Characterization and Antioxidant Activity Determination of Neutral and Acidic Polysaccharides from Panax Ginseng C. A. Meyer
Abstract
:1. Introduction
2. Results and Discussion
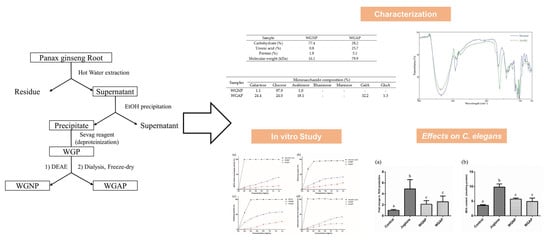
2.1. Extraction and Isolation of Polysaccharide from Panax Ginseng
2.2. Chemical Compositions and Molecular Weight of WGNP and WGAP
2.3. Fourier-Transform Infrared Spectroscopy (FT-IR) Spectrum of WGNP and WGAP
2.4. In vitro Antioxidant Activities of Polysaccharides
2.4.1. ABTS Radical Scavenging Activity
2.4.2. Reducing Power
2.4.3. Ferrous Ion Chelating Activity
2.4.4. Hydroxyl Radical Scavenging Activities
2.5. Antioxidant Activity of Polysaccharide on C. elegans
2.5.1. Lethality Assays of Polysaccharide on C. elegans
2.5.2. Antioxidant Effect of WGP on Oxidative Stress-Induced C. elegans
2.5.3. Lipid Peroxidation Assay
3. Materials and Methods
3.1. Materials and Reagent
3.2. Extraction and Purification of Polysaccharides
3.3. Characterization of WGPN and WGPA
3.3.1. Chemical Properties
3.3.2. Fourier-Transform Infrared Spectroscopy (FT-IR)
3.3.3. Monosaccharide Composition
3.4. In vitro Antioxidant Activities of Polysaccharides
3.4.1. ABTS Radical Scavenging Activity
3.4.2. Reducing Power Method
3.4.3. Ferrous Ion Chelating Activity
3.4.4. Hydroxyl Radical Scavenging Activity
3.5. Antioxidant Activity of Polysaccharide on C. elegans
3.5.1. C. elegans Strain and Culture Condition
3.5.2. Lethality Assay
3.5.3. Experimental Design
3.5.4. Intracellular ROS Assay
3.5.5. Lipid Peroxidation Assay
3.6. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| WGAP | White ginseng acidic polysaccharide |
| WGNP | White ginseng neutral polysaccharide |
| ROS | Reactive oxygen species |
| SOD | Superoxide dismutase |
| CAT | Catalase |
| GSH-Px | Glutathione peroxidase |
| ABTS | 2-2′-azino-bis-(3-ethylbenzothiazoline-6-sulfonic acid) |
| WGP | White ginseng polysaccharide |
| DEAE | Diethylaminoethyl |
| BCA | Bicinchoninic acid |
| HPGPC | High-performance gel permeation chromatography |
| FT-IR | Fourier-transform infrared spectroscopy |
| PMP | 1-phenyl-3-methyl-5-pyrazolone |
| NGM | Nematode growth media |
| FuDR | 2′-Deoxy-5-fluorouridine |
| MDA | Malondialdehyde |
| TBARS | Thiobarbituric acid reactive substance |
| H2DCFDA | 2,7-Dichlorodihydrofluorescein diacetate |
References
- Zhu, R.; Zhang, X.; Wang, Y.; Zhang, L.; Zhao, J.; Chen, G.; Fan, J.; Jia, Y.; Yan, F.; Ning, C. Characterization of polysaccharide fractions from fruit of Actinidia arguta and assessment of their antioxidant and antiglycated activities. Carbohy. Polym. 2019, 210, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Gao, T.; Yang, Y.; Meng, F.; Zhan, F.; Jiang, Q.; Sun, X. Anti-Cancer Activity of Porphyran and Carrageenan from Red Seaweeds. Molecules 2019, 24, 4286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hua, M.; Lu, J.; Qu, D.; Liu, C.; Zhang, L.; Li, S.; Chen, J.; Sun, Y. Structure, physicochemical properties and adsorption function of insoluble dietary fiber from ginseng residue: A potential functional ingredient. Food Chem. 2019, 286, 522–529. [Google Scholar] [CrossRef] [PubMed]
