In Vitro Inhibition of Hsp90 Protein by Benzothiazoloquinazolinequinones Is Enhanced in The Presence of Ascorbate. A Preliminary In Vivo Antiproliferative Study
Abstract
:1. Introduction
2. Results
2.1. Synthesis of Benzo[G]Benzothiazolo[2,3-B]Quinazoline-7,12-Quinones
2.2. In Vitro Antitumor Activity of Heterocyclic Quinones
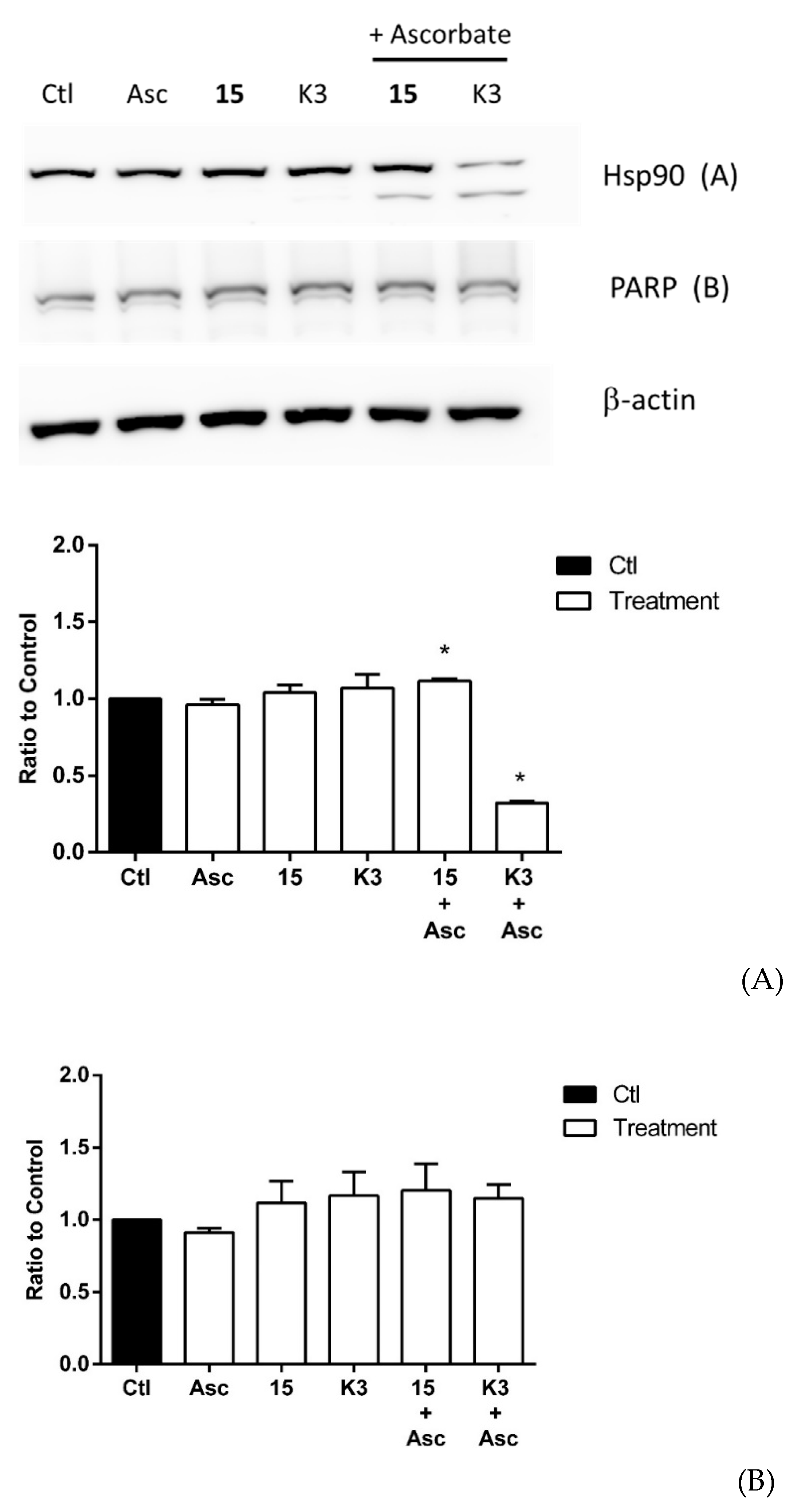
2.3. Effect of Selected Quinones on Intracellular Targets
2.4. In Vivo Antitumor Activity by 15
3. Materials and Methods
3.1. Synthesis Of Benzo[G]Benzothiazolo[2,3-B]Quinazoline-7,12-Quinones
3.2. Cell Lines and Cell Cultures
3.3. Cell Survival Assays
3.3.1. MTT Reduction Assay
3.3.2. Clonogenic Assays
3.4. Immunoblotting Procedures
3.5. Animals and Diet
3.6. Experimental Tumor Model
3.7. Statistical Analysis
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Verma, R.P. Anticancer Activities of 1,4-Naphthoquinones: A QSAR Study. Anti-Cancer Agents Med. Chem. 2006, 6, 489–499. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Huang, S.L.; Li, M.M.; Huang, Z.S.; Lee, K.S.; Gu, L.Q. Inhibition of thioredoxin reductase by mansonone F analogues: Implications for anticancer activity. Chem. Biol. Interact. 2009, 177, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Vaverkova, V.; Vrana, O.; Adam, V.; Pekarek, T.; Jampilek, J.; Babula, P. The Study of Naphthoquinones and Their Complexes with DNA by Using Raman Spectroscopy and Surface Enhanced Raman Spectroscopy: New Insight into Interactions of DNA with Plant Secondary Metabolites. BioMed. Res. Int. 2014, 2014, 461393. [Google Scholar] [CrossRef] [PubMed]
- Blunt, C.E.; Torcuk, C.; Liu, Y.; Lewis, W.; Siegel, D.; Ross, D.; Moody, C.J. Synthesis and Intracellular Redox Cycling of Natural Quinones and Their Analogues and Identification of Indoleamine-2,3-dioxygenase (IDO) as Potential Target for Anticancer Activity. Angew. Chem. Int. Ed. Engl. 2015, 54, 8740–8745. [Google Scholar] [CrossRef]
- Ho, S.H.; Sim, M.Y.; Yee, W.L.; Yang, T.; Yuen, S.P.; Go, M.L. Antiproliferative, DNA intercalation and redox cycling activities of dioxonaphtho[2,3-d]imidazolium analogs of YM155: A structure-activity relationship study. Eur. J. Med. Chem. 2015, 104, 42–56. [Google Scholar] [CrossRef]
