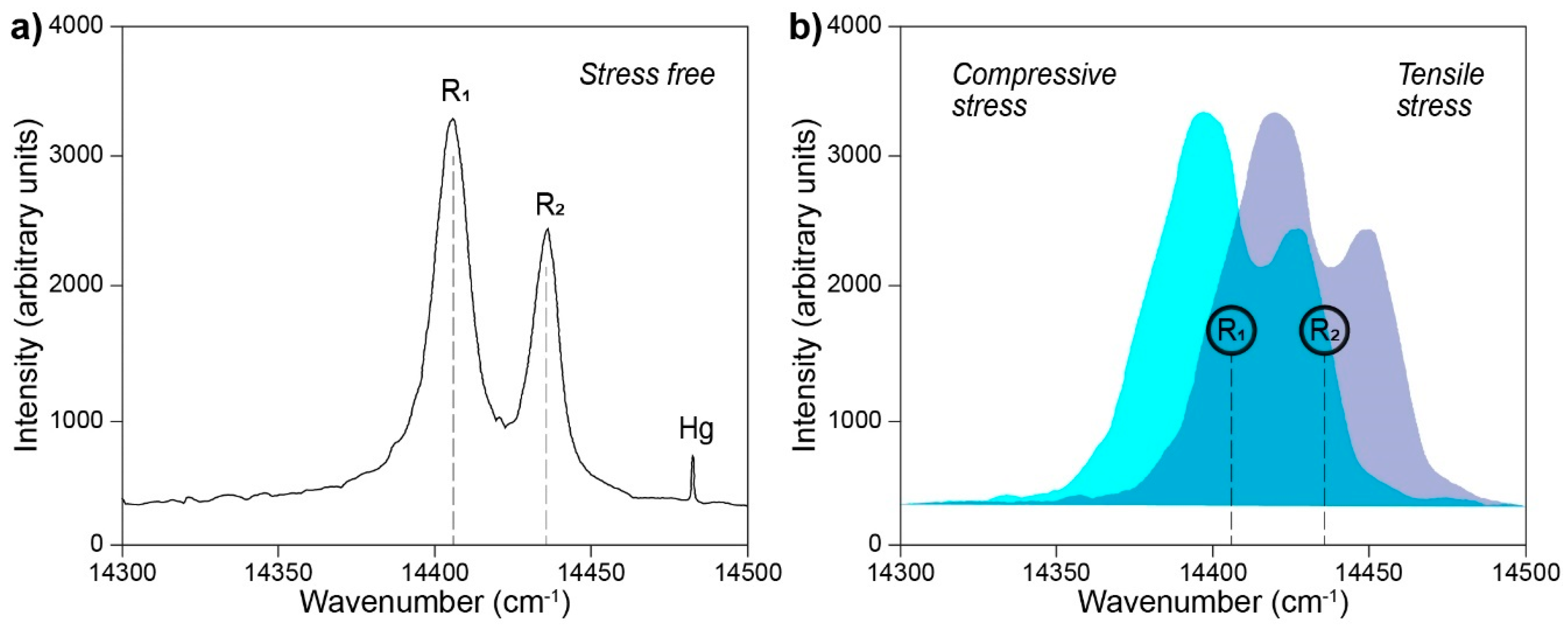
- Grabner, L. Spectroscopic technique for the measurement of residual stress in sintered Al2O3. J. Appl. Phys. 1978, 49, 580–583. [Google Scholar] [CrossRef]
- Ma, Q.; Clarke, D.R. Piezospectroscopic Determination of Residual Stresses in Polycrystalline Alumina. J. Am. Ceram. Soc. 1994, 77, 298–302. [Google Scholar] [CrossRef]
- Lima, C.R.C.; Dosta, S.; Guilemany, J.M.; Clarke, D.R. The application of photoluminescence piezospectroscopy for residual stresses measurement in thermally sprayed TBCs. Surf. Coat. Tech. 2017, 318, 147–156. [Google Scholar] [CrossRef]
- Pollock, T.M.; Lipkin, D.M.; Hemker, K.J. Multifunctional coating interlayers for thermal-barrier systems. Mrs Bull. 2012, 37, 923–931. [Google Scholar] [CrossRef]
- Kim, N.; Yun, H.-B. Noncontact mobile sensing for absolute stress in rail using photoluminescence piezospectroscopy. Struct. Health Monit. 2018, 17, 1213–1224. [Google Scholar] [CrossRef]
- Wang, X. Photoluminescence Piezo-Spectroscopy Method for Measurement of Residual Stresses. In Encyclopedia of Thermal Stresses; Hetnarski, R.B., Ed.; Springer Reference: Dordrecht, The Netherlands, 2014; pp. 3682–3690. ISBN 978-94-007-2738-0. [Google Scholar]
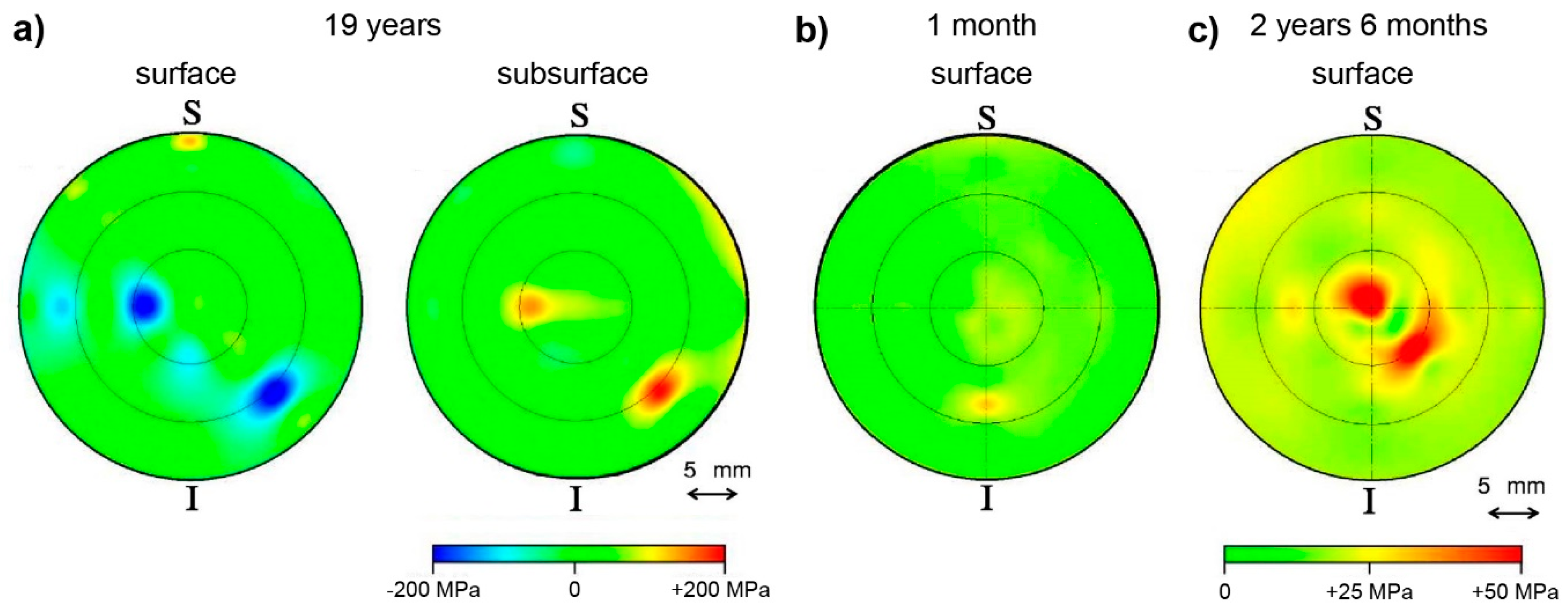
- Pezzotti, G.; Tateiwa, T.; Zhu, W.; Kumakura, T.; Yamada, K.; Yamamoto, K. Fluorescence spectroscopic analysis of surface and subsurface residual stress fields in alumina hip joints. J. Biomed. Opt. 2006, 11, 24009. [Google Scholar] [CrossRef] [PubMed]
- Shavandi, A.; Bekhit, A.E.-D.A.; Sun, Z.F.; Ali, A. A Review of Synthesis Methods, Properties and Use of Hydroxyapatite as a Substitute of Bone. JBBBE 2015, 25, 98–117. [Google Scholar] [CrossRef]
- Fihri, A.; Len, C.; Varma, R.S.; Solhy, A. Hydroxyapatite: A review of syntheses, structure and applications in heterogeneous catalysis. Coord. Chem. Rev. 2017, 347, 48–76. [Google Scholar] [CrossRef]
- Pajor, K.; Pajchel, L.; Kolmas, J. Hydroxyapatite and Fluorapatite in Conservative Dentistry and Oral Implantology-A Review. Materials 2019, 12, 2683. [Google Scholar] [CrossRef] [Green Version]
- Haider, A.; Haider, S.; Han, S.S.; Kang, I.-K. Recent advances in the synthesis, functionalization and biomedical applications of hydroxyapatite: A review. Rsc Adv. 2017, 7, 7442–7458. [Google Scholar] [CrossRef] [Green Version]
- Siddiqui, H.A.; Pickering, K.L.; Mucalo, M.R. A Review on the Use of Hydroxyapatite-Carbonaceous Structure Composites in Bone Replacement Materials for Strengthening Purposes. Materials 2018, 11, 1813. [Google Scholar] [CrossRef] [Green Version]
- Wei, G.; Gong, C.; Hu, K.; Wang, Y.; Zhang, Y. Biomimetic Hydroxyapatite on Graphene Supports for Biomedical Applications: A Review. Nanomaterials 2019, 9, 1435. [Google Scholar] [CrossRef] [Green Version]
- Dippel, B.; Mueller, R.T.; Pingsmann, A.; Schrader, B. Composition, constitution, and interaction of bone with hydroxyapatite coatings determined by FT Raman microscopy. Biospectroscopy 1998, 4, 403–412. [Google Scholar] [CrossRef]