- Park, S.E.; Seo, S.H.; Kim, E.J.; Park, D.H.; Park, K.M.; Cho, S.S.; Son, H.S. Metabolomic Approach for Discrimination of Cultivation Age and Ripening Stage in Ginseng Berry Using Gas Chromatography-Mass Spectrometry. Molecules 2019, 24, 3837. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, W.; Balan, P.; Popovich, D.G. Analysis of Ginsenoside Content (Panax ginseng) from Different Regions. Molecules 2019, 24, 3491. [Google Scholar] [CrossRef] [Green Version]
- Choi, J.; Kim, T.-H.; Choi, T.-Y.; Lee, M.S. Ginseng for health care: A systematic review of randomized controlled trials in Korean literature. PloS ONE 2013, 8, e59978. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.W.; Lim, H.-J.; Jun, J.H.; Choi, J.; Lee, M.S. Ginseng for Treating Hypertension: A Systematic Review and Meta-Analysis of Double Blind, Randomized, Placebo-Controlled Trials. Cur. Vascular Pharmaco. 2017, 15, 549–556. [Google Scholar] [CrossRef]
- Lee, C.H.; Kim, J.-H. A review on the medicinal potentials of ginseng and ginsenosides on cardiovascular diseases. J. Ginseng Res. 2014, 38, 161–166. [Google Scholar] [CrossRef] [Green Version]
- Wu, W.; Jiao, C.; Li, H.; Ma, Y.; Jiao, L.; Liu, S. LC-MS based metabolic and metabonomic studies of Panax ginseng. Phytochem. Anal. 2018, 29, 331–340. [Google Scholar] [CrossRef]
- Sun, C.; Chen, Y.; Li, X.; Tai, G.; Fan, Y.; Zhou, Y. Anti-hyperglycemic and anti-oxidative activities of ginseng polysaccharides in STZ-induced diabetic mice. Food Function 2014, 5, 845–848. [Google Scholar] [CrossRef]
- Luo, D.; Fang, B. Structural identification of ginseng polysaccharides and testing of their antioxidant activities. Carbohydr. Polym. 2008, 72, 376–381. [Google Scholar] [CrossRef]
- Kang, M.-C.; Kim, S.-Y.; Kim, E.-A.; Lee, J.-H.; Kim, Y.-S.; Yu, S.-K.; Chae, J.B.; Choe, I.-H.; Cho, J.H.; Jeon, Y.-J. Antioxidant activity of polysaccharide purified from Acanthopanax koreanum Nakai stems in vitro and in vivo zebrafish model. Carbohydr. Polym. 2015, 127, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Rani, V.; Deep, G.; Singh, R.K.; Palle, K.; Yadav, U.C. Oxidative stress and metabolic disorders: Pathogenesis and therapeutic strategies. Life Sci. 2016, 148, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Jomova, K.; Hudecova, L.; Lauro, P.; Simunkova, M.; Alwasel, S.H.; Alhazza, I.M.; Valko, M. A Switch between Antioxidant and Prooxidant Properties of the Phenolic Compounds Myricetin, Morin, 3’,4’-Dihydroxyflavone, Taxifolin and 4-Hydroxy-Coumarin in the Presence of Copper(II) Ions: A Spectroscopic, Absorption Titration and DNA Damage Study. Molecules 2019, 24, 4335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reynolds, A.; Laurie, C.; Mosley, R.L.; Gendelman, H.E. Oxidative stress and the pathogenesis of neurodegenerative disorders. Int. Rev. Neurobiol. 2007, 82, 297–325. [Google Scholar]
- Davalli, P.; Mitic, T.; Caporali, A.; Lauriola, A.; D’Arca, D. ROS, cell senescence, and novel molecular mechanisms in aging and age-related diseases. Oxid. Med. Cell. Longev. 2016, 2016, 3565127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lei, X.G.; Zhu, J.-H.; Cheng, W.-H.; Bao, Y.; Ho, Y.-S.; Reddi, A.R.; Holmgren, A.; Arnér, E.S. Paradoxical roles of antioxidant enzymes: Basic mechanisms and health implications. Physiol. Rev. 2015, 96, 307–364. [Google Scholar] [CrossRef] [Green Version]