- Samuni, A.; Goldstein, S. Redox properties and thiol reactivity of geldanamycin and its analogues in aqueous solutions. J. Phys. Chem. B. 2012, 116, 6404–6410. [Google Scholar] [CrossRef]
- Paz, M.M.; Zhang, X.; Lu, J.; Holmgren, A. A new mechanism of action for the anticancer drug mitomycin C: Mechanism-based inhibition of thioredoxin reductase. Chem. Res. Toxicol. 2012, 25, 1502–1511. [Google Scholar] [CrossRef]
- Ríos, D.; Benites, J.; Valderrama, J.A.; Farias, M.; Pedrosa, R.C.; Verrax, J.; Buc Calderon, P. Biological evaluation of 3-acyl-2-arylamino-1,4-naphthoquinones as inhibitors of Hsp90 chaperoning function. Curr. Top. Med. Chem. 2012, 12, 2094–2102. [Google Scholar] [CrossRef]
- Stulpinas, A.; Imbrasaitė, A.; Krestnikova, N.; Šarlauskas, J.; Čėnas, N.; Kalvelytė, A.V. Study of Bioreductive Anticancer Agent RH-1-Induced Signals Leading the Wild-Type p53-Bearing Lung Cancer A549 Cells to Apoptosis. Chem. Res. Toxicol. 2016, 29, 26–39. [Google Scholar] [CrossRef]
- Szatrowski, T.P.; Nathan, C.F. Production of large amounts of hydrogen peroxide by human tumor cells. Cancer Res. 1991, 51, 794–798. [Google Scholar]
- Verrax, J.; Pedrosa, R.C.; Beck, R.; Dejeans, N.; Taper, H.; Buc Calderon, P. In situ modulation of oxidative stress: A novel and efficient strategy to kill cancer cells. Curr. Med. Chem. 2009, 16, 1821–1830. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Rosen, D.G.; Zhou, Y.; Feng, L.; Yang, G.; Liu, J.; Huang, P. Mitochondrial manganese-superoxide dismutase expression in ovarian cancer: Role in cell proliferation and response to oxidative stress. J. Biol. Chem. 2005, 280, 39485–39492. [Google Scholar] [CrossRef] [Green Version]
- Oberley, T.D.; Oberley, L.W. Antioxidant enzyme levels in cancer. Histol. Histopathol. 1997, 12, 525–535. [Google Scholar] [PubMed]
- Benites, J.; Valderrama, J.A.; Taper, H.; Buc Calderon, P. An in vitro comparative study with furyl-1,4-quinones endowed with anticancer activities. Investig. New Drugs 2011, 29, 760–767. [Google Scholar] [CrossRef] [PubMed]
- Benites, J.; Toledo, H.; Salas, F.; Guerrero, A.; Ríos, D.; Valderrama, J.A.; Buc Calderon, P. In vitro inhibition of Helicobacter pylori growth by redox cycling phenylaminojuglones. Oxid. Med. Cell Longev. 2018, 2018, 1618051. [Google Scholar] [CrossRef] [Green Version]