- Döpner, S.; Müller, F.; Hildebrandt, P.; Müller, R.T. Integration of metallic endoprotheses in dog femur studied by near-infrared Fourier-transform Raman microscopy. Biomaterials 2002, 23, 1337–1345. [Google Scholar] [CrossRef]
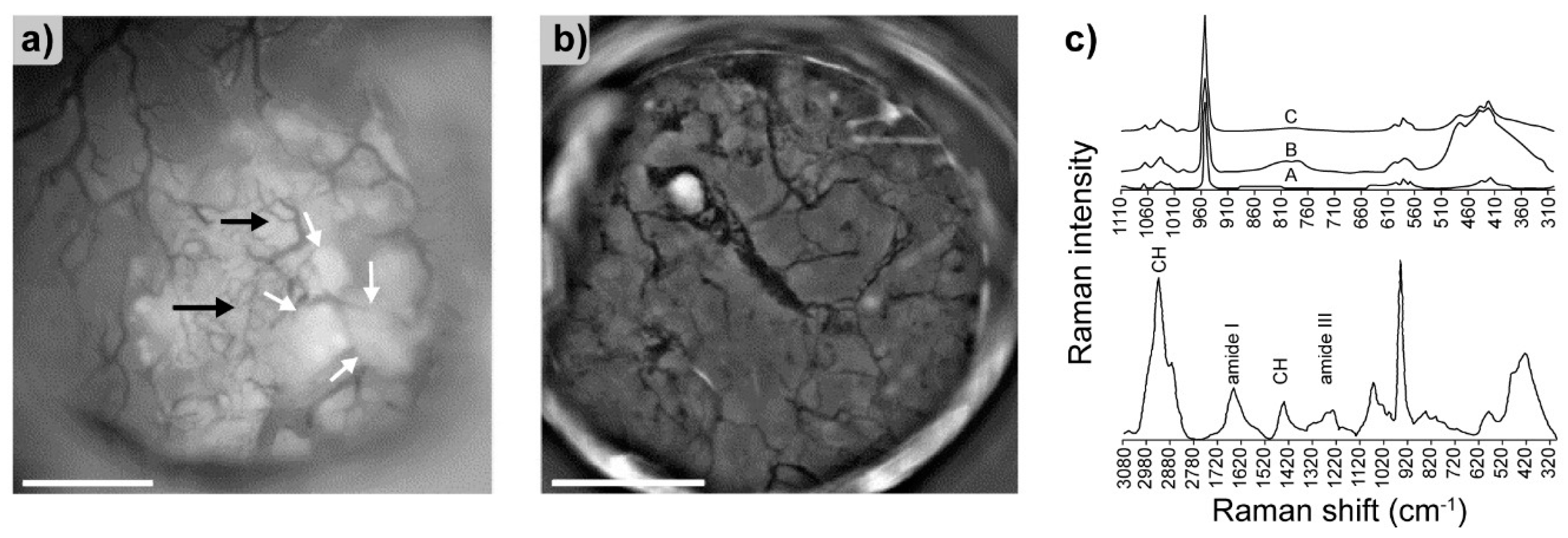
- Penel, G.; Delfosse, C.; Descamps, M.; Leroy, G. Composition of bone and apatitic biomaterials as revealed by intravital Raman microspectroscopy. Bone 2005, 36, 893–901. [Google Scholar] [CrossRef] [PubMed]
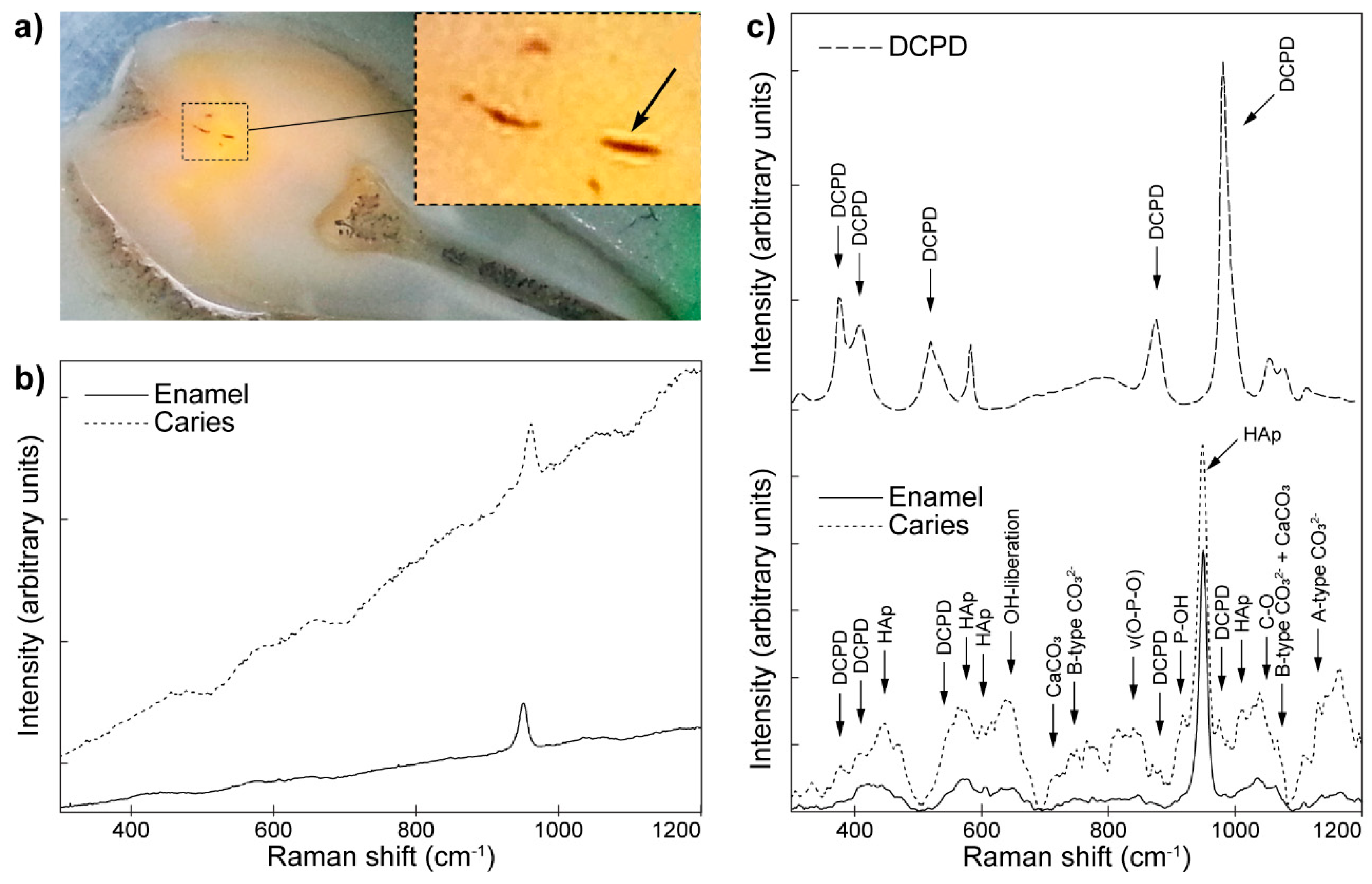
- Seredin, P.; Goloshchapov, D.; Prutskij, T.; Ippolitov, Y. Phase transformations in a human tooth tissue at the initial stage of caries. PLoS ONE 2015, 10, e0124008. [Google Scholar] [CrossRef] [PubMed]
- Mirabella, F.M. Modern Techniques in Applied Molecular Spectroscopy; Wiley: New York, NY, USA, 1998; ISBN 0471123595. [Google Scholar]
- Zeine, C.; Grobe, J. Diffuse reflectance infrared fourier transform (DRIFT) spectroscopy in the preservation of historical monuments: Studies on salt migration. Mikrochim. Acta 1997, 125, 279–282. [Google Scholar] [CrossRef]
- Chiarello, G.L.; Nachtegaal, M.; Marchionni, V.; Quaroni, L.; Ferri, D. Adding diffuse reflectance infrared Fourier transform spectroscopy capability to extended x-ray-absorption fine structure in a new cell to study solid catalysts in combination with a modulation approach. Rev. Sci. Instrum. 2014, 85, 74102. [Google Scholar] [CrossRef]
- Cumming, D.J.; Tumilson, C.; Taylor, S.F.R.; Chansai, S.; Call, A.V.; Jacquemin, J.; Hardacre, C.; Elder, R.H. Development of a diffuse reflectance infrared Fourier transform spectroscopy (DRIFTS) cell for the in situ analysis of co-electrolysis in a solid oxide cell. Faraday Discuss. 2015, 182, 97–111. [Google Scholar] [CrossRef] [Green Version]
- Ford, T.; Rizzo, A.; Hendriks, E.; Frøysaker, T.; Caruso, F. A non-invasive screening study of varnishes applied to three paintings by Edvard Munch using portable diffuse reflectance infrared Fourier transform spectroscopy (DRIFTS). Herit Sci 2019, 7, 200. [Google Scholar] [CrossRef] [Green Version]
- Deiner, L.J.; Farjami, E. Diffuse reflectance infrared spectroscopic identification of dispersant/particle bonding mechanisms in functional inks. J. Vis. Exp. 2015, e52744. [Google Scholar] [CrossRef] [Green Version]
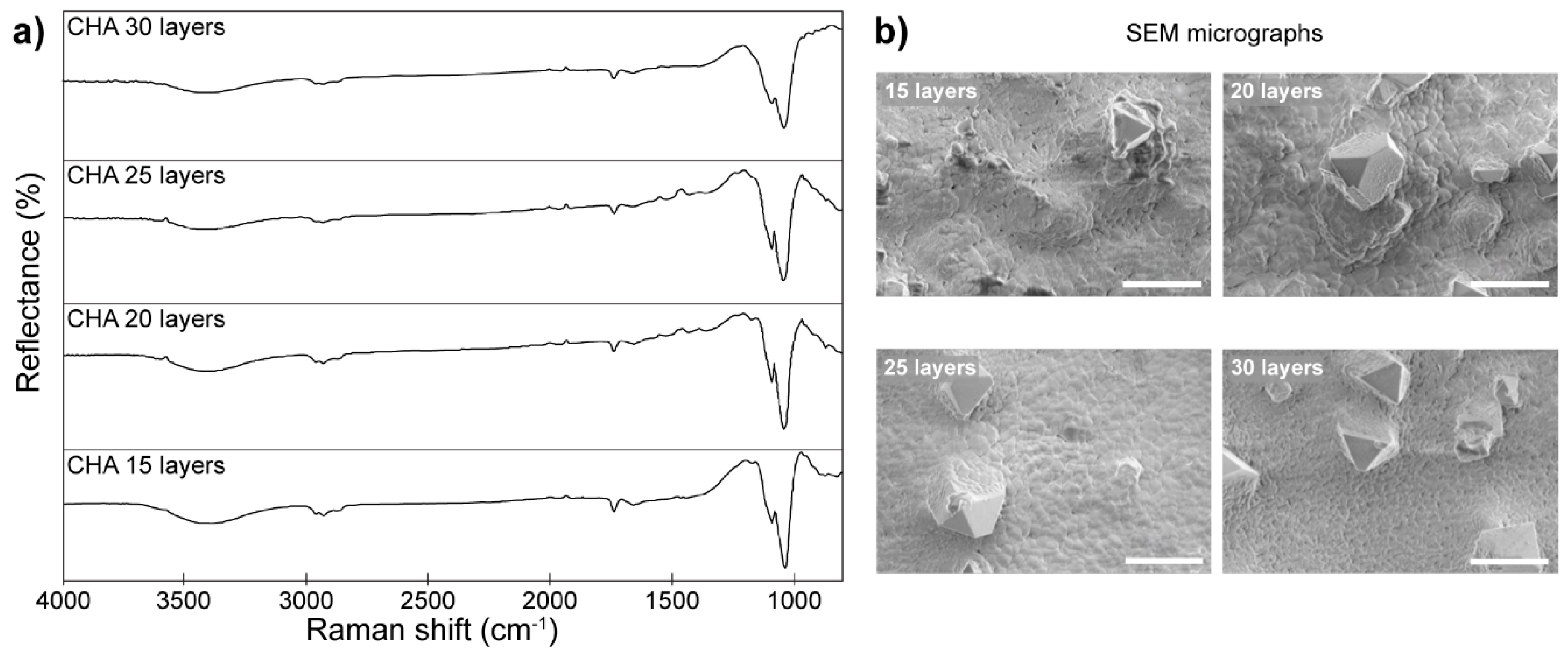
- Jonauske, V.; Stanionyte, S.; Chen, S.-W.; Zarkov, A.; Juskenas, R.; Selskis, A.; Matijosius, T.; Yang, T.C.K.; Ishikawa, K.; Ramanauskas, R.; et al. Characterization of Sol-Gel Derived Calcium Hydroxyapatite Coatings Fabricated on Patterned Rough Stainless Steel Surface. Coatings 2019, 9, 334. [Google Scholar] [CrossRef] [Green Version]
- Rigo, E.C.S.; Boschi, A.O.; Yoshimoto, M.; Allegrini, S.; Konig, B.; Carbonari, M.J. Evaluation in vitro and in vivo of biomimetic hydroxyapatite coated on titanium dental implants. Mater. Sci. Eng. C 2004, 24, 647–651. [Google Scholar] [CrossRef]
- Marín-Pareja, N.; Salvagni, E.; Guillem-Marti, J.; Aparicio, C.; Ginebra, M.-P. Collagen-functionalised titanium surfaces for biological sealing of dental implants: Effect of immobilisation process on fibroblasts response. Colloids Surf. B Biointerfaces 2014, 122, 601–610. [Google Scholar] [CrossRef] [PubMed]
