Preparation of Electrospun Gelatin Mat with Incorporated Zinc Oxide/Graphene Oxide and Its Antibacterial Activity
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of ZnO/GO-Gelatin Fibers
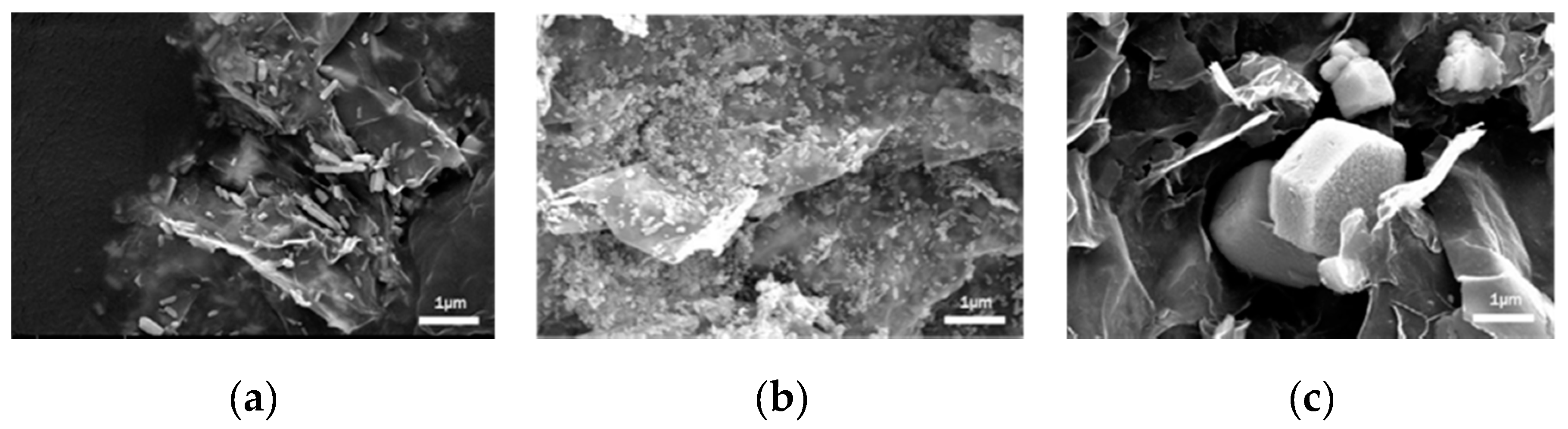
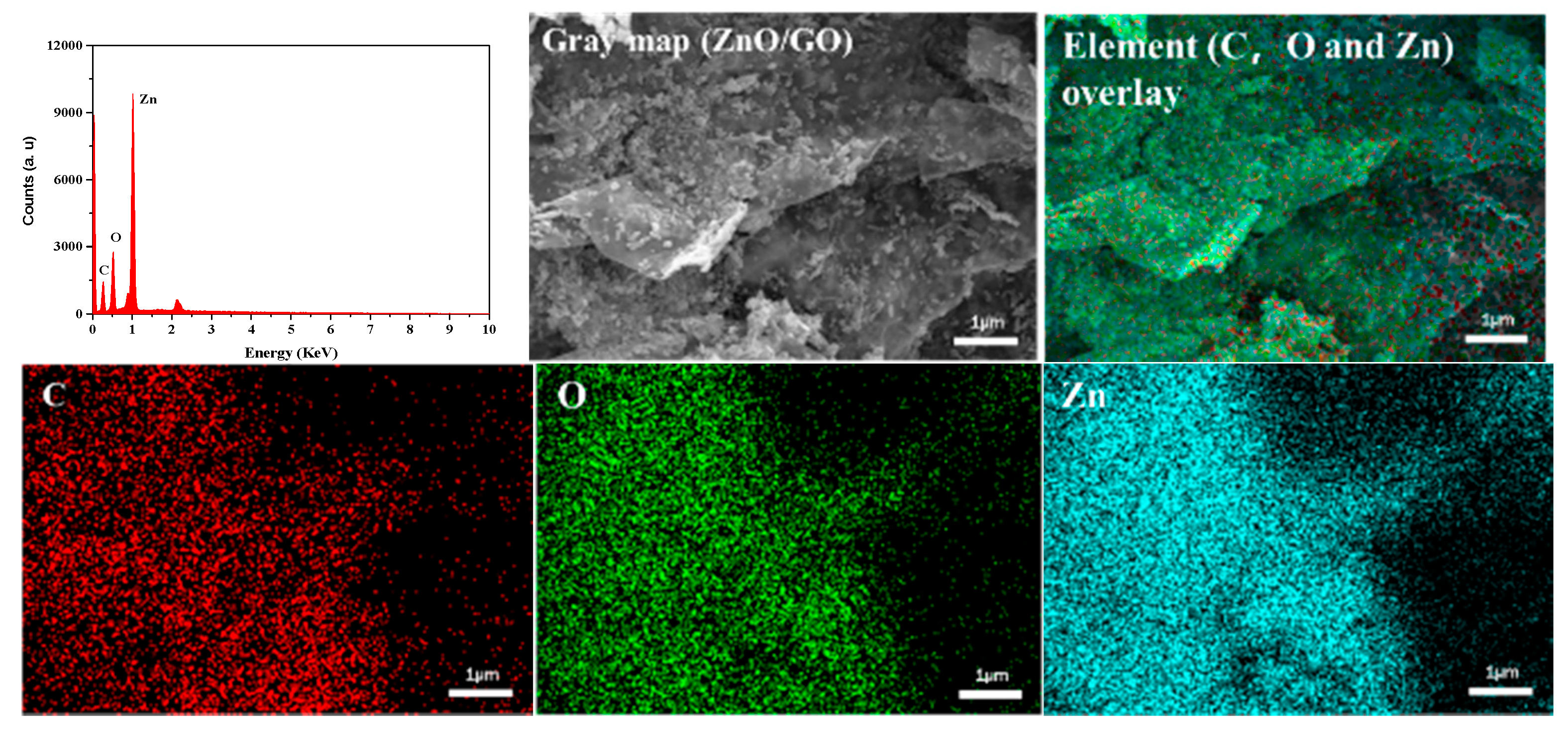
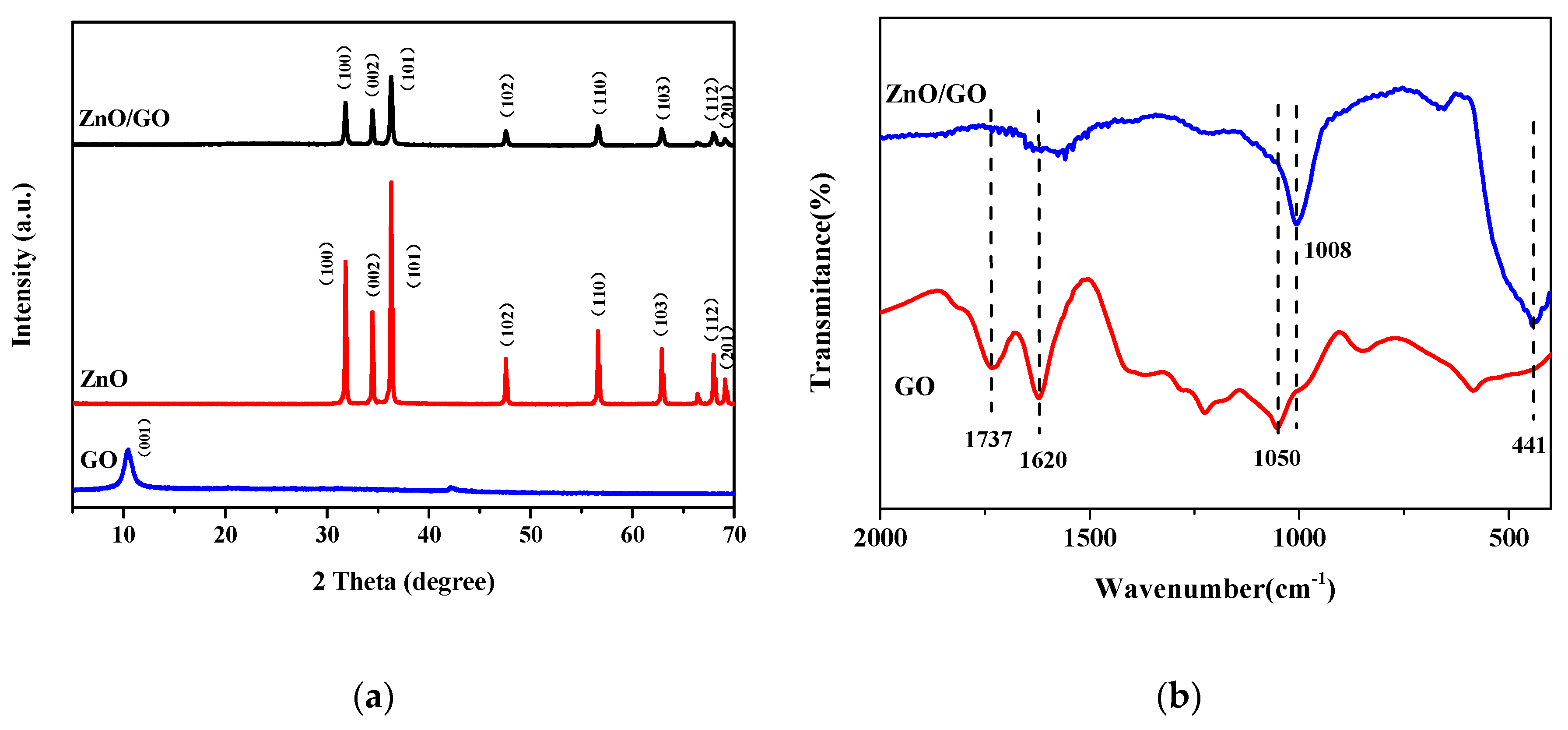
2.1.1. Characterization of ZnO/GO Nanocomposites
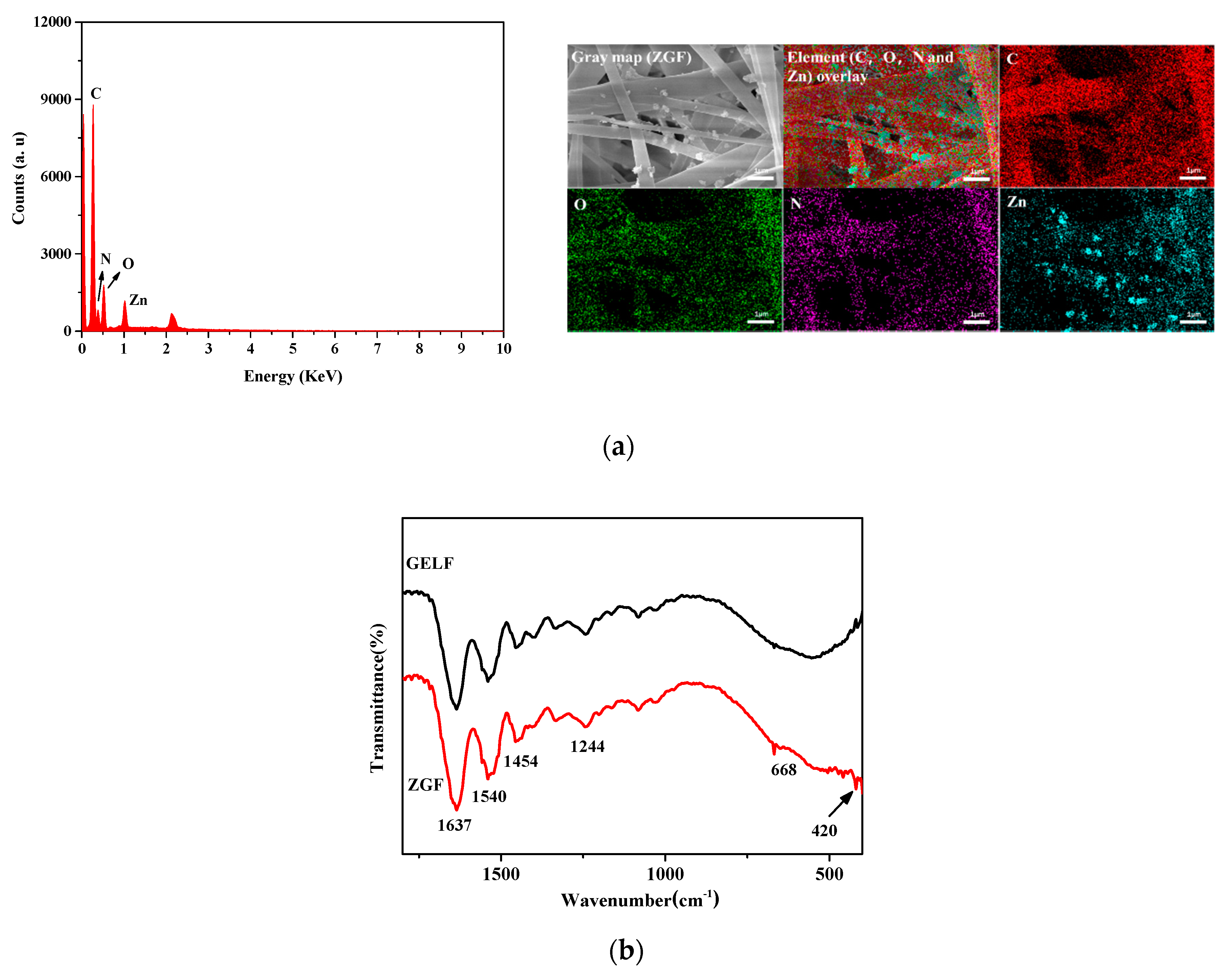
2.1.2. Characterization of ZnO/GO-Gelatin Fibers
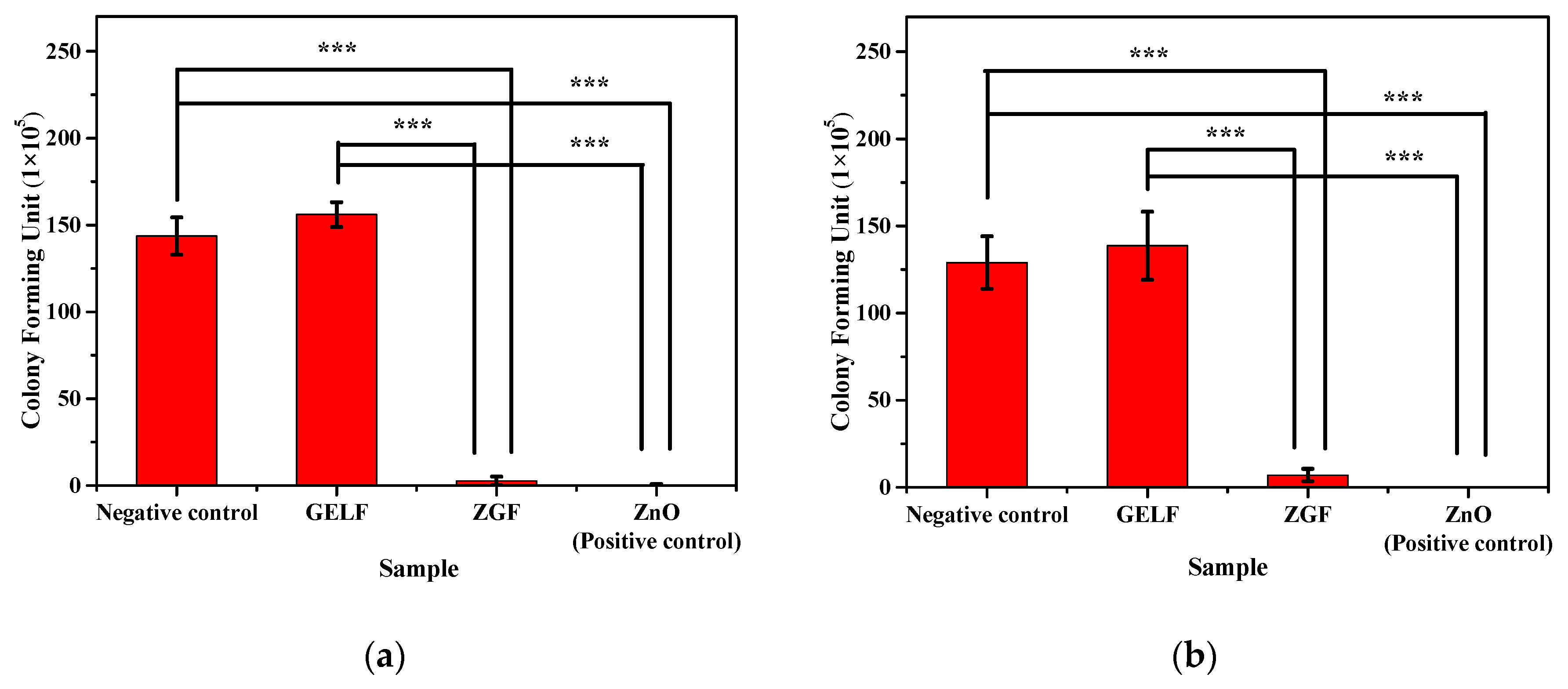
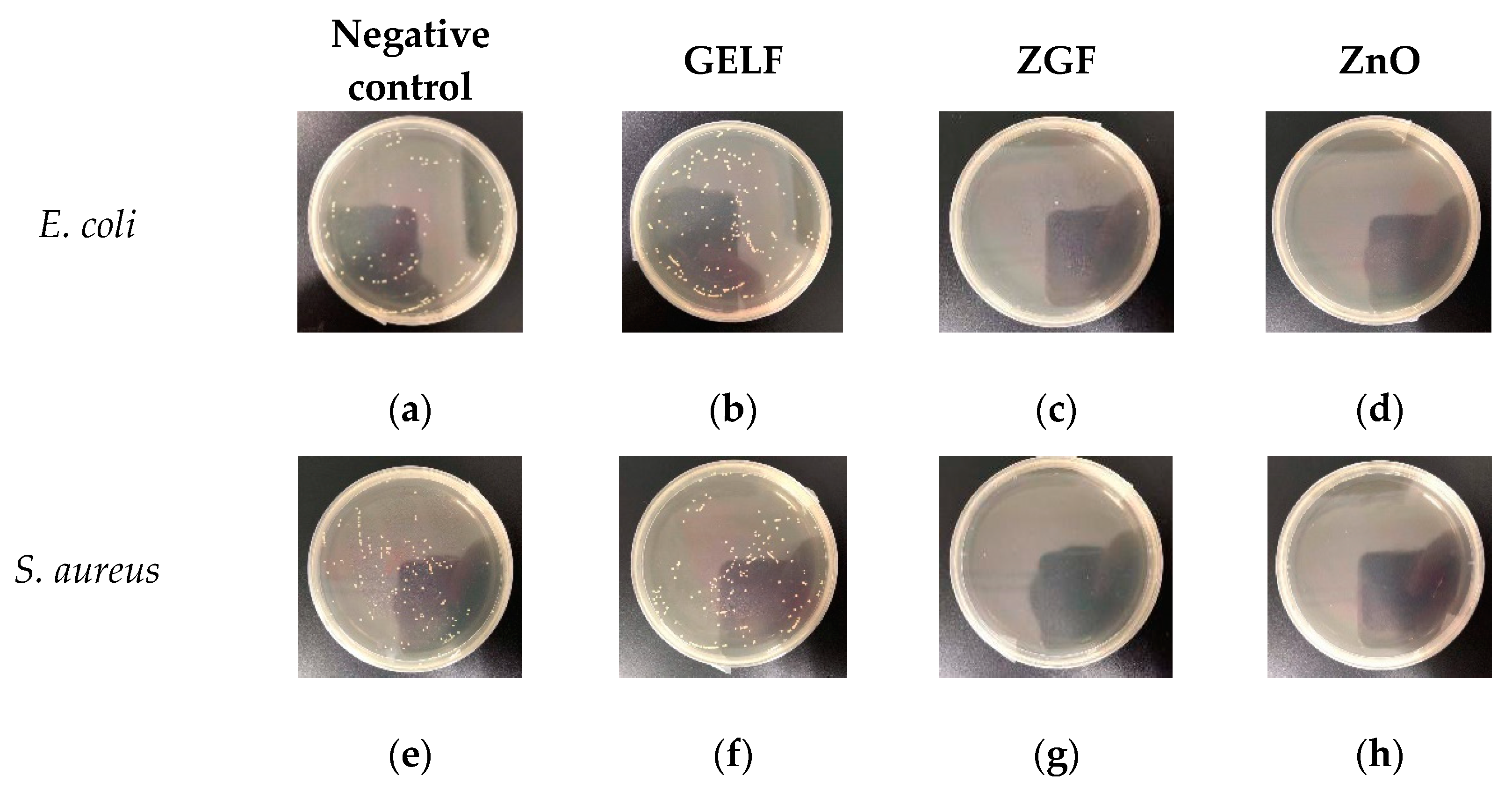
2.2. Antibacterial Assay
3. Materials and Methods
3.1. Materials
3.2. Preparation of ZnO/GO Nanocomposites
3.3. Preparation of ZnO/GO-Gelatin
3.4. Characterization
3.5. Stability of ZGF in PBS
3.6. Antibacterial Assay
3.7. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kong, X.; Fu, J.; Shao, K.; Wang, L.; Lan, X.; Shi, J. Biomimetic hydrogel for rapid and scar-free healing of skin wounds inspired by the healing process of oral mucosa. Acta Biomater. 2019, 100, 255–269. [Google Scholar] [CrossRef] [PubMed]
- Liang, D.; Lu, Z.; Yang, H.; Gao, J.; Chen, R. Novel Asymmetric Wettable AgNPs/Chitosan Wound Dressing: In Vitro and In Vivo Evaluation. ACS Appl. Mater. Interfaces 2016, 8, 3958–3968. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Qu, Y.; Chen, H.; Xu, R.; Yu, Q.; Yang, P. Self-assembled proteinaceous wound dressings attenuate secondary trauma and improve wound healing in vivo. J. Mater. Chem. B 2018, 6, 4645–4655. [Google Scholar] [CrossRef]