- Valderrama, J.A.; Rios, D.; Muccioli, G.G.; Buc Calderon, P.; Brito, I.; Benites, J. Hetero-annulation reaction between 2-acylnaphthoquinones and 2-aminobenzothiazoles. A new synthetic route to antiproliferative benzo[g]benzothiazolo[2,3-b]quinazoline-7,12-quinones. Tetrahedron Lett. 2015, 56, 5103–5105. [Google Scholar] [CrossRef]
- Tang, C.; Li, C.; Zhang, S.; Hu, Z.; Wu, J.; Dong, C.; Huang, J.; Zhou, H.B. Novel Bioactive Hybrid Compound Dual Targeting Estrogen Receptor and Histone Deacetylase for the Treatment of Breast Cancer. J. Med. Chem. 2015, 58, 4550–4572. [Google Scholar] [CrossRef]
- Nagamoto, A.; Kubota, Y.; Shuin, T.; Kondo, I.; Moriyama, M.; Satomi, Y.; Fukushima, S.; Fukuoka, H.; Ishizuka, E.; Furuhata, A. Phase II study of 5-FU tablets for bladder tumors. Jap. J. Cancer Chemother. 1989, 16, 845–849. [Google Scholar]
- Fossa, S.D.; Gudmundsen, T.E. Single drug chemotherapy with 5-FU and adriamycin in metastatic bladder carcinoma. BJU Int. 1981, 53, 320–323. [Google Scholar] [CrossRef]
- Beck, R.; Dejeans, N.; Glorieux, C.; Pedrosa, R.C.; Vásquez, D.; Valderrama, J.A.; Buc Calderon, P.; Verrax, J. Molecular chaperone Hsp90 as a target for oxidant-based anticancer therapies. Curr. Med. Chem. 2011, 18, 2816–2825. [Google Scholar] [CrossRef]
- Beck, R.; Dejeans, N.; Glorieux, C.; Creton, M.; Delaive, E.; Dieu, M.; Raes, M.; Levêque, P.; Gallez, B.; Depuydt, M.; et al. Hsp90 is cleaved by reactive oxygen species at a highly conserved N-terminal amino acid motif. PLoS One 2012, 7, e40795. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beck, R.; Verrax, J.; Gonze, T.; Zappone, M.; Pedrosa, R.C.; Taper, H.; Feron, O.; Buc Calderon, P. Hsp90 cleavage by an oxidative stress leads to its client proteins degradation and cancer cell death. Biochem. Pharmacol. 2009, 77, 375–383. [Google Scholar] [CrossRef] [PubMed]
- Verrax, J.; Stockis, J.; Tison, A.; Taper, H.S.; Buc Calderon, P. Oxidative stress by ascorbate/menadione association kills K562 human chronic myelogenous leukaemia cells and inhibits its tumour growth in nude mice. Biochem. Pharmacol. 2006, 72, 671–680. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Franken, N.A.P.; Rodermond, H.M.; Stap, J.; Haveman, J.; Van Bree, C. Clonogenic assay of cells in vitro. Nat. Protoc. 2006, 1, 2315–2319. [Google Scholar] [CrossRef]
