Removal of Volatile Phenols From Wine Using Crosslinked Cyclodextrin Polymers
Abstract
:1. Introduction
2. Results and Discussion
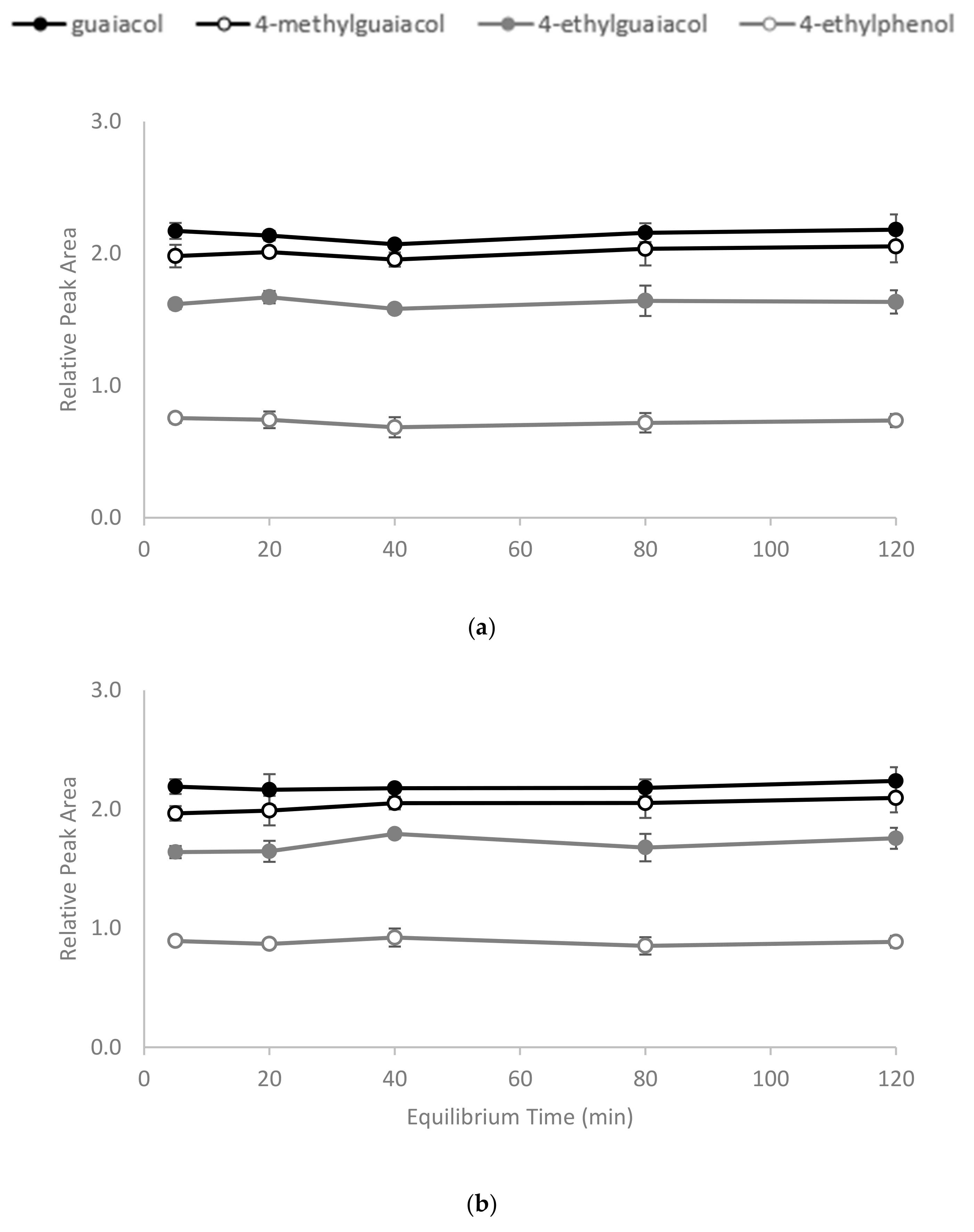
2.1. Preparation of CD Polymers and Determination of Time Required to Achieve Equilibrium
2.2. Determination of CD Polymer Adsorption Capacity in Model Wine
2.3. Batch Adsorption of Volatile Phenols by CD Polymers in Wine and Comparison with CD Addition
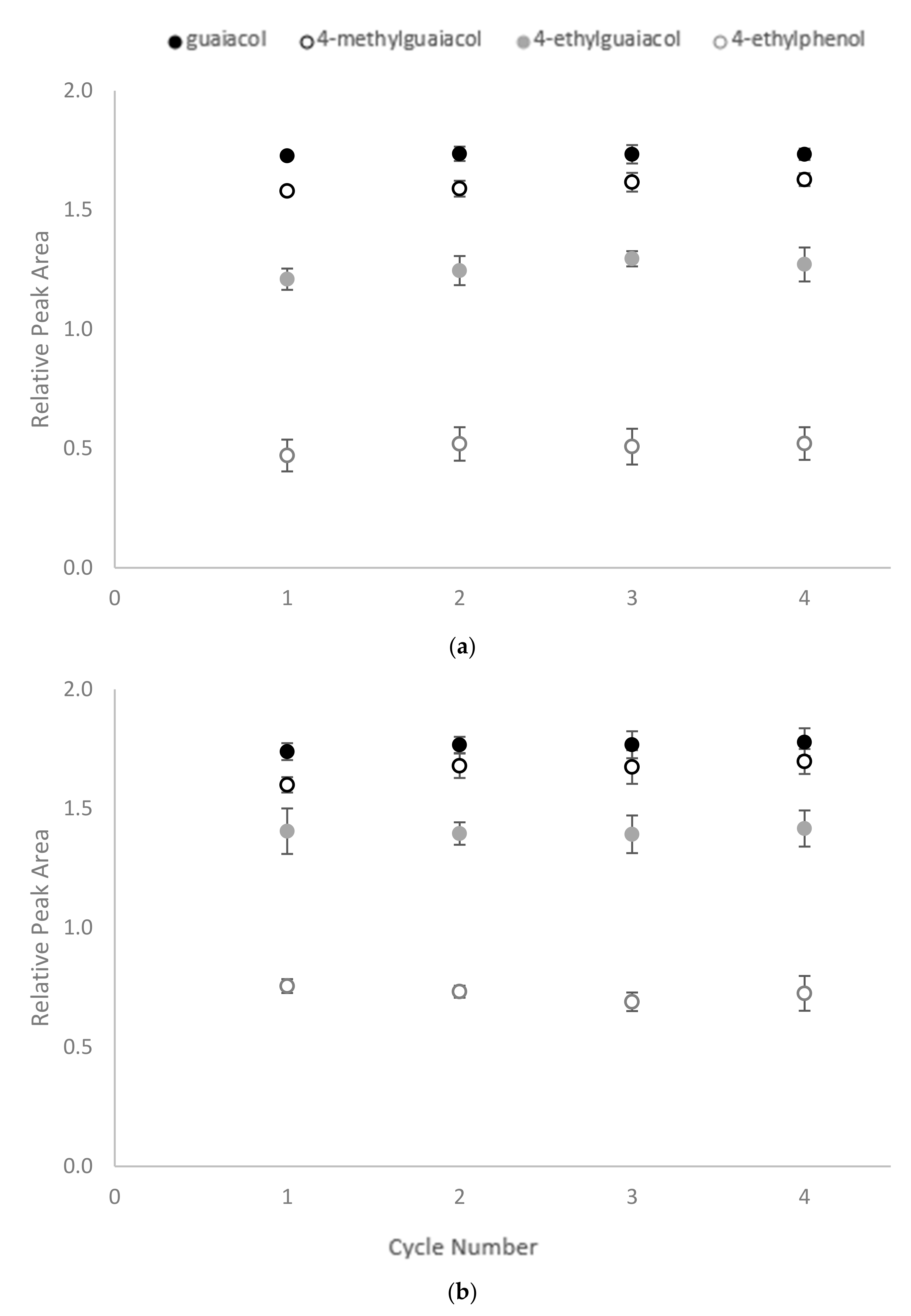
2.4. Reusability Test
3. Materials and Methods
3.1. Chemicals
3.2. Preparation of HDI Crosslinked CD Polymers (CD-HDI)
3.3. Adsorption Experiments with CD Polymers
3.4. Binding Experiments Comparing CDs and CD Polymers
3.5. Four-Phase HS-SPME GC-MS Analysis
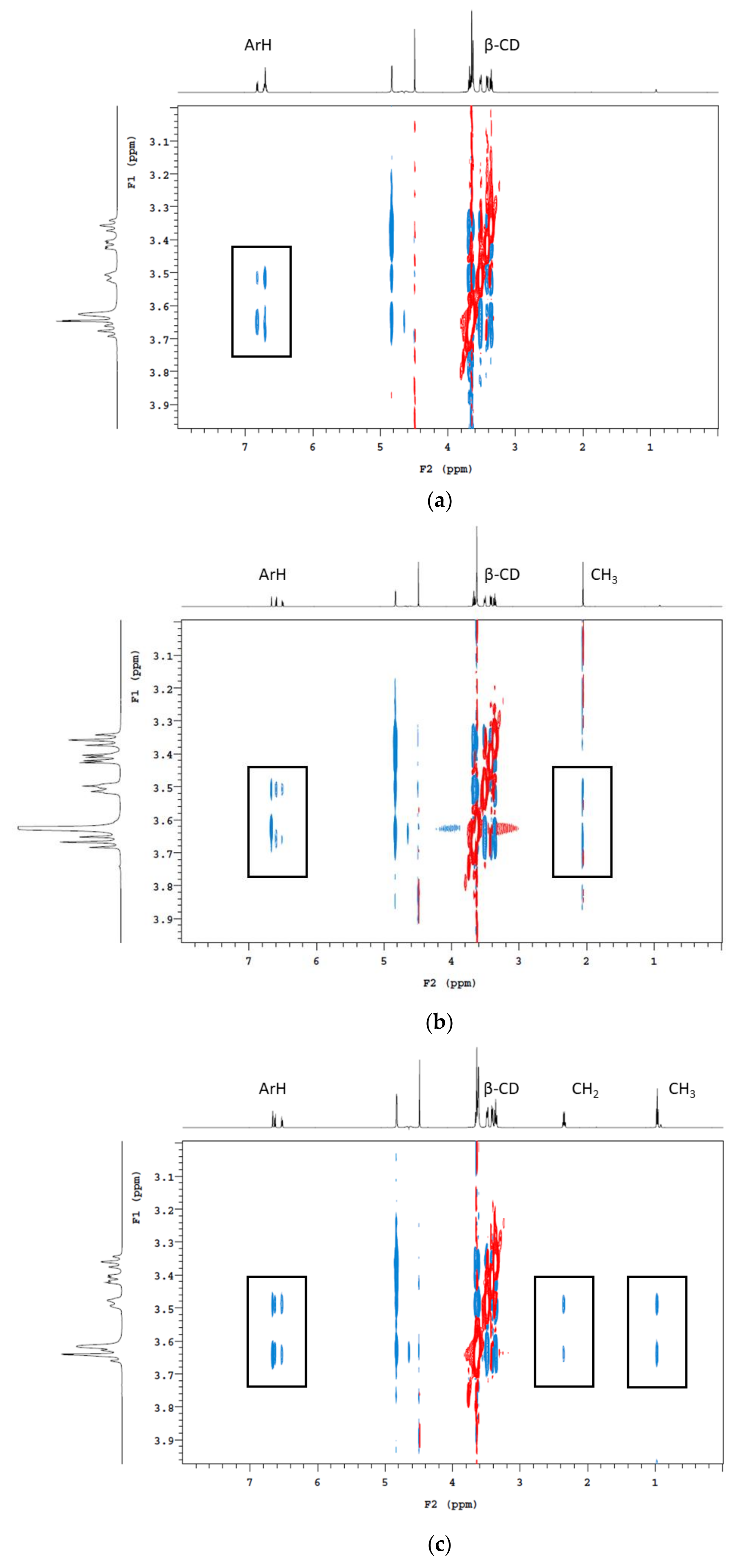
3.6. Nuclear Magnetic Resonance Analysis
3.7. Data Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- De Orduna, R.M. Climate change associated effects on grape and wine quality and production. Food Res. Int. 2010, 43, 1844–1855. [Google Scholar] [CrossRef]
- Carrasco-Sánchez, V.; John, A.; Marican, A.; Santos, L.S.; Laurie, V.F. Removal of 4-ethylphenol and 4-ethylguaiacol with polyaniline-based compounds in wine-like model solutions and red wine. Molecules 2015, 20, 14312–14325. [Google Scholar] [CrossRef] [Green Version]
- Chatonnet, P.; Dubourdieu, D.; Boidron, J.N. The influence of Brettanomyces/Dekkera sp. yeasts and lactic acid bacteria on the ethylphenol content of red wines. Am. J. Enol. Vitic. 1995, 46, 463–468. [Google Scholar]