- Pisoschi, A.M.; Pop, A. The role of antioxidants in the chemistry of oxidative stress: A review. Eur. J. Med. Chem. 2015, 97, 55–74. [Google Scholar] [CrossRef]
- Kim, H.M.; Lee, D.-K.; Long, N.P.; Kwon, S.W.; Park, J.H. Uptake of nanopolystyrene particles induces distinct metabolic profiles and toxic effects in Caenorhabditis elegans. Environ. Pollut. 2019, 246, 578–586. [Google Scholar] [CrossRef]
- Nimse, S.B.; Pal, D. Free radicals, natural antioxidants, and their reaction mechanisms. Rsc Adv. 2015, 5, 27986–28006. [Google Scholar] [CrossRef] [Green Version]
- Matés, J.M.; Pérez-Gómez, C.; De Castro, I.N. Antioxidant enzymes and human diseases. Clin. Biochem. 1999, 32, 595–603. [Google Scholar]
- Manzocco, L.; Calligaris, S.; Mastrocola, D.; Nicoli, M.C.; Lerici, C.R. Review of non-enzymatic browning and antioxidant capacity in processed foods. Trends Food Sci. Technol. 2000, 11, 340–346. [Google Scholar] [CrossRef]
- Dong, J.l.; Wang, L.; LÜ, J.; Zhu, Y.y.; Shen, R.l. Structural, antioxidant and adsorption properties of dietary fiber from foxtail millet (Setaria italica) bran. J. Sci. Food Agric. 2019, 99, 3886–3894. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Zhang, W.; Cheng, J.; Lu, Z. Antioxidant and physicochemical properties of soluble dietary fiber from garlic straw as treated by energy-gathered ultrasound. Int. J. Food Prop. 2019, 22, 678–688. [Google Scholar] [CrossRef] [Green Version]
- Kalisz, S.; Oszmiański, J.; Kolniak-Ostek, J.; Grobelna, A.; Kieliszek, M.; Cendrowski, A. Effect of a variety of polyphenols compounds and antioxidant properties of rhubarb (Rheum rhabarbarum). LWT 2020, 118, 108775. [Google Scholar] [CrossRef]
- Grobelna, A.; Kalisz, S.; Kieliszek, M. The effect of the addition of blue honeysuckle berry juice to apple juice on the selected quality characteristics, anthocyanin stability, and antioxidant properties. Biomolecules 2019, 9, 744. [Google Scholar] [CrossRef] [Green Version]
- Bermúdez-Oria, A.; Rodríguez-Gutiérrez, G.; Fernández-Prior, Á.; Vioque, B.; Fernández-Bolaños, J. Strawberry dietary fiber functionalized with phenolic antioxidants from olives. Interactions between polysaccharides and phenolic compounds. Food Chem. 2019, 280, 310–320. [Google Scholar]
- Li, K.; Li, S.; Wang, D.; Li, X.; Wu, X.; Liu, X.; Du, G.; Li, X.; Qin, X.; Du, Y. Extraction, Characterization, Antitumor and Immunological Activities of Hemicellulose Polysaccharide from Astragalus radix Herb Residue. Molecules 2019, 24, 3644. [Google Scholar] [CrossRef] [Green Version]
- Xu, W.; Choi, H.-K.; Huang, L. State of Panax ginseng research: A global analysis. Molecules 2017, 22, 1518. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.J.; Kim, P.; Shin, C.Y. A comprehensive review of the therapeutic and pharmacological effects of ginseng and ginsenosides in central nervous system. J. Ginseng Res. 2013, 37, 8. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Fan, Y.; Wang, W.; Liu, N.; Zhang, H.; Zhu, Z.; Liu, A. Polysaccharides from Lycium barbarum leaves: Isolation, characterization and splenocyte proliferation activity. Int. J. Biol. Macromol. 