- Farokhi, M.; Mottaghitalab, F.; Fatahi, Y.; Khademhosseini, A.; Kaplan, D.L. Overview of Silk Fibroin Use in Wound Dressings. Trends Biotechnol. 2018, 36, 907–922. [Google Scholar] [CrossRef]
- Memic, A.; Abudula, T.; Mohammed, H.; Joshi Navare, K.; Colombani, T. Latest Progress in Electrospun Fibers for Wound Healing Applications. ACS Appl. Bio. Mater. 2019, 2, 952–969. [Google Scholar] [CrossRef]
- Lin, Z.; Wu, T.; Wang, W.; Li, B.; Wang, M.; Chen, Li. Biofunctions of antimicrobial peptide-conjugated alginate/hyaluronic acid/collagen wound dressings promote wound healing of a mixed-bacteria-infected wound. Int. J. Biol. Macromol. 2019, 140, 330–342. [Google Scholar] [CrossRef]
- Ou, Q.; Miao, Y.; Yang, F.; Lin, X.; Zhang, L.M.; Wang, Y. Zein/gelatin/nanohydroxyapatite nanofibrous scaffolds are biocompatible and promote osteogenic differentiation of human periodontal ligament stem cells. Biomater. Sci. 2019, 7, 1973–1983. [Google Scholar] [CrossRef] [Green Version]
- Li, D.; Sun, H.; Jiang, L.; Zhang, K.; Liu, W.; Zhu, Y.; Fangteng, J.; Shi, C.; Zhao, L.; Sun, H.; et al. Enhanced Biocompatibility of PLGA Fibers with Gelatin/Nano-Hydroxyapatite Bone Biomimetics Incorporation. ACS Appl. Mater. Interfaces 2014, 6, 9402–9410. [Google Scholar] [CrossRef]