- van Leeuwen, C.; Darriet, P. The impact of climate change on viticulture and wine quality. J. Wine Econ. 2016, 11, 150–167. [Google Scholar] [CrossRef] [Green Version]
- Kennison, K.R.; Wilkinson, K.L.; Williams, H.G.; Smith, J.H.; Gibberd, M.R. Smoke-derived taint in wine: Effect of postharvest smoke exposure of grapes on the chemical composition and sensory characteristics of wine. J. Agric. Food Chem. 2007, 55, 10897–10901. [Google Scholar] [CrossRef] [PubMed]
- Kennison, K.R.; Gibberd, M.R.; Pollnitz, A.P.; Wilkinson, K.L. Smoke-derived taint in wine: The release of smoke-derived volatile phenols during fermentation of Merlot juice following grapevine exposure to smoke. J. Agric. Food Chem. 2008, 56, 7379–7383. [Google Scholar] [CrossRef] [PubMed]
- Ristic, R.; Fudge, A.L.; Pinchbeck, K.A.; De Bei, R.; Fuentes, S.; Hayasaka, Y.; Tyerman, S.D.; Wilkinson, K.L. Impact of grapevine exposure to smoke on vine physiology and the composition and sensory properties of wine. Theor. Exp. Plant Phys. 2016, 28, 67–83. [Google Scholar] [CrossRef]
- Noestheden, M.; Thiessen, K.; Dennis, E.G.; Zandberg, W.F. Quantitating organoleptic volatile phenols in smoke-exposed Vitis vinifera berries. J. Agric. Food Chem. 2017, 65, 8418–8425. [Google Scholar] [CrossRef]
- Parker, M.; Osidacz, P.; Baldock, G.A.; Hayasaka, Y.; Black, C.A.; Pardon, K.H.; Jeffery, D.W.; Geue, J.P.; Herderich, M.J.; Francis, I.L. Contribution of several volatile phenols and their glycoconjugates to smoke-related sensory properties of red wine. J. Agric. Food Chem. 2012, 60, 2629–2637. [Google Scholar] [CrossRef]
