Physicochemical Properties of Dried Apple Slices: Impact of Osmo-Dehydration, Sonication, and Drying Methods
Abstract
:1. Introduction
2. Results and Discussion
2.1. Drying Kinetics
2.2. Dry Matter Content and Water Activity
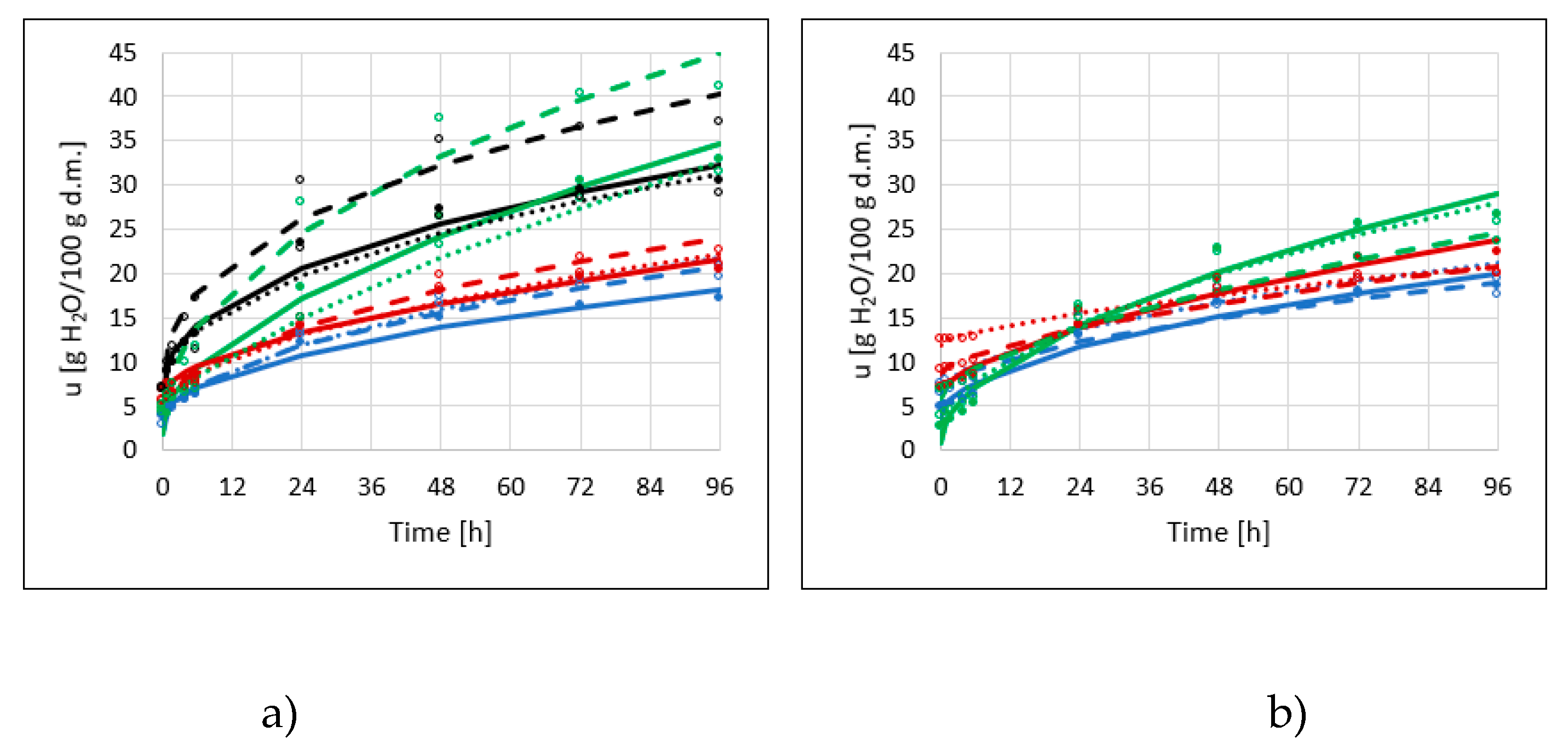
2.3. Hygroscopic Properties
2.4. Color Changes
2.5. Selected Chemical Analysis
2.6. Product Safety
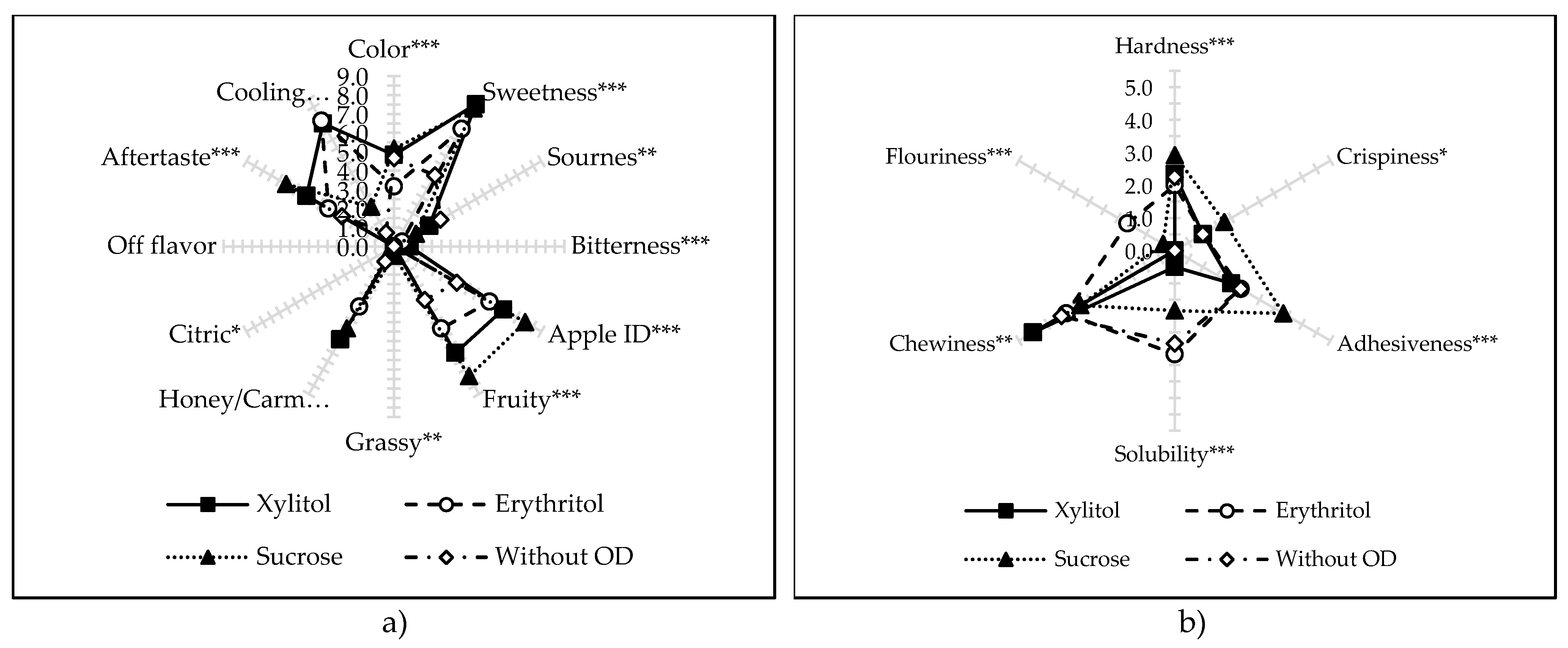
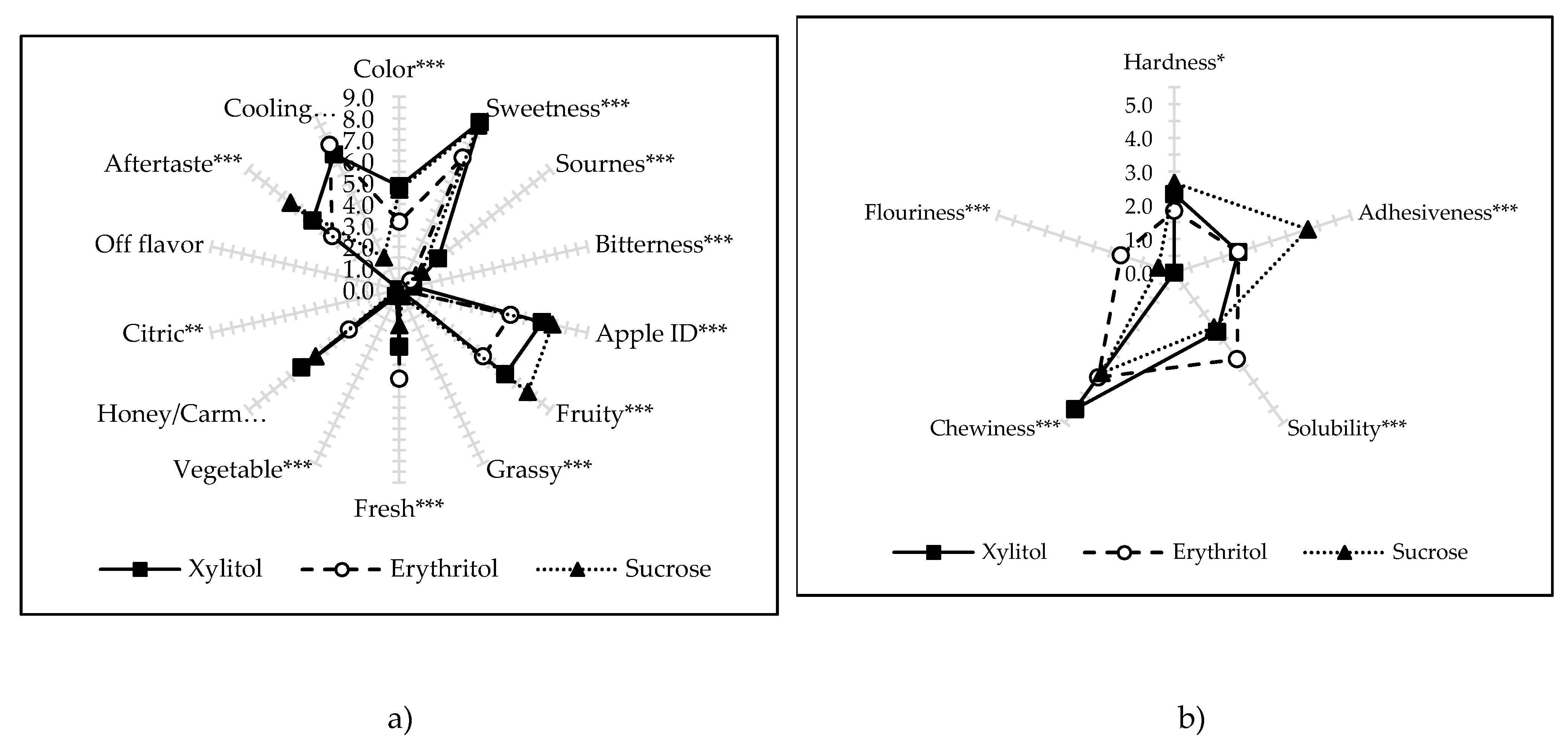
2.7. Descriptive Sensory Analysis (DSA)
3. Materials and Methods
3.1. Sample Preparation
3.2. Pre-Treatment Procedure
3.3. Drying Procedure
3.4. Mathematical Modeling
3.5. Water Activity
3.6. Hygroscopicity
3.7. Color Measurement
3.8. Descriptive Sensory Analysis (DSA)
3.9. Selected Chemical Analysis
3.10. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| OD | osmotic dehydration |
| US | ultrasound |
| CD | convective drying |
| VM | vacuum-microwave drying |
| CD/VM | combined drying (convective-vacuum-microwave drying) |
Appendix A
| No | Sample Code | Drying Method | Type of Hypertonic Solution | Sonication |
|---|---|---|---|---|
| 1. | XCD | CD | Xylitol | − |
| 2. | XVM | VM | Xylitol | |
| 3. | XCD/VM | CD/VM | Xylitol | |
| 4. | ECD | CD | Erythritol | − |
| 5. | EVM | VM | Erythritol | |
| 6. | ECD/VM | CD/VM | Erythritol | |
| 7. | SCD | CD | Sucrose | − |
| 8. | SVM | VM | Sucrose | |
| 9. | SCD/VM | CD/VM | Sucrose | |
| 10. | RCD | CD | Without OD | − |
| 11. | RVM | VM | Without OD | |
| 12. | RCD/VM | CD/VM | Without OD | |
| 13. | XCD_US | CD | Xylitol | + |
| 14. | XVM_US | VM | Xylitol | |
| 15. | XCD/VM_US | CD/VM | Xylitol | |
| 16. | ECD_US | CD | Erythritol | + |
| 17. | EVM_US | VM | Erythritol | |
| 18. | ECD/VM_US | CD/VM | Erythritol | |
| 19. | SCD_US | CD | Sucrose | + |
| 20. | SVM_US | VM | Sucrose | |
| 21. | SCD/VM_US | CD/VM | Sucrose |
References