- Sazo, R.; Maenaka, K.; Gu, W.; Wood, P.; Bunge, M. Fabrication of growth factor- and extracellular matrix-loaded, gelatin-based scaffolds and their biocompatibility with Schwann cells and dorsal root ganglia. Biomaterials 2012, 33, 8529–8539. [Google Scholar] [CrossRef] [Green Version]
- Goh, E.S.; Mah, J.W.; Yoon, T.L. Effects of Hubbard term correction on the structural parameters and electronic properties of wurtzite ZnO. Comp. Mater. Sci. 2017, 138, 111–116. [Google Scholar] [CrossRef]
- Harun, K.; Salleh, N.A.; Deghfel, B.; Yaakob, M.K.; Mohamad, A.A. DFT + U calculations for electronic, structural, and optical properties of ZnO wurtzite structure: A review. Results Phys. 2020, 16, 102829. [Google Scholar] [CrossRef]
- Wahid, F.; Duan, Y.-X.; Hu, X.-H.; Chu, L.-Q.; Jia, S.-R.; Cui, J.-D.; Zhong, C. A facile construction of bacterial cellulose/ZnO nanocomposite films and their photocatalytic and antibacterial properties. Int. J. Biol. Macromol. 2019, 132, 692–700. [Google Scholar] [CrossRef] [PubMed]
- Lakshmi Prasanna, V.; Vijayaraghavan, R. Insight into the Mechanism of Antibacterial Activity of ZnO: Surface Defects Mediated Reactive Oxygen Species Even in the Dark. Langmuir 2015, 31, 9155–9162. [Google Scholar] [CrossRef] [PubMed]