- Du Toit, W.; Pretorius, I.; Lonvaud-Funel, A. The effect of sulphur dioxide and oxygen on the viability and culturability of a strain of Acetobacter pasteurianus and a strain of Brettanomyces bruxellensis isolated from wine. J. Appl. Microbiol. 2005, 98, 862–871. [Google Scholar] [CrossRef]
- Ristic, R.; Osidacz, P.; Pinchbeck, K.; Hayasaka, Y.; Fudge, A.; Wilkinson, K. The effect of winemaking techniques on the intensity of smoke taint in wine. Aust. J. Grape Wine Res. 2011, 17, S29–S40. [Google Scholar] [CrossRef]
- Fudge, A.L.; Schiettecatte, M.; Ristic, R.; Hayasaka, Y.; Wilkinson, K.L. Amelioration of smoke taint in wine by treatment with commercial fining agents. Aust. J. Grape Wine Res. 2012, 18, 302–307. [Google Scholar] [CrossRef]
- van Wyk, C.; Rogers, I.M. “A phenolic” off-odour in white table wines: Causes and methods to diminish its occurrence. S. Afr. J. Enol. Vitic. 2000, 21, 52–57. [Google Scholar] [CrossRef] [Green Version]
- Chassagne, D.; Guilloux-Benatier, M.; Alexandre, H.; Voilley, A. Sorption of wine volatile phenols by yeast lees. Food Chem. 2005, 91, 39–44. [Google Scholar] [CrossRef]
- Pradelles, R.; Alexandre, H.; Ortiz-Julien, A.; Chassagne, D. Effects of yeast cell-wall characteristics on 4-ethylphenol sorption capacity in model wine. J. Agric. Food Chem. 2008, 56, 11854–11861. [Google Scholar] [CrossRef]
- Larcher, R.; Puecher, C.; Rohregger, S.; Malacarne, M.; Nicolini, G. 4-Ethylphenol and 4-ethylguaiacol depletion in wine using esterified cellulose. Food Chem. 2012, 132, 2126–2130. [Google Scholar] [CrossRef]