2012, 51, 417–422. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Feng, S.; Shen, S.; Wang, H.; Yuan, M.; Liu, J.; Huang, Y.; Ding, C. The antioxidant activities effect of neutral and acidic polysaccharides from Epimedium acuminatum Franch. on Caenorhabditis elegans. Carbohydr. Polym. 2016, 144, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Pütter, J.; Becker, R. Methods of Enzymatic Analysis. Becker R 1983, 2, 286–293. [Google Scholar]
- Nam, W.; Kim, S.P.; Nam, S.H.; Friedman, M. Structure-Antioxidative and Anti-Inflammatory Activity Relationships of Purpurin and Related Anthraquinones in Chemical and Cell Assays. Molecules 2017, 22, 265. [Google Scholar] [CrossRef] [PubMed]
- Kong, Z.L.; Kuo, H.P.; Johnson, A.; Wu, L.C.; Chang, K.L.B. Curcumin-Loaded Mesoporous Silica Nanoparticles Markedly Enhanced Cytotoxicity in Hepatocellular Carcinoma Cells. Int. J. Mol. Sci. 2019, 20, 2918. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sridhar, K.; Charles, A.L. In vitro antioxidant activity of Kyoho grape extracts in DPPH and ABTS assays: Estimation methods for EC50 using advanced statistical programs. Food Chem. 2019, 275, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Liu, X.; Lian, C.; Ke, J.; Liu, J. Triterpenes and Aromatic Meroterpenoids with Antioxidant Activity and Neuroprotective Effects from Ganoderma lucidum. Molecules 2019, 24, 4353. [Google Scholar] [CrossRef] [Green Version]
- Hajji, M.; Hamdi, M.; Sellimi, S.; Ksouda, G.; Laouer, H.; Li, S.; Nasri, M. Structural characterization, antioxidant and antibacterial activities of a novel polysaccharide from Periploca laevigata root barks. Carbohydr. Polym. 2019, 206, 380–388. [Google Scholar] [CrossRef]
- Chen, J.; Huang, G. Antioxidant activities of garlic polysaccharide and its phosphorylated derivative. Int. J. Biol. Macromol. 2019, 125, 432–435. [Google Scholar] [CrossRef]
- Liu, J.; Luo, J.; Ye, H.; Sun, Y.; Lu, Z.; Zeng, X. In vitro and in vivo antioxidant activity of exopolysaccharides from endophytic bacterium Paenibacillus polymyxa EJS-3. Carbohydr. Polym. 2010, 82, 1278–1283. [Google Scholar] [CrossRef]
- Staub, A. Removeal of protein-Sevag method. Methods Carbohydr. Chem. 1965, 5, 5–6. [Google Scholar]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.t.; Smith, F. Colorimetric method for determination of sugars and related substances. Analytical chemistry 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Blumenkrantz, N.; Asboe-Hansen, G. New method for quantitative determination of uronic acids. Anal. Biochem. 1973, 54, 484–489. [Google Scholar] [CrossRef]
- Smith, P.K.; Krohn, R.I.; Hermanson, G.; Mallia, A.; Gartner, F.; Provenzano, M.; Fujimoto, E.; Goeke, N.; Olson, B.; Klenk, D. Measurement of protein using bicinchoninic acid. Anal. Biochem. 1985, 150, 76–85. [Google Scholar] [CrossRef]
- Guo, N.; Bai, Z.; Jia, W.; Sun, J.; Wang, W.; Chen, S.; Wang, H. Quantitative Analysis of Polysaccharide Composition in Polyporus umbellatus by HPLC-ESI-TOF-MS. Molecules 2019, 24, 2526. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Liang, J.; Luan, G.; Zhang, S.; Zhuoma, Y.; Xie, J.; Zhou, W. Quantitative Analyses of Nine Phenolic Compounds and Their Antioxidant Activities from Thirty-Seven Varieties of Raspberry Grown in the Qinghai-Tibetan Plateau Region. Molecules 2019, 24, 3932. [Google Scholar] [CrossRef] [Green Version]