- Sinha, N.K. Apple: Production, Chemistry, and Processing. In Handbook of Food Products Manufacturing; Hui, Y.H., Ed.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2007; pp. 773–791. [Google Scholar]
- de la Fuente-Blanco, S.; Riera-Franco de Sarabia, E.; Acosta-Aparicio, V.M.; Blanco-Blanco, A.; Gallego-Juarez, J.A. Food drying process by power ultrasound. Ultrasonics 2006, 44, 523–527. [Google Scholar] [CrossRef] [PubMed]
- Schössler, K.; Jäger, H.; Knorr, D. Effect of continuous and intermittent ultrasound on drying time and effective diffusivity during convective drying of apple and red bell pepper. J. Food Eng. 2012, 108, 103–110. [Google Scholar] [CrossRef]
- Wojdyło, A.; Figiel, A.; Oszmiański, J. Effect of Drying Methods with the Application of Vacuum Microwaves on the Bioactive Compounds, Color, and Antioxidant Activity of Strawberry Fruits. J. Agric. Food Chem. 2009, 57, 1337–1343. [Google Scholar] [CrossRef] [PubMed]
- Chong, C.H.; Figiel, A.; Law, C.L.; Wojdyło, A. Combined Drying of Apple Cubes by Using of Heat Pump, Vacuum-Microwave, and Intermittent Techniques. Food Bioprocess. Technol. 2014, 7, 975–989. [Google Scholar] [CrossRef] [Green Version]
- Ramya, V.; Jain, N.K. A Review on Osmotic Dehydration of Fruits and Vegetables: An Integrated Approach. J. Food Process. Eng. 2017, 40, e12440. [Google Scholar] [CrossRef]
- Cichowska, J.; Figiel, A.; Stasiak-Różańska, L.; Witrowa-Rajchert, D. Modeling of Osmotic Dehydration of Apples in Sugar Alcohols and Dihydroxyacetone (DHA) Solutions. Foods 2019, 8, 20. [Google Scholar] [CrossRef] [Green Version]
- Cichowska, J.; Witrowa-Rajchert, D.; Stasiak-Rózánska, L.; Figiel, A. Ultrasound-assisted osmotic dehydration of apples in polyols and dihydroxyacetone (DHA) solutions. Molecules 2019, 24, 3429. [Google Scholar] [CrossRef] [Green Version]