- Fudge, A.L.; Ristic, R.; Wollan, D.; Wilkinson, K.L. Amelioration of smoke taint in wine by reverse osmosis and solid phase adsorption. Aust. J. Grape Wine Res. 2011, 17, S41–S48. [Google Scholar] [CrossRef]
- Garde-Cerdán, T.; Zalacain, A.; Lorenzo, C.; Alonso, J.L.; Salinas, M.R. Molecularly imprinted polymer-assisted simple clean-up of 2, 4, 6-trichloroanisole and ethylphenols from aged red wines. Am. J. Enol. Vitic. 2008, 59, 396–400. [Google Scholar]
- Botelho, G.; Valiau, C.; Moreira da Silva, A. Effect of cyclodextrins on off-odours removal of red wine: An innovative approach. Ciência Téc. Vitiv. 2011, 26, 63–68. [Google Scholar]
- Szejtli, J. Introduction and general overview of cyclodextrin chemistry. Chem. Rev. 1998, 98, 1743–1754. [Google Scholar] [CrossRef]
- Buvári, A.; Barcza, L. Complex formation of phenol, aniline, and their nitro derivatives with β-cyclodextrin. J. Chem. Soc. Perkin Trans. 2 1988, 4, 543–545. [Google Scholar] [CrossRef]
- Del Valle, E.M.M. Cyclodextrins and their uses: A review. Process. Biochem. 2004, 39, 1033–1046. [Google Scholar] [CrossRef]
- Astray, G.; Mejuto, J.C.; Morales, J.; Rial-Otero, R.; Simal-Gándara, J. Factors controlling flavors binding constants to cyclodextrins and their applications in foods. Food Res. Int. 2010, 43, 1212–1218. [Google Scholar] [CrossRef]
- Astray, G.; Gonzalez-Barreiro, C.; Mejuto, J.C.; Rial-Otero, R.; Simal-Gándara, J. A review on the use of cyclodextrins in foods. Food Hydrocoll. 2009, 23, 1631–1640. [Google Scholar] [CrossRef]
- Buschmann, H.-J.; Schollmeyer, E. Applications of cyclodextrins in cosmetic products: A review. J. Cosmet. Sci. 2002, 53, 185–191. [Google Scholar]