- Benites, J.; Valderrama, J.A.; Ramos, M.; Muccioli, G.G.; Buc Calderon, P. Targeting Akt as strategy to kill cancer cells using 3-substituted 5-anilino[c]isoxazolequinone: A preliminary study. Biomed. Pharmacother. 2018, 97, 778–783. [Google Scholar] [CrossRef]
- Taper, H.S.; Woolley, G.W.; Teller, M.N.; Lardis, M.P. A new transplantable mouse liver tumor of spontaneous origin. Cancer Res. 1966, 26, 143–148. [Google Scholar]
- Fritzler, M.J.; Church, R.B.; Wagenaar, E.B. Ultrastructure of taper hepatoma ascites cells. J. Electron. Microsc. (Tokyo) 1973, 22, 73–90. [Google Scholar]
- Geran, R.I.; Greenberg, N.H.; Macdonald, M.M.; Schumacher, A.M.; Abbott, B.J. Protocols for screening chemical agents and natural products against animal tumors and other biological systems. Cancer Chemother. Rep. 1972, 3, 1–88. [Google Scholar]
- Taper, H.S.; de Gerlache, J.; Lans, M.; Roberfroid, M. Non-toxic potentiation of cancer chemotherapy by combined C and K3 vitamin pre-treatment. Int. J. Cancer 1987, 40, 575–579. [Google Scholar] [CrossRef]
- Jordan, B.F.; Gregoire, V.; Demeure, R.J.; Sonveaux, P.; Feron, O.; O’Hara, J.; Vanhulle, V.P.; Delzenne, N.; Gallez, B. Insulin increases the sensitivity of tumors to irradiation: Involvement of an increase in tumor oxygenation mediated by a nitric oxide-dependent decrease of the tumor cells oxygen consumption. Cancer Res. 2002, 62, 3555–3561. [Google Scholar] [PubMed]
- Verrax, J.; Cadrobbi, J.; Marques, C.; Taper, H.S.; Habraken, Y.; Piette, J.; Buc Calderon, P. Ascorbate potentiates the cytotoxicity of menadione leading to an oxidative stress that kills cancer cells by a non-apoptotic caspase-3 independent form of cell death. Apoptosis 2004, 9, 223–233. [Google Scholar] [CrossRef] [PubMed]
- O’Brian, C.A.; Liskamp, R.M.; Solomon, D.H.; Weinstein, I.B. Inhibition of protein kinase C by tamoxifen. Cancer Res. 1985, 45, 2462–2465. [Google Scholar] [PubMed]
- O’Brian, C.A.; Liskamp, R.M.; Solomon, D.H.; Weinstein, I.B. Triphenylethylenes: A new class of protein kinase C inhibitors. J. Natl. Cancer Inst. 1986, 76, 1243–1246. [Google Scholar]
- Longley, D.B.; Harkin, D.P.; Johnston, P.G. 5-fluorouracil: Mechanisms of action and clinical strategies. Nat. Rev. Cancer 2003, 3, 330–338. [Google Scholar] [CrossRef]