- Mierzwa, D.; Kowalski, S.J. Ultrasound-assisted osmotic dehydration and convective drying of apples: Process kinetics and quality issues. Chem. Process. Eng. - Inz. Chem. i Proces. 2016, 37, 383–391. [Google Scholar] [CrossRef]
- Fijalkowska, A.; Nowacka, M.; Witrowa-Rajchert, D. The physical, optical and reconstitution properties of apples subjected to ultrasound before drying. Ital. J. Food Sci. 2017, 29, 343–356. [Google Scholar]
- Fernandes, F.A.N.; Gallão, M.I.; Rodrigues, S. Effect of osmotic dehydration and ultrasound pre-treatment on cell structure: Melon dehydration. LWT - Food Sci. Technol. 2008, 41, 604–610. [Google Scholar] [CrossRef]
- Figiel, A. Drying kinetics and quality of beetroots dehydrated by combination of convective and vacuum-microwave methods. J. Food Eng. 2010, 98, 461–470. [Google Scholar] [CrossRef]
- Figiel, A.; Michalska, A. Overall quality of fruits and vegetables products affected by the drying processes with the assistance of vacuum-microwaves. Int. J. Mol. Sci. 2017, 18, 71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Figiel, A. Dehydration of Apples By a Combination of Convective and Vacuum-Microwave Drying. Pol. J. Food Nutr. Sci. 2007, 57, 131–135. [Google Scholar]
- Calín-Sánchez, Á.; Lech, K.; Szumny, A.; Figiel, A.; Carbonell-Barrachina, A.A. Volatile composition of sweet basil essential oil (Ocimum basilicum L.) as affected by drying method. Food Res. Int. 2012, 48, 217–225. [Google Scholar] [CrossRef]
- Wiktor, A.; Gondek, E.; Jakubczyk, E.; Dadan, M.; Nowacka, M.; Rybak, K.; Witrowa-Rajchert, D. Acoustic and mechanical properties of carrot tissue treated by pulsed electric field, ultrasound and combination of both. J. Food Eng. 2018, 238, 12–21. [Google Scholar] [CrossRef]
- Witrowa-Rajchert, D.; Fabisiak, A.; Stawczyk, J.; Li, S. Wpływ metody suszenia i temperatury procesu na właściwości higroskopijne suszu jabłkowego. Inżynieria Rol. 2006, 7, 457–463. [Google Scholar]