- Brayner, R.; Si, A.D.; Yéprémian, C.; Djediat, C.; Fiévet, F. ZnO Nanoparticles: Synthesis, Characterization, and Ecotoxicological Studies. Langmuir 2010, 26, 6522–6528. [Google Scholar] [CrossRef] [PubMed]
- Sekar, V.; Vaseeharan, B. Antibiofilm, anticancer and ecotoxicity properties of collagen based ZnO nanoparticles. Adv. Powder Technol. 2018, 29, 2331–2345. [Google Scholar]
- Liu, Y.; Li, Y.; Deng, L.; Zou, L.; Feng, F.; Zhang, H. Hydrophobic Ethylcellulose/Gelatin Fibers Containing Zinc Oxide Nanoparticles for Antimicrobial Packaging. J. Agric. Food. Chem. 2018, 66, 9498–9506. [Google Scholar] [CrossRef]
- Rath, G.; Hussain, T.; Chauhan, G.; Garg, T.; Goyal, A.K. Development and characterization of cefazolin loaded zinc oxide nanoparticles composite gelatin nanofiber mats for postoperative surgical wounds. Mater. Sci. Eng. C 2016, 58, 242–253. [Google Scholar] [CrossRef]
- Wan, C.; Frydrych, M.; Chen, B. Strong and bioactive gelatin–graphene oxide nanocomposites. Soft Matter 2011, 7, 6159–6166. [Google Scholar] [CrossRef]
- Williams, G.; Seger, B.; Kamat, P.V. TiO2-Graphene Nanocomposites. UV-Assisted Photocatalytic Reduction of Graphene Oxide. ACS Nano 2008, 2, 1487–1491. [Google Scholar] [CrossRef]