- Raphel, J.; Karlsson, J.; Galli, S.; Wennerberg, A.; Lindsay, C.; Haugh, M.G.; Pajarinen, J.; Goodman, S.B.; Jimbo, R.; Andersson, M.; et al. Engineered protein coatings to improve the osseointegration of dental and orthopaedic implants. Biomaterials 2016, 83, 269–282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raphel, J.; Parisi-Amon, A.; Heilshorn, S. Photoreactive elastin-like proteins for use as versatile bioactive materials and surface coatings. J. Mater. Chem. 2012, 22, 19429–19437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Truc, N.T.; Minh, H.H.; Khanh, L.L.; Thuy, V.M.; van Toi, V.; van Man, T.; Nam, H.C.N.; Quyen, T.N.; Hiep, N.T. Modification of type I collagen on TiO2 surface using electrochemical deposition. Surf. Coat. Tech. 2018, 344, 664–672. [Google Scholar] [CrossRef]
- Ao, H.-Y.; Xie, Y.-T.; Yang, S.-B.; Wu, X.-D.; Li, K.; Zheng, X.-B.; Tang, T.-T. Covalently immobilised type I collagen facilitates osteoconduction and osseointegration of titanium coated implants. J. Orthop. Translat. 2016, 5, 16–25. [Google Scholar] [CrossRef] [Green Version]
- Konermann, A.; Götz, W.; Le, M.; Dirk, C.; Lossdörfer, S.; Heinemann, F. Histopathological Verification of Osteoimmunological Mediators in Peri-Implantitis and Correlation to Bone Loss and Implant Functional Period. J. Oral Implantol. 2016, 42, 61–68. [Google Scholar] [CrossRef]
- Shnaiderman-Shapiro, A.; Dayan, D.; Buchner, A.; Schwartz, I.; Yahalom, R.; Vered, M. Histopathological spectrum of bone lesions associated with dental implant failure: Osteomyelitis and beyond. Head Neck Pathol. 2015, 9, 140–146. [Google Scholar] [CrossRef] [Green Version]
- Vlahović, Z.; Marković, A.; Lazić, Z.; Šćepanović, M.; Đinić, A.; Kalanović, M. Histopathological comparative analysis of periimplant bone inflammatory response after dental implant insertion using flap and flapless surgical technique. An experimental study in pigs. Clin. Oral Implant. Res. 2017, 28, 1067–1073. [Google Scholar] [CrossRef]
- Linag, Y.; Li, H.; Xu, J.; Li, X.I.N.; Li, X.; Yam, Y.; Qi, M.; Hi, M.I.N. Strontium coating by electrochemical deposition improves implant osseointegration in osteopenic models. Exp. Ther. Med. 2014, 9, 172–176. [Google Scholar] [CrossRef]
- Chen, R.; Willcox, M.D.P.; Ho, K.K.K.; Smyth, D.; Kumar, N. Antimicrobial peptide melimine coating for titanium and its in vivo antibacterial activity in rodent subcutaneous infection models. Biomaterials 2016, 85, 142–151. [Google Scholar] [CrossRef]
- Summers, R.B. A new concept in maxillary implant surgery: The osteotome technique. Compendium 1994, 15, 152, 154–156, 158 passim; quiz 162. [Google Scholar] [PubMed]
- Yu, Y.; Jin, G.; Xue, Y.; Wang, D.; Liu, X.; Sun, J. Multifunctions of dual Zn/Mg ion co-implanted titanium on osteogenesis, angiogenesis and bacteria inhibition for dental implants. Acta Biomater. 2017, 49, 590–603. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gryshkov, O.; Klyui, N.I.; Temchenko, V.P.; Kyselov, V.S.; Chatterjee, A.; Belyaev, A.E.; Lauterboeck, L.; Iarmolenko, D.; Glasmacher, B. Porous biomorphic silicon carbide ceramics coated with hydroxyapatite as prospective materials for bone implants. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 68, 143–152. [Google Scholar] [CrossRef]