- Challa, R.; Ahuja, A.; Ali, J.; Khar, R.K. Cyclodextrins in drug delivery: An updated review. AAPS PharmSciTech 2005, 6, E329–E357. [Google Scholar] [CrossRef]
- Marques, H.M.C. A review on cyclodextrin encapsulation of essential oils and volatiles. Flavour Fragr. J. 2010, 25, 313–326. [Google Scholar] [CrossRef]
- Crini, G.; Cosentino, C.; Bertini, S.; Naggi, A.; Torri, G.; Vecchi, C.; Janus, L.; Morcellet, M. Solid state NMR spectroscopy study of molecular motion in cyclomaltoheptaose (β-cyclodextrin) crosslinked with epichlorohydrin1. Carbohydr. Res. 1998, 308, 37–45. [Google Scholar] [CrossRef]
- Crini, G.; Janus, L.; Morcellet, M.; Torri, G.; Morin, N. Sorption properties toward substituted phenolic derivatives in water using macroporous polyamines containing β-cyclodextrin. J. Appl. Polym. Sci. 1999, 73, 2903–2910. [Google Scholar] [CrossRef]
- Yamasaki, H.; Makihata, Y.; Fukunaga, K. Efficient phenol removal of wastewater from phenolic resin plants using crosslinked cyclodextrin particles. J. Chem. Technol. Biotechnol. 2006, 81, 1271–1276. [Google Scholar] [CrossRef]
- Zhao, D.; Zhao, L.; Zhu, C.-S.; Huang, W.-Q.; Hu, J.-L. Water-insoluble β-cyclodextrin polymer crosslinked by citric acid: Synthesis and adsorption properties toward phenol and methylene blue. J. Incl. Phenom. Macrocycl. Chem. 2009, 63, 195–201. [Google Scholar] [CrossRef]
- Alsbaiee, A.; Smith, B.J.; Xiao, L.; Ling, Y.; Helbling, D.E.; Dichtel, W.R. Rapid removal of organic micropollutants from water by a porous β-cyclodextrin polymer. Nature 2016, 529, 190–194. [Google Scholar] [CrossRef] [PubMed]