- Raghupathi, K.R.; Koodali, R.T.; Manna, A.C. Size-Dependent Bacterial Growth Inhibition and Mechanism of Antibacterial Activity of Zinc Oxide Nanoparticles. Langmuir 2011, 27, 4020–4028. [Google Scholar] [CrossRef]
- Lian, P.; Zhu, X.; Liang, S.; Zhong, L.; Wang, H. Large reversible capacity of high quality graphene sheets as an anode material for lithium-ion batteries. Electrochim. Acta 2010, 55, 3909–3914. [Google Scholar] [CrossRef]
- Wu, Z.; Wang, L. Graphene oxide (GO) doping hexagonal flower-like ZnO as potential enhancer of photocatalytic ability. Mater. Lett. 2019, 234, 287–290. [Google Scholar] [CrossRef]
- Paul, R.; Gayen, R.N.; Biswas, S.; Bhat, S.V.; Bhunia, R. Enhanced UV detection by transparent graphene oxide/ZnO composite thin films. RSC Adv. 2016, 6, 61661–61672. [Google Scholar] [CrossRef]
- Church, R.B.; Hu, K.; Magnacca, G.; Cerruti, M. Intercalated Species in Multilayer Graphene Oxide: Insights Gained from In Situ FTIR Spectroscopy with Probe Molecule Delivery. J. Phys. Chem. C 2016, 120, 23207–23211. [Google Scholar] [CrossRef]
- Boukhoubza, I.; Khenfouch, M.; Achehboune, M.; Mothudi, B.M.; Zorkani, I.; Jorio, A. Graphene oxide/ZnO nanoparticles/graphene oxide sandwich structure: The origins and mechanisms of photoluminescence. J. Alloy. Compd. 2019, 797, 1320–1326. [Google Scholar] [CrossRef]