- Xiang, Y.; Li, J.; Liu, X.; Cui, Z.; Yang, X.; Yeung, K.W.K.; Pan, H.; Wu, S. Construction of poly(lactic-co-glycolic acid)/ZnO nanorods/Ag nanoparticles hybrid coating on Ti implants for enhanced antibacterial activity and biocompatibility. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 79, 629–637. [Google Scholar] [CrossRef] [PubMed]
- Spiers, K.; Cardamone, T.; Furness, J.B.; Clark, J.C.M.; Patrick, J.F.; Clark, G.M. An X-ray fluorescence microscopic analysis of the tissue surrounding the multi-channel cochlear implant electrode array. Cochlear Implant. Int. 2016, 17, 129–131. [Google Scholar] [CrossRef]
- Bilo, F.; Borgese, L.; Prost, J.; Rauwolf, M.; Turyanskaya, A.; Wobrauschek, P.; Kregsamer, P.; Streli, C.; Pazzaglia, U.; Depero, L.E. Atomic layer deposition to prevent metal transfer from implants: An X-ray fluorescence study. Appl. Surf. Sci. 2015, 359, 215–220. [Google Scholar] [CrossRef]
- Uo, M. Dissolution of nickel and tissue response observed by X-ray scanning analytical microscopy. Biomaterials 1999, 20, 747–755. [Google Scholar] [CrossRef]
- Zhao, C.; Pandit, S.; Fu, Y.; Mijakovic, I.; Jesorka, A.; Liu, J. Graphene oxide based coatings on nitinol for biomedical implant applications: Effectively promote mammalian cell growth but kill bacteria. Rsc Adv. 2016, 6, 38124–38134. [Google Scholar] [CrossRef]
- Burmeister, J.S.; Olivier, L.A.; Reichert, W.M.; Truskey, G.A. Application of total internal reflection fluorescence microscopy to study cell adhesion to biomaterials. Biomaterials 1998, 19, 307–325. [Google Scholar] [CrossRef]
- Truskey, G.A.; Burmeister, J.S.; Grapa, E.; Reichert, W.M. Total internal reflection fluorescence microscopy (TIRFM). II. Topographical mapping of relative cell/substratum separation distances. J. Cell Sci. 1992, 103 Pt 2, 491–499. [Google Scholar]
- Berdichevski, A.; Simaan Yameen, H.; Dafni, H.; Neeman, M.; Seliktar, D. Using bimodal MRI/fluorescence imaging to identify host angiogenic response to implants. Proc. Natl. Acad. Sci. USA 2015, 112, 5147–5152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lovati, A.B.; Lopa, S.; Talò, G.; Previdi, S.; Recordati, C.; Mercuri, D.; Segatti, F.; Zagra, L.; Moretti, M. In vivo evaluation of bone deposition in macroporous titanium implants loaded with mesenchymal stem cells and strontium-enriched hydrogel. J. Biomed. Mater. Res. Part B Appl. Biomater. 2015, 103, 448–456. [Google Scholar] [CrossRef] [PubMed]
- Markovic, S.; Belz, J.; Kumar, R.; Cormack, R.A.; Sridhar, S.; Niedre, M. Near-infrared fluorescence imaging platform for quantifying in vivo nanoparticle diffusion from drug loaded implants. Int. J. Nanomed. 2016, 11, 1213–1223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kubitscheck, U. Fluorescence Microscopy: From Principles to Biological Applications/Edited by Ulrich Kubitscheck, 2nd ed.; Wiley-VCH: Weinheim, Germany, 2017; ISBN 9783527687756. [Google Scholar]