- Neckers, L. Hsp90 inhibitors as novel cancer chemotherapeutic agents. Trends Mol. Med. 2002, 8, 555–561. [Google Scholar] [CrossRef]
- Trepel, J.; Mollapour, M.; Giaccone, G.; Neckers, L. Targeting the dynamic Hsp90 complex in cancer. Nat. Rev. Cancer 2010, 10, 537–549. [Google Scholar] [CrossRef] [Green Version]
Sample Availability: Samples of the synthesized compounds 17, 20, 24, 25, 26 are available from the authors. |


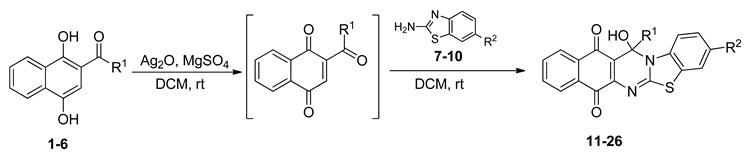
| Acylhydroquinone | Aminobenzothiazol | Product | R1 | R2 | Yield (%) a |
|---|---|---|---|---|---|
| 1 | 7 | 11 | Me | H | 67 b |
| 2 | 7 | 12 | 1-Propyl | H | 78 b |
| 3 | 7 | 13 | 1-Pentyl | H | 47 b |
| 4 | 7 | 14 | 1-Heptyl | H | 41 b |
| 1 | 8 | 15 | Me | Me | 61 b |
| 2 | 8 | 16 | 1-Propyl | Me | 68 b |
| 3 | 8 | 17 | 1-Pentyl | Me | 87 |
| 4 | 8 | 18 | 1-Heptyl | Me | 84 b |
| 1 | 9 | 19 | Me | OMe | 43 b |
| 2 | 9 | 20 | 1-Propyl | OMe | 61 |
| 3 | 9 | 21 | 1-Pentyl | OMe | 75 b |
| 4 | 9 | 22 | 1-Heptyl | OMe | 60 b |
| 5 | 7 | 23 | 2-Furyl | H | 55 |
| 5 | 9 | 24 | 2-Furyl | OMe | 63 |
| 6 | 7 | 25 | 2- Thiophen | H | 63 |
| 6 | 10 | 26 | 2- Thiophen | F | 63 |
| IC50 ± SEM (Standard Error of the Mean) a (μM) | ||||||
|---|---|---|---|---|---|---|
| Structure | No. | T24 | DU-145 | MCF-7 | MSIb | AG 1523 |
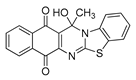 | 11 | 4.65 ± 0.43 | 9.59 ± 0.32 | 6.83 ± 1.29 | 1.52 | 10.71 ± 0.57 |
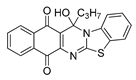 | 12 | 4.86 ± 0.50c | 11.58 ± 2.05c | 4.25 ± 0.50c | 1.28 | 8.82 ± 0.27 |
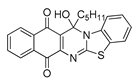 | 13 | 6.85 ± 0.54 | 7.99 ± 0.17 | 5.83 ± 0.25 | 1.24 | 8.55 ± 0.35 |
 | 14 | 8.42 ± 1.27 | 9.04 ± 0.69 | 6.84 ± 0.79 | 0.90 | 7.28 ± 0.39 |
 | 15 | 0.22 ± 0.06 c | 0.11 ± 0.03 c | 2.98 ± 0.52 c | 29.4 | 32.31 ± 3.31 |
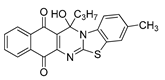 | 16 | 1.36 ± 0.15c | 0.85 ± 0.10c | 1.77 ± 0.26c | 2.62 | 3.46 ± 0.08 |
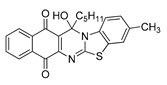 | 17 | 6.86 ± 0.33 | 8.46 ± 0.31 | 5.26 ± 0.22 | 1.09 | 7.50 ± 0.69 |
 | 18 | 5.60 ± 0.29 | 7.55 ± 0.69 | 7.12 ± 0.65 | 0.89 | 6.02 ± 0.29 |
 | 19 | 1.32 ± 0.11c | 0.79 ± 0.11c | 2.72 ± 0.18c | 3.97 | 6.40 ± 0.46 |
 | 20 | 1.89 ± 0.15 | 1.13 ± 0.05 | 1.67 ± 0.12 | 3.17 | 4.99 ± 0.59 |
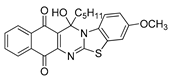 | 21 | 7.09 ± 0.14 | 7.43 ± 0.28 | 4.63 ± 0.30 | 1.78 | 11.32 ± 1.10 |