- Li, Y.T.; Xu, J.M.; Tang, Z.J.; Xu, T.T.; Li, X.J. Nearly white light photoluminescence from ZnO/rGO nanocomposite prepared by a one-step hydrothermal method. J. Alloy. Compd. 2017, 715, 122–128. [Google Scholar] [CrossRef]
- Chen, Y.; Ma, Y.; Lu, W.; Guo, Y.; Zhu, Y.; Lu, H.; Song, Y. Environmentally Friendly Gelatin/β-Cyclodextrin Composite Fiber Adsorbents for the Efficient Removal of Dyes from Wastewater. Molecules 2018, 23, 2473. [Google Scholar] [CrossRef] [Green Version]
- Kenawy, E.; Omer, A.M.; Tamer, T.M.; Elmeligy, M.A.; Mohy Eldin, M.S. Fabrication of biodegradable gelatin/chitosan/cinnamaldehyde crosslinked membranes for antibacterial wound dressing applications. Int. J. Biol. Macromol. 2019, 139, 440–448. [Google Scholar] [CrossRef]
- Gupta, N.; Santhiya, D. In situ mineralization of bioactive glass in gelatin matrix. Mater. Lett. 2017, 188, 127–129. [Google Scholar] [CrossRef]
- Lonkar, S.P.; Pillai, V.; Abdala, A. Solvent-free synthesis of ZnO-graphene nanocomposite with superior photocatalytic activity. Appl. Surf. Sci. 2019, 465, 1107–1113. [Google Scholar] [CrossRef]
- Chen, Y.; Lu, W.; Guo, Y.; Zhu, Y.; Song, Y. Electrospun Gelatin Fibers Surface Loaded ZnO Particles as a Potential Biodegradable Antibacterial Wound Dressing. Nanomaterials 2019, 9, 525. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shankar, S.; Jaiswal, L.; Selvakannan, P.R.; Ham, K.S.; Rhim, J.W. Gelatin-based dissolvable antibacterial films reinforced with metallic nanoparticles. RSC Adv. 2016, 6, 67340–67352. [Google Scholar] [CrossRef]