- Liu, J.; Sun, Y.-Q.; Zhang, H.; Shi, H.; Shi, Y.; Guo, W. Sulfone-Rhodamines: A New Class of Near-Infrared Fluorescent Dyes for Bioimaging. Acs Appl. Mater. Interfaces 2016, 8, 22953–22962. [Google Scholar] [CrossRef]
- Yu, J.; Su, X.; Zhu, C.; Pan, Q.; Yang, J.; Ma, J.; Shen, L.; Cao, H.; Li, L. GFP Labeling and Hepatic Differentiation Potential of Human Placenta-Derived Mesenchymal Stem Cells. Cell. Physiol. Biochem. 2015, 35, 2299–2308. [Google Scholar] [CrossRef]
- Beija, M.; Afonso, C.A.M.; Martinho, J.M.G. Synthesis and applications of Rhodamine derivatives as fluorescent probes. Chem. Soc. Rev. 2009, 38, 2410–2433. [Google Scholar] [CrossRef] [Green Version]
- Xie, L.; Chen, Y.; Wu, W.; Guo, H.; Zhao, J.; Yu, X. Fluorescent coumarin derivatives with large stokes shift, dual emission and solid state luminescent properties: An experimental and theoretical study. Dye. Pigm. 2012, 92, 1361–1369. [Google Scholar] [CrossRef]
- Zhang, X.-F.; Zhang, J.; Liu, L. Fluorescence properties of twenty fluorescein derivatives: Lifetime, quantum yield, absorption and emission spectra. J. Fluoresc. 2014, 24, 819–826. [Google Scholar] [CrossRef]
- Reiss, P.; Quemard, G.; Carayon, S.; Bleuse, J.; Chandezon, F.; Pron, A. Luminescent ZnSe nanocrystals of high color purity. Mater. Chem. Phys. 2004, 84, 10–13. [Google Scholar] [CrossRef]
- Cui, R.; Liu, H.-H.; Xie, H.-Y.; Zhang, Z.-L.; Yang, Y.-R.; Pang, D.-W.; Xie, Z.-X.; Chen, B.-B.; Hu, B.; Shen, P. Living Yeast Cells as a Controllable Biosynthesizer for Fluorescent Quantum Dots. Adv. Funct. Mater. 2009, 19, 2359–2364. [Google Scholar] [CrossRef]
- Chen, H.-S.; Hsu, C.-K.; Hong, H.-Y. InGaN-CdSe-ZnSe quantum dots white LEDs. Ieee Photon. Technol. Lett. 2006, 18, 193–195. [Google Scholar] [CrossRef]
- Suzuki, T.; Fujikura, K.; Higashiyama, T.; Takata, K. DNA staining for fluorescence and laser confocal microscopy. J. Histochem. Cytochem. 1997, 45, 49–53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chazotte, B. Labeling cytoskeletal F-actin with rhodamine phalloidin or fluorescein phalloidin for imaging. Cold Spring Harb. Protoc. 2010, 2010, pdb.prot4947. [Google Scholar] [CrossRef] [PubMed]
- Macri-Pellizzeri, L.; de Melo, N.; Ahmed, I.; Grant, D.; Scammell, B.; Sottile, V. Live Quantitative Monitoring of Mineral Deposition in Stem Cells Using Tetracycline Hydrochloride. Tissue Eng. Part C Methods 2018, 24, 171–178. [Google Scholar] [CrossRef] [Green Version]
- Stiefel, P.; Schmidt-Emrich, S.; Maniura-Weber, K.; Ren, Q. Critical aspects of using bacterial cell viability assays with the fluorophores SYTO9 and propidium iodide. BMC Microbiol. 2015, 15, 36. [Google Scholar] [CrossRef] [Green Version]
- Cell Cycle- Materials and Methods; Pagano, M. (Ed.) Springer Verlag: New York, NY, USA, 1995; ISBN 9780387580661. [Google Scholar]
- Chalfie, M.; Kain, S. Green Fluorescent Protein: Properties, Applications and Protocols/Edited by Martin Chalfie, Steven, P. Kain, 2nd ed.; Wiley: New York, NY, USA, 2006; ISBN 0471736821. [Google Scholar]
- Hoffmann, C.; Gaietta, G.; Zürn, A.; Adams, S.R.; Terrillon, S.; Ellisman, M.H.; Tsien, R.Y.; Lohse, M.J. Fluorescent labeling of tetracysteine-tagged proteins in intact cells. Nat. Protoc. 2010, 5, 1666–1677. [Google Scholar] [CrossRef]