- Konopacka, D.; Plocharski, W.; Beveridge, T. Water sorption and crispness of fat-free apple chips. J. Food Sci. 2002, 67, 87–92. [Google Scholar] [CrossRef]
- Nowacka, M.; Witrowa-Rajchert, D. Changes of hygroscopic properties of dried apples during storage condition. Acta Agrophysica 2010, 15, 359–370. [Google Scholar]
- Fijalkowska, A.; Nowacka, M.; Wiktor, A.; Sledz, M.; Witrowa-Rajchert, D. Ultrasound as a Pretreatment Method to Improve Drying Kinetics and Sensory Properties of Dried Apple. J. Food Process. Eng. 2016, 39, 256–265. [Google Scholar] [CrossRef]
- Calín-Sánchez, Á.; Kharaghani, A.; Lech, K.; Figiel, A.; Carbonell-Barrachina, A.A.; Tsotsas, E. Drying Kinetics and Microstructural and SensoryProperties of Black Chokeberry (Aronia melanocarpa) as Affected by Drying Method. Food Bioprocess. Technol. 2015, 8, 63–74. [Google Scholar] [CrossRef]
- Cichowska, J.; Woźniak, Ł; Figiel, A.; Witrowa-Rajchert, D. The Influence of Osmotic Dehydration in Polyols Solutions on Sugar Profiles and Color Changes of Apple Tissue. Period. Polytech. Chem. Eng. 2019, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Tetzloff, W.; Dauchy, F.; Medimagh, S.; Carr, D.; Bar, A. Tolerance to Subchronic, High-Dose Ingestion of Erythritol in Human Volunteers. Regul. Toxicol. Pharmacol. 1996, 24, S286–S295. [Google Scholar] [CrossRef] [PubMed]
- Grembecka, M. Sugar alcohols — their role in the modern world of sweeteners: A review. Eur. Food Res. Technol. 2015, 241, 1–14. [Google Scholar] [CrossRef] [Green Version]
- EFSA Panel on Dietetic Products N and A (NDA) Scientific Opinion on the substantiation of health claims related to the sugar replacers xylitol, sorbitol, mannitol, maltitol, lactitol, isomalt, erythritol, D-tagatose, isomaltulose, sucralose and polydextrose and maintenance of tooth mineralisation by decreasing tooth demineralisation. EFSA Journal 2011, 9, 2076.