- Banthia, S.; Hazra, C.; Sen, R.; Das, S.; Das, K. Electrodeposited functionally graded coating inhibits Gram-positive and Gram-negative bacteria by a lipid peroxidation mediated membrane damage mechanism. Mater. Sci. Eng. C 2019, 102, 623–633. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; He, Y.; Irwin, P.L.; Jin, T.; Shi, X. Antibacterial Activity and Mechanism of Action of Zinc Oxide Nanoparticles against Campylobacter jejuni. Appl. Environ. Microbiol. 2011, 77, 2325–2331. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.-W.; Cao, A.; Jiang, Y.; Zhang, X.; Liu, J.-H.; Liu, Y.; Wang, H. Superior Antibacterial Activity of Zinc Oxide/Graphene Oxide Composites Originating from High Zinc Concentration Localized around Bacteria. ACS Appl. Mater. Interfaces 2014, 6, 2791–2798. [Google Scholar] [CrossRef]
- El-Shafai, N.; El-Khouly, M.; El-Kemary, M.; Ramadan, M.; Eldesoukey, I.; Masoud, M. Graphene oxide decorated with zinc oxide nanoflower, silver and titanium dioxide nanoparticles: Fabrication, characterization, DNA interaction, and antibacterial activity. RSC Adv. 2019, 9, 3704–3714. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Wang, Y.; Ma, J.; Peng, Y.; Wang, A. A review on bidirectional analogies between the photocatalysis and antibacterial properties of ZnO. J. Alloy. Compd. 2019, 783, 898–918. [Google Scholar] [CrossRef]
- Thangavel, S.; Krishnamoorthy, K.; Krishnaswamy, V.; Raju, N.; Kim, S.J.; Venugopal, G. Graphdiyne-ZnO Nanohybrids as an Advanced Photocatalytic Material. J. Phys. Chem. C 2015, 119, 22057–22065. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds ZnO/GO and ZGF are available from the authors. |




© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.; Chen, Y.; Lu, W.; Xu, Y.; Guo, Y.; Yang, G. Preparation of Electrospun Gelatin Mat with Incorporated Zinc Oxide/Graphene Oxide and Its Antibacterial Activity. Molecules 2020, 25, 1043. https://doi.org/10.3390/molecules25051043
Li H, Chen Y, Lu W, Xu Y, Guo Y, Yang G. Preparation of Electrospun Gelatin Mat with Incorporated Zinc Oxide/Graphene Oxide and Its Antibacterial Activity. Molecules. 2020; 25(5):1043. https://doi.org/10.3390/molecules25051043
Chicago/Turabian StyleLi, Honghai, Yu Chen, Weipeng Lu, Yisheng Xu, Yanchuan Guo, and Geng Yang. 2020. "Preparation of Electrospun Gelatin Mat with Incorporated Zinc Oxide/Graphene Oxide and Its Antibacterial Activity" Molecules 25, no. 5: 1043. https://doi.org/10.3390/molecules25051043