- Lichtman, J.W.; Conchello, J.-A. Fluorescence microscopy. Nat. Methods 2005, 2, 910–919. [Google Scholar] [CrossRef]
- Nkenke, E.; Kloss, F.; Wiltfang, J.; Schultze-Mosgau, S.; Radespiel-Troger, M.; Loos, K.; Neukam, F.W. Histomorphometric and fluorescence microscopic analysis of bone remodelling after installation of implants using an osteotome technique. Clin. Oral. Implant. Res. 2002, 13, 595–602. [Google Scholar] [CrossRef]
- Hennig, B.; Toborek, M.; McClain, C.J. Antiatherogenic properties of zinc: Implications in endothelial cell metabolism. Nutrition 1996, 12, 711–717. [Google Scholar] [CrossRef]
- Zreiqat, H.; Howlett, C.R.; Zannettino, A.; Evans, P.; Schulze-Tanzil, G.; Knabe, C.; Shakibaei, M. Mechanisms of magnesium-stimulated adhesion of osteoblastic cells to commonly used orthopaedic implants. J. Biomed. Mater. Res. 2002, 62, 175–184. [Google Scholar] [CrossRef]
- Michette, A.G.; Morrison, G.R.; Buckley, C.J. X-Ray Microscopy III: Proceedings of the Third International Conference, London, UK, 3–7 September 1990; Springer: Berlin/Heidelberg, Germany, 1992; ISBN 9783662138946. [Google Scholar]
- Nakasako, M. X-Ray Diffraction Imaging of Biological Cells; Springer: Tokyo, Japan, 2018; ISBN 4431566163. [Google Scholar]
- Hawkes, P.W.; Spence, J.C.H. Springer Handbook of Microscopy; Springer: Cham, Switzerland, 2019; ISBN 9783030000684. [Google Scholar]
- Rivers, M.L.; Sutton, S.R.; Jones, K.W. Synchrotron X-ray fluorescence microscopy. Synchrotron Radiat. News 1991, 4, 23–26. [Google Scholar] [CrossRef]
- Cheremisinoff, N.P. Elemental and Structural Characterization Tests. In Polymer Characterization: Laboratory Techniques and Analysis/by Nicholas, P. Cheremisinoff; Cheremisinoff, N.P., Ed.; Noyes Publications: Westwood, NJ, USA, 1996; pp. 43–81. ISBN 9780815514039. [Google Scholar]
- Matsumoto, B. Cell Biological Applications of Confocal Microscopy, 2nd ed.; Academic Press: Amsterdam, The Netherlands, 2002; ISBN 9780124802773. [Google Scholar]
- Pawley, J.B. Handbook of Biological Confocal Microscopy, 3rd ed.; Springer: New York, NY, USA, 2005; ISBN 9780387259215. [Google Scholar]
- Price, R.L.; Jerome, W.G. Basic Confocal Microscopy, 2nd ed.; Springer Science+Business Media, LLC: New York, NY, USA, 2018; ISBN 9780387781747. [Google Scholar]
- Nwaneshiudu, A.; Kuschal, C.; Sakamoto, F.H.; Anderson, R.R.; Schwarzenberger, K.; Young, R.C. Introduction to confocal microscopy. J. Investig. Dermatol. 2012, 132, e3. [Google Scholar] [CrossRef] [Green Version]
- Khosla, S.; Melton, L.J. Clinical practice. Osteopenia. N. Engl. J. Med. 2007, 356, 2293–2300. [Google Scholar] [CrossRef] [PubMed]
- Jurczak, P.; Witkowska, J.; Rodziewicz-Motowidlo, S.; Lach, S. Proteins, peptides and peptidomimetics as active agents in implant surface functionalization. Adv. Colloid Interface Sci. 2019, 102083. [Google Scholar] [CrossRef]
- Super-Resolution Microscopy: Methods and Protocols; Erfle, H. (Ed.) Springer: New York, NY, USA, 2017; ISBN 1493972650. [Google Scholar]
- Fluorescence Microscopy: Super-Resolution and Other Novel Techniques, 1st ed.; Cornea, A.; Conn, P.M. (Eds.) Academic/Elsevier Press: London, UK, 2014; ISBN 9780124167131. [Google Scholar]