- Grembecka, M. Sugar Alcohols as Sugar Substitutes in Food Industry. In Sweeteners; Springer International Publishing: Cham, Switzerland, 2016; pp. 1–27. [Google Scholar]
- Oku, T.; Okazaki, M. Laxative threshold of sugar alcohol erythritol in human subjects. Nutr. Res. 1996, 16, 577–589. [Google Scholar] [CrossRef]
- Bornet, F.R.J.; Blayo, A.; Dauchy, F.; Slama, G. Gastrointestinal Response and Plasma and Urine Determinations in Human Subjects Given Erythritol. Regul. Toxicol. Pharmacol. 1996, 24, 296–302. [Google Scholar] [CrossRef] [Green Version]
- Storey, D.; Lee, A.; Bornet, F.; Brouns, F. Gastrointestinal tolerance of erythritol and xylitol ingested in a liquid. Eur. J. Clin. Nutr. 2007, 61, 349–354. [Google Scholar] [CrossRef]
- Culbert, S.J.; Wang, Y.M.; Fritsche, H.A.; Carr, D.; Lantin, E.; van Eys, J. Oral xylitol in American adults. Nutr. Res. 1986, 6, 913–922. [Google Scholar] [CrossRef]
- Lee, B.D.; Park, M.K. Effects and Safety of Xylitol on Middle Ear Epithelial Cells. J. Int. Adv. Otol. 2014, 10, 19–24. [Google Scholar] [CrossRef]
- Wang, Y.M.; van Eys, J. Nutritional Significance of Fructose and Sugar Alcohols. Annu. Rev. Nutr. 1981, 1, 437–475. [Google Scholar] [CrossRef]
- Oku, T.; Nakamura, S. Threshold for Transitory Diarrhea Induced by Ingestion of Xylitol and Lactitol in Young Male and Female Adults. J. Nutr. Sci. Vitaminol. 2007, 53, 13–20. [Google Scholar] [CrossRef] [PubMed]
- de Cock, P.E. Erythritol. In Sweeteners and Sugar Alternatives in Food Technology; Donnell, K.O., Kearsley, M.W., Eds.; Black Well Publishing: Hoboken, NJ, USA, 2012; pp. 215–242. [Google Scholar]
- Lech, K.; Figiel, A.; Wojdyło, A.; Korzeniowska, M.; Serowik, M.; Szarycz, M. Drying Kinetics and Bioactivity of Beetroot Slices Pretreated in Concentrated Chokeberry Juice and Dried with Vacuum Microwaves. Dry. Technol. 2015, 33, 1644–1653. [Google Scholar] [CrossRef]
- Vázquez-Araújo, L.; Nuncio-Jáuregui, P.N.; Cherdchu, P.; Hernandez, F.; Chambers, E.; Carbonell-Barrachina, A.A. Physicochemical and descriptive sensory characterization of Spanish pomegranates: Aptitudes for processing and fresh consumption. Int. J. Food Sci. Technol. 2013, 49, 1663–1672. [Google Scholar] [CrossRef]
Sample Availability: Samples of the osmotic agents: erythritol, xylitol, sucrose are available from the authors. |

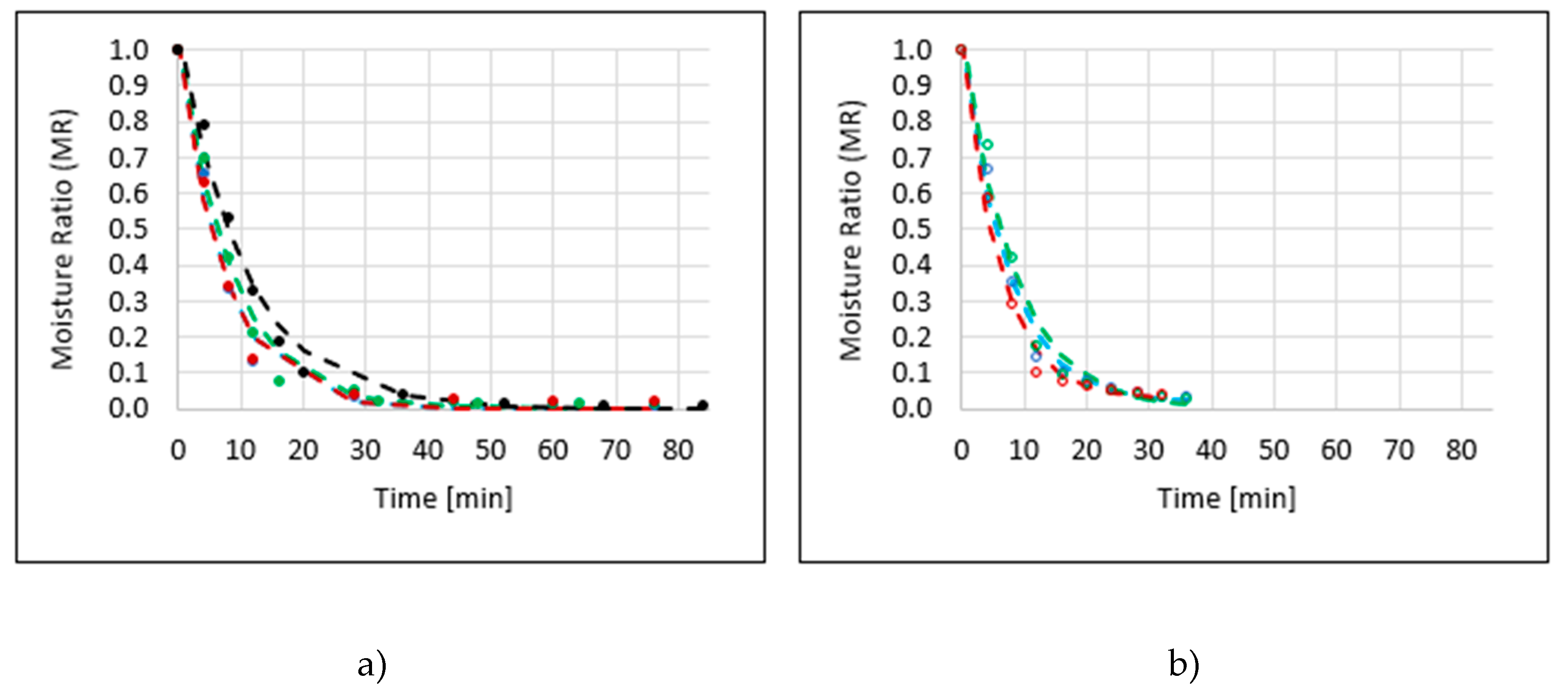
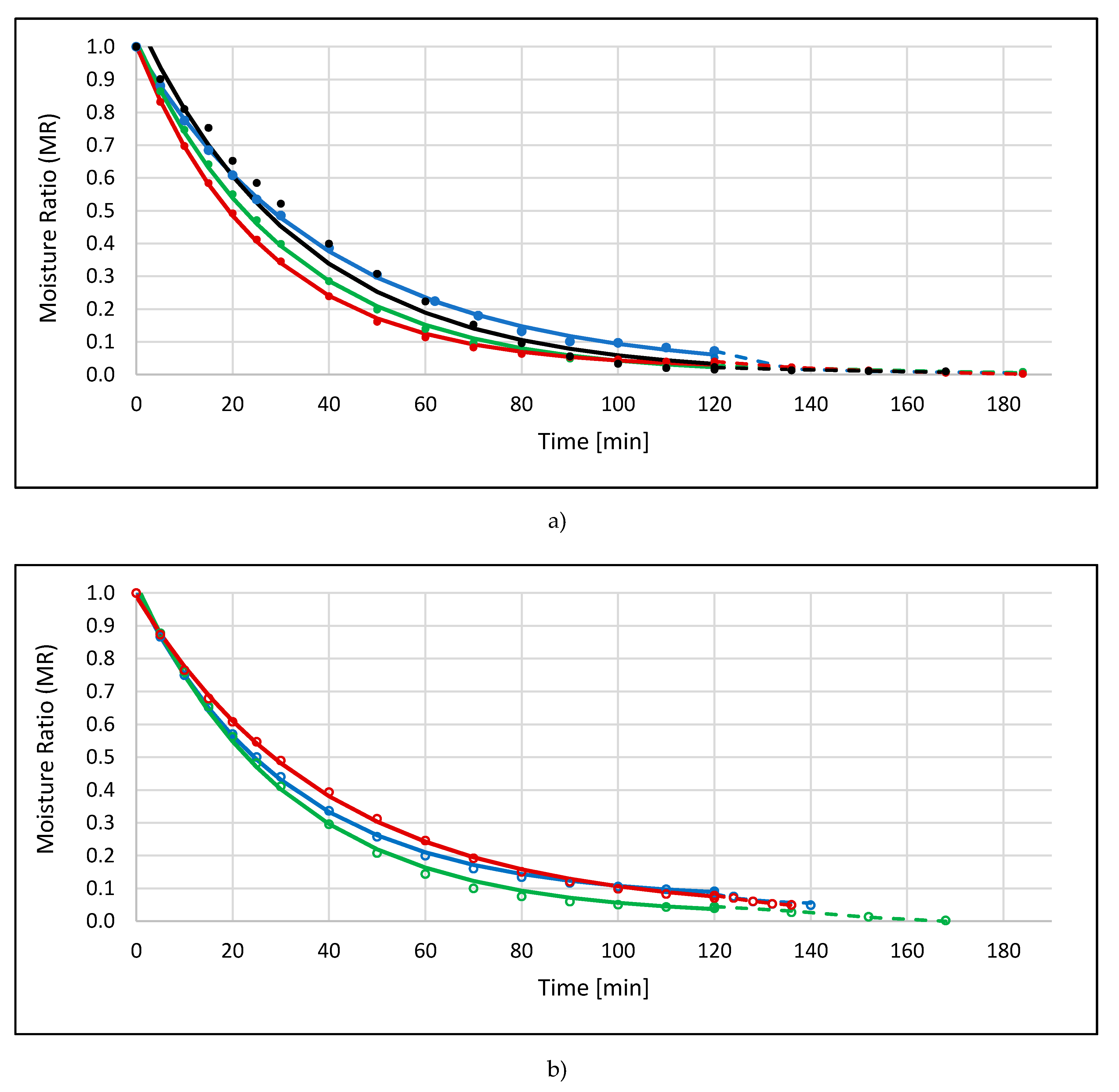