- Ming, J.; Yaxin, C.; Jinrong, L.; Hui, Z. 1, 10-Phenanthroline-Fe2+ Oxidative Assay of Hydroxyl Radical Produced by H2O2/Fe2+. Progress Biochem. Biophys. 1996, 6. [Google Scholar]
- Kim, H.M.; Long, N.P.; Yoon, S.J.; Nguyen, H.T.; Kwon, S.W. Metabolomics and phenotype assessment reveal cellular toxicity of triclosan in Caenorhabditis elegans. Chemosphere 2019, 236, 124306. [Google Scholar] [CrossRef]
- Li, H.; Roxo, M.; Cheng, X.; Zhang, S.; Cheng, H.; Wink, M. Pro-oxidant and lifespan extension effects of caffeine and related methylxanthines in Caenorhabditis elegans. Food Chem.: X 2019, 1, 100005. [Google Scholar] [CrossRef]
- Kim, H.M.; Long, N.P.; Yoon, S.J.; Anh, N.H.; Kim, S.J.; Park, J.H.; Kwon, S.W. Omics approach reveals perturbation of metabolism and phenotype in Caenorhabditis elegans triggered by perfluorinated compounds. Sci. Total Environ. 2019, 703, 135500. [Google Scholar] [CrossRef]
- Azevedo, B.C.; Roxo, M.; Borges, M.C.; Peixoto, H.; Crevelin, E.J.; Bertoni, B.W.; Contini, S.H.T.; Lopes, A.A.; França, S.C.; Pereira, A.M.S.; et al. Antioxidant Activity of an Aqueous Leaf Extract from Uncaria tomentosa and Its Major Alkaloids Mitraphylline and Isomitraphylline in Caenorhabditis elegans. Molecules 2019, 24, 3299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Esterbauer, H.; Cheeseman, K.H. Determination of aldehydic lipid peroxidation products: Malonaldehyde and 4-hydroxynonenal. Methods Enzymol. 1990, 186, 407–421. [Google Scholar] [PubMed]
Sample Availability: Not available. |




| Sample | WGNP | WGAP |
|---|---|---|
| Carbohydrate (%) | 77.4 ± 0.6 | 28.2 ± 1.8 |
| Uronic Acid (%) | 0.8 ± 0.1 | 25.7 ± 0.8 |
| Protein (%) | 1.8 ± 0.2 | 5.1 ± 0.1 |
| Molecular Weight (kDa) | 16.1–70.4 | 50.0–80.0 |
| Samples | Monosaccharide Composition (%) | ||||||
|---|---|---|---|---|---|---|---|
| Galactose | Glucose | Arabinose | Rhamnose | Mannose | GalA | GluA | |
| WGNP | 1.1 | 97.9 | 1.0 | - | - | - | - |
| WGAP | 24.4 | 24.0 | 18.1 | - | - | 32.2 | 1.3 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.M.; Song, Y.; Hyun, G.H.; Long, N.P.; Park, J.H.; Hsieh, Y.S.Y.; Kwon, S.W. Characterization and Antioxidant Activity Determination of Neutral and Acidic Polysaccharides from Panax Ginseng C. A. Meyer. Molecules 2020, 25, 791. https://doi.org/10.3390/molecules25040791
Kim HM, Song Y, Hyun GH, Long NP, Park JH, Hsieh YSY, Kwon SW. Characterization and Antioxidant Activity Determination of Neutral and Acidic Polysaccharides from Panax Ginseng C. A. Meyer. Molecules. 2020; 25(4):791. https://doi.org/10.3390/molecules25040791
Chicago/Turabian StyleKim, Hyung Min, Yanxue Song, Gyu Hwan Hyun, Nguyen Phuoc Long, Jeong Hill Park, Yves S.Y. Hsieh, and Sung Won Kwon. 2020. "Characterization and Antioxidant Activity Determination of Neutral and Acidic Polysaccharides from Panax Ginseng C. A. Meyer" Molecules 25, no. 4: 791. https://doi.org/10.3390/molecules25040791