- Birk, U.J. Super-resolution Microscopy: A Practical Guide; Wiley-VCH: Mainz, Germany; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2017; ISBN 9783527802067. [Google Scholar]
- Axelrod, D. Total internal reflection fluorescence microscopy in cell biology. Traffic 2001, 2, 764–774. [Google Scholar] [CrossRef]
- Poulter, N.S.; Pitkeathly, W.T.E.; Smith, P.J.; Rappoport, J.Z. The physical basis of total internal reflection fluorescence (TIRF) microscopy and its cellular applications. Methods Mol. Biol. 2015, 1251, 1–23. [Google Scholar] [CrossRef]
- Fu, Y.; Winter, P.W.; Rojas, R.; Wang, V.; McAuliffe, M.; Patterson, G.H. Axial superresolution via multiangle TIRF microscopy with sequential imaging and photobleaching. Proc. Natl. Acad. Sci. USA 2016, 113, 4368–4373. [Google Scholar] [CrossRef] [Green Version]
- Mattheyses, A.L.; Simon, S.M.; Rappoport, J.Z. Imaging with total internal reflection fluorescence microscopy for the cell biologist. J. Cell Sci. 2010, 123, 3621–3628. [Google Scholar] [CrossRef] [Green Version]
- Thompson, N.L.; Lagerholm, B.C. Total internal reflection fluorescence: Applications in cellular biophysics. Curr. Opin. Biotech. 1997, 8, 58–64. [Google Scholar] [CrossRef]
- Degasne, I.; Baslé, M.F.; Demais, V.; Huré, G.; Lesourd, M.; Grolleau, B.; Mercier, L.; Chappard, D. Effects of roughness, fibronectin and vitronectin on attachment, spreading, and proliferation of human osteoblast-like cells (Saos-2) on titanium surfaces. Calcif. Tissue Int. 1999, 64, 499–507. [Google Scholar] [CrossRef]
- Massia, S.P.; Hubbell, J.A. An RGD spacing of 440 nm is sufficient for integrin alpha V beta 3-mediated fibroblast spreading and 140 nm for focal contact and stress fiber formation. J. Cell Biol. 1991, 114, 1089–1100. [Google Scholar] [CrossRef] [PubMed]








| Time | Weight % | Ratio | |
|---|---|---|---|
| Ca | P | Ca/P | |
| 15.07 ± 2.83 | 7.83 ± 1.56 | 1.93 ± 0.10 | |
| 19.77 ± 1.65 | 10.27 ± 1.05 | 1.93 ± 0.07 | |
| 17.33 ± 2.39 | 8.90 ± 0.80 | 1.94 ± 0.10 | |
| 21.33 ± 1.88 | 11.03 ± 0.70 | 1.93 ± 0.05 | |
| Product | Code | Surface Treatment * | Manufacturer |
|---|---|---|---|
| Allfit | ALF | Al2O3 blasted | Dr. Idhe Dental |
| Ice | ICE | Smooth machined | 3i |
| IMZ TPS | TPS | Titanium plasma sprayed | Friedrichsfeld |
| Laser Lok | LLK | Tricalcium phosphate/hydroxyapatite blasted | Biohorizons |
| PrimaConnex | PRC | Calcium phosphate blasted | Lifecore Biomedical |
| Ospol | OSP | Calcium-anodized | Ospol |
| Osseospeed TX | OSS | Titanium oxide blasted/fluoride treated | Astra Tech |
| Osseotite Full | OTF | Acid-etched (double) | 3i |
| Replace Select | RPS | Calcium phosphate anodized | Nobel Biocare |
| SLA | SLA | Al2O3 blasted/acid etched | Institute Straumann |
| Trilobe | TRB | Al2O3 blasted | Southern Implants |
| Fluorescent Technique | Staining Method | Monitored Cells | Monitored Tissues | Principle Of The Study | Ref. |
|---|---|---|---|---|---|
| Fluorescence microscopy | Oxytetracycline, alizarin complexion, calcein, xylenol | - | Implant-surrounding fibrous tissue | Evaluation of the osteotome technique | [114] |