| Sample Code | Model | b1 | k1 | b2 | k2 | R2 | RMSE | Drying Time [min] |
|---|---|---|---|---|---|---|---|---|
| Non-US | ||||||||
| Erythritol | ||||||||
| CD | two-term exponential | 0.8304 | 0.0251 | 0.1658 | 0.0064 | 0.9998 | 0.0037 | 405 |
| VM | two-term exponential | 0.5857 | 0.1373 | 0.4381 | 0.1373 | 0.9887 | 0.0345 | 76 |
| CD/VM | two-term exponential | 0.0549 | 0.1665 | 0.0263 | 0.0093 | 0.9992 | 0.0090 | 184 |
| Xylitol | ||||||||
| CD | two-term exponential | 0.5670 | 0.0316 | 0.4486 | 0.0319 | 0.9990 | 0.0093 | 315 |
| VM | two-term exponential | 0.5960 | 0.1172 | 0.4321 | 0.1172 | 0.9893 | 0.0342 | 76 |
| CD/VM | two-term exponential | 0.0190 | 0.0144 | 0.0190 | 0.0125 | 0.8953 | 0.0018 | 184 |
| Sucrose | ||||||||
| CD | two-term exponential | 0.9779 | 0.0376 | 0.0256 | 0.0022 | 0.9997 | 0.0048 | 375 |
| VM | two-term exponential | 0.6467 | 0.1375 | 0.3707 | 0.1373 | 0.9913 | 0.0308 | 76 |
| CD/VM | two-term exponential | 0.0305 | 0.1018 | 0.0498 | 0.0084 | 0.9977 | 0.0005 | 184 |
| Without OD | two-term exponential | 0.3843 | 0.0940 | 0.6628 | 0.0939 | 0.9869 | 0.0378 | 84 |
| CD | two-term exponential | 0.3411 | 0.0255 | 0.7059 | 0.0257 | 0.9936 | 0.0255 | 345 |
| VM | ||||||||
| CD/VM | Henderson–Pabis | 0.0221 | 0.0211 | – | – | 0.9113 | 0.0016 | 168 |
| US | ||||||||
| Erythritol | ||||||||
| CD | two-term exponential | 0.9262 | 0.0319 | 0.0786 | 0.0011 | 0.9997 | 0.0045 | 615 |
| VM | two-term exponential | 1.0097 | 0.1389 | 0.0164 | 0.0001 | 0.9893 | 0.0321 | 36 |
| CD/VM | two-term exponential | −0.2943 | 0.0399 | 0.3452 | 0.0399 | 0.9443 | 0.0063 | 140 |
| Xylitol | ||||||||
| CD | two-term exponential | 1.0115 | 0.0319 | 0.0134 | −0.0007 | 0.9986 | 0.0103 | 465 |
| VM | two-term exponential | 0.9930 | 0.1225 | 0.0510 | 0.1230 | 0.9785 | 0.0447 | 36 |
| CD/VM | Henderson-Pabis | 0.0810 | 0.0298 | - | - | 0.9868 | 0.0025 | 168 |
| Sucrose | ||||||||
| CD | two-term exponential | 0.9513 | 0.0253 | 0.0390 | 0.0024 | 0.9996 | 0.0054 | 615 |
| VM | two-term exponential | 0.9908 | 0.1657 | 0.0256 | 0.0001 | 0.9890 | 0.0277 | 32 |
| CD/VM | two-term exponential | 0.0548 | 0.0498 | 0.0394 | 0.0000 | 0.9895 | 0.0011 | 136 |
| Dry matter [%] | Water Activity [-] | |||
|---|---|---|---|---|
| Non-US | US | Non-US | US | |
| Erythritol | ||||
| CD | 96.37 ± 0.19 c, A | 95.37 ± 0.04 b, A | 0.296 ± 0.00 a, B | 0.316 ± 0.01 b, A |
| VM | 96.92 ± 0.31 d, B | 93.10 ± 1.20 a, A | 0.256 ± 0.03 a, A | 0.404 ± 0.08 a, A |