- Romo, A.; Penas, F.J.; Isasi, J.R.; Garcia-Zubiri, I.X.; González-Gaitano, G. Extraction of phenols from aqueous solutions by β-cyclodextrin polymers. Comparison of sorptive capacities with other sorbents. React. Funct. Polym. 2008, 68, 406–413. [Google Scholar] [CrossRef]
- Li, J.-M.; Meng, X.-G.; Hu, C.-W.; Du, J. Adsorption of phenol, p-chlorophenol and p-nitrophenol onto functional chitosan. Bioresour. Technol. 2009, 100, 1168–1173. [Google Scholar] [CrossRef] [PubMed]
- Dang, C.; Wilkinson, K.L.; Jiranek, V.; Taylor, D.K. Development and evaluation of a HS-SPME GC-MS method for determining the retention of volatile phenols by cyclodextrin in model wine. Molecules 2019, 24, 3432. [Google Scholar] [CrossRef] [Green Version]
- Crini, G. Kinetic and equilibrium studies on the removal of cationic dyes from aqueous solution by adsorption onto a cyclodextrin polymer. Dyes Pigment. 2008, 77, 415–426. [Google Scholar] [CrossRef]
- Yamasaki, H.; Makihata, Y.; Fukunaga, K. Preparation of crosslinked β-cyclodextrin polymer beads and their application as a sorbent for removal of phenol from wastewater. J. Chem. Technol. Biotechnol. 2008, 83, 991–997. [Google Scholar] [CrossRef] [Green Version]
- Langmuir, I. The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef] [Green Version]
- Redlich, O.; Peterson, D.L. A useful adsorption isotherm. J. Phys. Chem. 1959, 63, 1024. [Google Scholar] [CrossRef]
- Castro-Mejías, R.; Natera-Marín, R.; Valme García-Moreno, M.; Barroso, C.G. Optimisation of headspace solid-phase microextraction for the analysis of volatile phenols in wine. J. Chromatogr. A 2003, 995, 11–20. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |

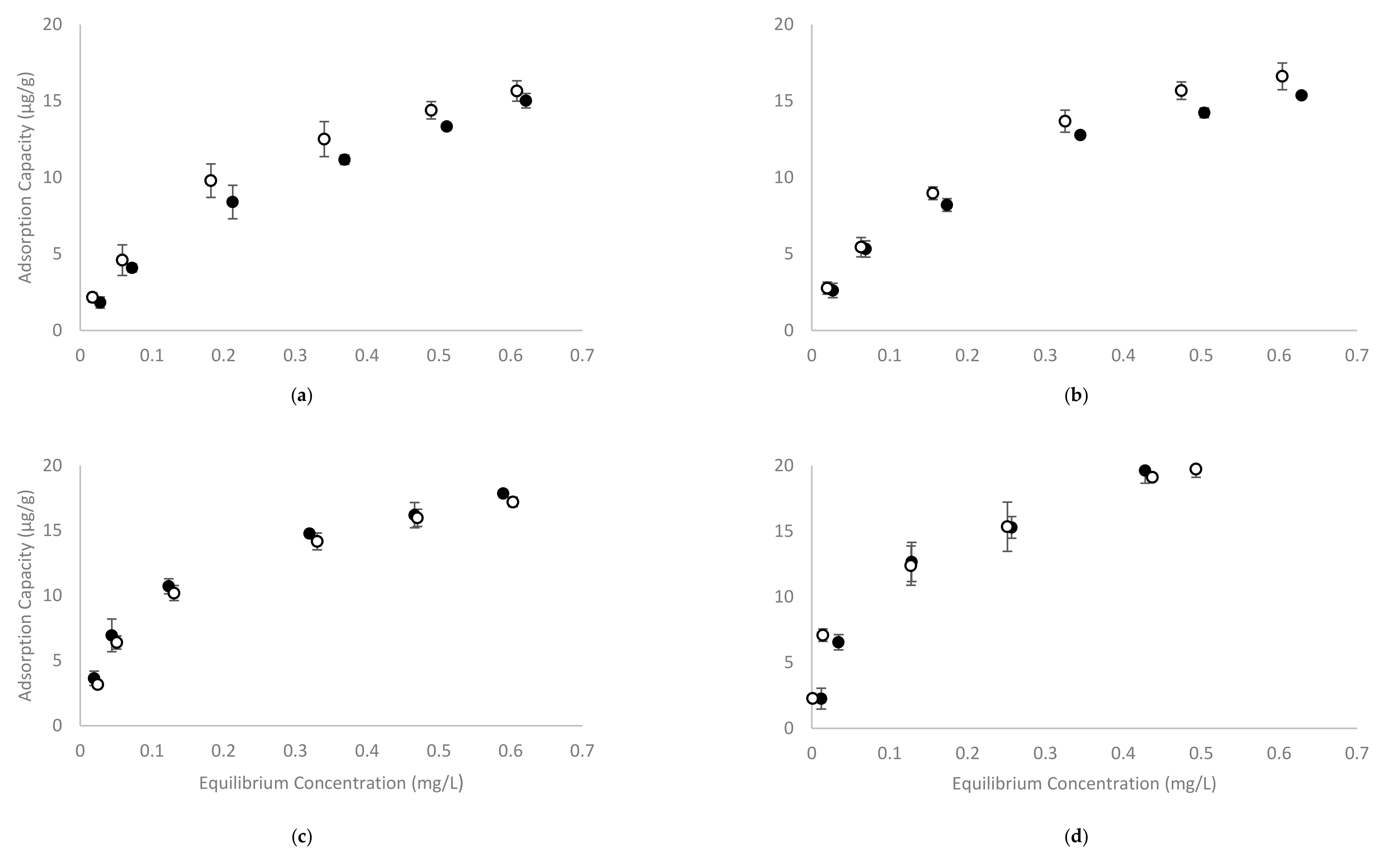
| Langmuir Adsorption Isotherm | Freundlich Adsorption Isotherm | ||||||
|---|---|---|---|---|---|---|---|
| qm (mg/g) | KL (L/mg) | r | Kf | 1/n | r | ||
| guaiacol | β-CD-HDI | 22.3 | 3.0 | 0.989 | 21.7 | 0.67 | 0.992 |
| γ-CD-HDI | 19.6 | 5.8 | 0.992 | 22.3 | 0.56 | 0.991 | |
| 4-methylguaiacol | β-CD-HDI | 20.1 | 4.9 | 0.990 | 21.5 | 0.56 | 0.987 |
| γ-CD-HDI | 21.2 | 5.8 | 0.988 | 23.5 | 0.54 | 0.995 | |
| 4-ethylguaiacol | β-CD-HDI | 20.0 | 10.4 | 0.995 | 23.9 | 0.44 | 0.968 |
| γ-CD-HDI | 20.5 | 7.7 | 0.997 | 24.2 | 0.50 | 0.959 | |
| 4-ethylphenol | β-CD-HDI | 25.1 | 8.1 | 0.986 | 34.0 | 0.56 | 0.957 |
| γ-CD-HDI | 20.6 | 23.1 | 0.976 | 25.9 | 0.35 | 0.985 | |
| Guaiacol | 4-methylguaiacol | 4-ethylguaiacol | 4-ethylphenol | ||||||
|---|---|---|---|---|---|---|---|---|---|
| control | 2.34 a ± 0.03 | 2.18 a ± 0.04 | 1.89 a ± 0.03 | 0.97 a ± 0.03 | |||||
| 1% w/v | 2.07 b ± 0.03 | 89% | 1.96 a ± 0.01 | 90% | 1.63 b ± 0.01 | 86% | 0.81 b ± 0.01 | 84% | |
| β-CD-HDI | 2% w/v | 1.69 c ± 0.05 | 72% | 1.57 b ± 0.03 | 72% | 1.25 c ± 0.02 | 66% | 0.55 c ± 0.01 | 57% |
| 5% w/v | 1.29 d ± 0.05 | 55% | 1.17 c ± 0.05 | 55% | 0.82 d ± 0.02 | 43% | 0.23 d ± 0.01 | 23% | |
| 1% w/v | 1.97 b ± 0.06 | 84% | 1.90 a ± 0.05 | 87% | 1.63 b ± 0.05 | 86% | 0.83 ab ± 0.04 | 86% | |
| γ-CD-HDI | 2% w/v | 1.61 c ± 0.10 | 69% | 1.50 b ± 0.10 | 69% | 1.31 c ± 0.00 | 69% | 0.64 c ± 0.00 | 66% |