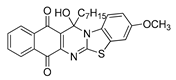 | 22 | 5.95 ± 0.45 | 6.62 ± 0.19 | 3.52 ± 0.32 | 1.11 | 5.97 ± 0.24 |
 | 23 | 9.29 ± 0.77 | 19.95 ± 0.50 | 8.62 ± 0.72 | 1.72 | 21.70 ± 0.27 |
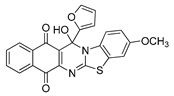 | 24 | 10.41 ± 1.00 | 4.69 ± 0.33 | 11.69 ± 0.65 | 10.1 | 90.12 ± 1.21 |
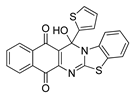 | 25 | 8.72 ± 0.41 | 7.85 ± 0.60 | 7.32 ± 0.36 | 1.60 | 12.77 ± 0.31 |
 | 26 | 13.37 ± 2.37 | 10.75 ± 1.01 | 7.46 ± 0.53 | 1.88 | 19.79 ± 0.83 |
| 5-Fluorouracil | - | 3.61 ± 0.77 | 7.10 ± 1.45 | 12.68 ± 1.9 | 0.84 | 6.59 ± 1.04 |
| Tamoxifen | - | 27.9 ± 4.8 | 23.0 ± 4.1 | 19.6 ± 2.6 | 0.95 | 22.5 ± 2.3 |
| Dose (µM) | 15 | 16 | 19 | 20 |
|---|---|---|---|---|
| 0.00 | 195 ± 16 (100) | 202 ± 28 (100) | 191 ± 24 (100) | 213 ± 23 (100) |
| 0.025 | 180 ± 11 (92) | 204 ± 19 (101) | 195 ± 16 (102) | 204 ± 17 (96) |
| 0.125 | 162 ± 18 (83) | 216 ± 29 (107) | 183 ± 21 (96) | 214 ± 25 (100) |
| 0.25 | 72 ± 5 * (37) | 189 ± 12 (94) | 138 ± 18 * (72) | 225 ± 19 (106) |
| 1.25 | 22 ± 9 * (11) | 79 ± 9 * (39) | 68 ± 12 * (36) | 118 ± 22 * (55) |
| 2.5 | 16 ± 3 * (8) | 58 ± 12 * (29) | 30 ± 16 * (16) | 89 ± 10 * (42) |
| 5.0 | 2 ± 1 * (1) | 23 ± 15 * (11) | 35 ± 11 * (18) | 46 ± 7 * (22) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valderrama, J.A.; Ríos, D.; Muccioli, G.G.; Buc Calderon, P.; Benites, J. In Vitro Inhibition of Hsp90 Protein by Benzothiazoloquinazolinequinones Is Enhanced in The Presence of Ascorbate. A Preliminary In Vivo Antiproliferative Study. Molecules 2020, 25, 953. https://doi.org/10.3390/molecules25040953
Valderrama JA, Ríos D, Muccioli GG, Buc Calderon P, Benites J. In Vitro Inhibition of Hsp90 Protein by Benzothiazoloquinazolinequinones Is Enhanced in The Presence of Ascorbate. A Preliminary In Vivo Antiproliferative Study. Molecules. 2020; 25(4):953. https://doi.org/10.3390/molecules25040953
Chicago/Turabian StyleValderrama, Jaime A., David Ríos, Giulio G. Muccioli, Pedro Buc Calderon, and Julio Benites. 2020. "In Vitro Inhibition of Hsp90 Protein by Benzothiazoloquinazolinequinones Is Enhanced in The Presence of Ascorbate. A Preliminary In Vivo Antiproliferative Study" Molecules 25, no. 4: 953. https://doi.org/10.3390/molecules25040953