| CD/VM | 96.38 ± 0.32 c, A, B | 93.95 ± 1.63 b, A | 0.284 ± 0.01 b, A, B | 0.342 ± 0.03 b, A |
| Xylitol | ||||
| CD | 95.35 ± 0.18 b, A | 97.40 ± 0.12 c, A | 0.298 ± 0.01 a, A | 0.217 ± 0.04 a, A |
| VM | 96.05 ± 0.26 c, A | 93.62 ± 1.70 a, A | 0.285 ± 0.00 a, A | 0.329 ± 0.01 a, B |
| CD/VM | 96.56 ± 0.52 b, c, A | 96.32 ± 1.66 b, A | 0.295 ± 0.00 a, A | 0.225 ± 0.01 a, A |
| Sucrose | ||||
| CD | 93.40 ± 0.15 a, A | 93.34 ± 0.43 a, C | 0.298 ± 0.01 a, A | 0.331 ± 0.01 b, A |
| VM | 94.75 ± 0.02 b, B | 91.61 ± 0.24 a, B | 0.277 ± 0.03 a, A | 0.345 ± 0.01 a, A |
| CD/VM | 94.80 ± 0.16 b, B | 88.85 ± 0.53 a, A | 0.289 ± 0.01 b, A | 0.392 ± 0.01 c, B |
| Without OD | ||||
| CD | 93.74 ± 0.31 a, A | – | 0.287 ± 0.01 a, A | – |
| VM | 93.69 ± 0.41 a, A | – | 0.288 ± 0.00 a, A | – |
| CD/VM | 93.91 ± 0.47 a, A | – | 0.284 ± 0.00 a, b, A | – |
| L* | ∆E | |||
|---|---|---|---|---|
| Non-US | US | Non-US | US | |
| Erythritol | ||||
| CD | 80.32 ± 1.57 a, A | 74.58 ± 2.79 b, A | 4.79 ± 1.34 a, A | 14.94 ± 0.59 a, A |
| VM | 82.03 ± 2.29 a, A | 72.15 ± 3.81 a, A | 2.48 ± 0.33 a, A | 15.24 ± 2.14 a, A |
| CD/VM | 76.93 ± 3.01 a, A | 75.29 ± 2.72 a, A | 7.62 ± 1.81 a, A | 11.07 ± 3.34 a, A |
| Xylitol | ||||
| CD | 79.88 ± 0.95 a, A | 74.81 ± 1.96 c, A | 8.01 ± 0.72 a, A | 18.89 ± 1.04 c, A |
| VM | 74.14 ± 0.32 a, A | 68.62 ± 6.66 a, A | 12.98 ± 1.81 a, b, A | 20.09 ± 4.20 a, A |
| CD/VM | 77.27 ± 2.70 a, A | 72.72 ± 3.58 a, A | 7.66 ± 2.60 a, A | 15.28 ± 5.97 a, A |
| Sucrose | ||||
| CD | 80.49 ± 0.99 a, A | 72.55 ± 2.05 a, A | 9.72 ± 1.71 a, A | 18.45 ± 2.01 b, A |
| VM | 73.42 ± 6.05 a, A | 70.32 ± 1.73 a, A | 10.07 ± 5.34 a, b, A | 17.28 ± 1.43 a, A |
| CD/VM | 76.49 ± 3.83 a, A | 75.64 ± 2.23 a, A | 9.03 ± 2.02 a, A | 12.40 ± 6.04 a, A |
| Without OD | ||||
| CD | 84.80 ± 0.31 b, B | – | 7.87 ± 1.18 a, A | – |
| VM | 68.90 ± 2.77 a, A | – | 20.16 ± 1.31 b, B | – |
| CD/VM | 83.79 ± 0.91 a, B | – | 7.71 ± 0.60 a, A | – |
| ECD/VM | XCD/VM | SCD/VM | RCD/VM | Raw Apple | |
|---|---|---|---|---|---|
| Total protein content (N × 6.25) [g/100 g] | 1.43 ± 0.09 a | 1.22 ± 0.03 a | 1.52 ± 0.04 a, b | 1.77 ± 0.11 b | 0.34 ± 0.00 |
| Total ash [g/100 g] | 0.51 ± 0.00 b | 0.47 ± 0.01 a | 0.46 ± 0.00 a | 0.56 ± 0.01 c | 0.17 ± 0.00 |
| Total fat [g/100 g] | 0.33 ± 0.06 a | 0.36 ± 0.09 a | 0.25 ± 0.03 a | 0.33 ± 0.06 a | 0.06 ± 0.00 |
| Total dietary fiber [g/100 g] | 6.81 ± 0.30 a | 7.42 ± 0.08 a | 7.23 ± 0.01 a | 8.52 ± 0.08 b | 1.74 ± 0.03 |