| 5% w/v | 1.27 d ± 0.04 | 54% | 1.11 c ± 0.10 | 51% | 0.88 d ± 0.08 | 46% | 0.36 d ± 0.06 | 37% | |
| Guaiacol | 4-methylguaiacol | 4-ethylguaiacol | 4-ethylphenol | ||||||
|---|---|---|---|---|---|---|---|---|---|
| control | 2.43 a ± 0.06 | 2.30 a ± 0.09 | 1.99 a ± 0.08 | 1.04 a ± 0.05 | |||||
| 5 g/L | 2.06 bc ± 0.02 | 85% | 1.88 b ± 0.07 | 82% | 1.62 b ± 0.07 | 81% | 0.79 b ± 0.01 | 76% | |
| β-CD | 10 g/L | 1.96 bc ± 0.02 | 81% | 1.80 b ± 0.07 | 78% | 1.38 bc ± 0.07 | 69% | 0.49 c ± 0.01 | 47% |
| 20 g/L | 1.87 d ± 0.01 | 77% | 1.73 bc ± 0.02 | 75% | 1.15 c ± 0.03 | 58% | 0.33 c ± 0.00 | 32% | |
| 5 g/L | 2.19 b ± 0.06 | 90% | 1.97 ab ± 0.06 | 85% | 1.64 b ± 0.05 | 82% | 0.89 ab ± 0.03 | 86% | |
| γ-CD | 10 g/L | 1.83 cd ± 0.08 | 75% | 1.68 bc ± 0.10 | 73% | 1.41 bc ± 0.09 | 71% | 0.76 b ± 0.07 | 73% |
| 20 g/L | 1.61 d ± 0.05 | 66% | 1.45 c ± 0.10 | 63% | 1.13 c ± 0.04 | 57% | 0.49 c ± 0.01 | 48% | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dang, C.; Jiranek, V.; Taylor, D.K.; Wilkinson, K.L. Removal of Volatile Phenols From Wine Using Crosslinked Cyclodextrin Polymers. Molecules 2020, 25, 910. https://doi.org/10.3390/molecules25040910
Dang C, Jiranek V, Taylor DK, Wilkinson KL. Removal of Volatile Phenols From Wine Using Crosslinked Cyclodextrin Polymers. Molecules. 2020; 25(4):910. https://doi.org/10.3390/molecules25040910
Chicago/Turabian StyleDang, Chao, Vladimir Jiranek, Dennis K. Taylor, and Kerry L. Wilkinson. 2020. "Removal of Volatile Phenols From Wine Using Crosslinked Cyclodextrin Polymers" Molecules 25, no. 4: 910. https://doi.org/10.3390/molecules25040910