| Rhodamine-phalloidin, DAPI, fluorochrome-conjugated secondary antibody | rBMSC, HUVEC | - | Evaluation of the osteogenic and pro-angiogenic properties of Zn/Mg coating | [115] | |
| Bisbenzimide trihydrochloride, phalloidin Atto 488 | NIH 3T3 | - | Evaluation of the properties and cytocompatibility of silicon carbide ceramics | [116] | |
| FITC-phalloidin, DAPI, propidium iodide, acridine orange | MC3T3-E1, S. aureus, E. coli | - | Evaluation of the cytocompatibility and antibacterial properties of ZnO/Ag/PLGA coating | [117] | |
| X-ray fluorescence microscopy | - | - | Implant-surrounding fibrous tissue | Evaluate the level of corrosion of electrode implants | [118] |
| - | - | Implant-surrounding bone tissue | Monitoring of the release of cobalt and chromium from Co-Cr alloy implant | [119] | |
| - | - | Implant-surrounding connective tissue | Monitoring of the release of nickel from nickel-based implants | [120] | |
| Confocal microscopy | Tetracycline hydrochloride, calcein | - | Implant-surrounding bone tissue | Visualization of the process of the new bone formation | [112] |
| Phalloidin, DAPI, live/dead assay kit (in vitro), calcein (in vivo) | hMSC | Implant-surrounding bone tissue | Description of the influence of a coating on the new bone formation | [108] | |
| Immunostaining with goat anti-mouse IgG-FITC, rhodamine-phalloidin, DAPI, Live/Dead Bacterial Viability Kit | MC3T3-E1, E. coli | - | Evaluation of cytocompatibility and antibacterial properties of graphene/gelatin-based coating | [121] | |
| Total internal reflection fluorescence microscopy | 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanineperchlorate (DiIC18(3)) | Bovine aortic endothelial cells | - | Description of dynamics of cell surface adhesion | [122,123] |
| 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanineperchlorate (DiIC18(3)) | Endothelial cells | - | Description of dynamics of cell-implant contact | [122,123] | |
| Imaging systems | Cy5.5 N-hydroxysuccinimide | - | Hydrogel-surrounding soft tissue | Integration of biodegradable hydrogel implants containing VEGF | [124] |
| OsteoSenseTM750 | - | Bone tissue accumulated on the implants | Bone deposition on surface-modified titanium implants | [125] | |
| Cy7.5-NHS ester | - | Implant-surrounding soft tissue | Visualization and quantification of diffusion of fluorescently doped NPs | [126] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lach, S.; Jurczak, P.; Karska, N.; Kubiś, A.; Szymańska, A.; Rodziewicz-Motowidło, S. Spectroscopic Methods Used in Implant Material Studies. Molecules 2020, 25, 579. https://doi.org/10.3390/molecules25030579
Lach S, Jurczak P, Karska N, Kubiś A, Szymańska A, Rodziewicz-Motowidło S. Spectroscopic Methods Used in Implant Material Studies. Molecules. 2020; 25(3):579. https://doi.org/10.3390/molecules25030579
Chicago/Turabian StyleLach, Sławomir, Przemysław Jurczak, Natalia Karska, Agnieszka Kubiś, Aneta Szymańska, and Sylwia Rodziewicz-Motowidło. 2020. "Spectroscopic Methods Used in Implant Material Studies" Molecules 25, no. 3: 579. https://doi.org/10.3390/molecules25030579