| Total carbohydrates [g/100 g] | 49.54 | 49.47 | 80.84 | 77.64 | 14.06 |
| Sugars: [g/100 g] | 46.55 | 48.65 | 78.28 | 77.56 | 12.93 |
| fructose g/100 g] | 22.32 ± 0.11 b | 22.46 ± 0.08 b | 20.38 ± 0.09 a | 36.04 ± 0.25 c | 6.86 ± 0.05 |
| glucose [g/100 g] | 7.39 ± 0.11 b | 6.22 ± 0.04 a | 6.20 ± 0.07 a | 11.06 ± 0.11 c | 2.32 ± 0.01 |
| sucrose [g/100 g] | 16.84 ± 0.05 a | 19.98 ± 0.09 b | 51.70 ± 0.14 d | 30.46 ± 0.04 c | 3.75 ± 0.04 |
| Polyols: [g/100 g] | 34.85 ± 0.29 c | 35.79 ± 0.30 c | 2.05 ± 0.02 a | 3.33 ± 0.08 b | 0.68 ± 0.02 |
| Sorbitol + xylitol [g/100 g] | 2.26 ± 0.04 a | 35.79 ± 0.30 c | 2.05 ± 0.02 a | 3.33 ± 0.08 b | 0.68 ± 0.02 |
| erythritol [g/100 g] | 32.59 ± 0.25 b | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 |
| Sodium (Na) [mg/kg] | 16.9 ± 0.14 a | 19.65 ± 0.21 b | 19.95 ± 0.35 b | 17.15 ± 0.49 a | 2.8 ± 0.14 |
| Energy value [kJ/100 g] | 225.8 | 306.7 | 351.1 | 345.0 | 63.3 |
| Energy value [kcal/100 g] | 955.5 | 1292.3 | 1487.8 | 1460.8 | 267.7 |
| Erythritol | Xylitol | |||
|---|---|---|---|---|
| According [23] | According [33] | According [30,32] | According [33] | |
| Adult female 60 kg | 60 g of erythritol contains in 184 g of the product → 12 packages | 41 g of erythritol contains in 126 g of the product → 8 packages | 100 g of xylitol contains in 279 g of the product → 18 packages | 25 g of xylitol contains in 70 g of the product → 5 packages |
| Adult male 75 kg | 75 g of erythritol contains in 215 g of the product → 14 packages | 35 g of erythritol contains in 107 g of the product → 7 packages | 100 g of xylitol contains in 279 g of the product → 18 packages | 29 g of xylitol contains in 81 g of the product → 5 packages |
| 10-years girl 30 kg | 30 g of erythritol contains in 92 g of the product → 6 packages | 20 g of erythritol contains in 61 g of the product → 4 packages | 65 g of xylitol contains in 182 g of the product → 12 packages | 13 g of xylitol contains in 36 g of the product → 2 packages |
| 10-years boy 30 kg | 30 g of erythritol contains in 92 g of the product → 6 packages | 14 g of erythritol contains in 43 g of the product → 3 packages | 65 g of xylitol contains in 182 g of the product → 12 packages | 11 g of xylitol contains in 31 g of the product → 2 packages |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cichowska-Bogusz, J.; Figiel, A.; Carbonell-Barrachina, A.A.; Pasławska, M.; Witrowa-Rajchert, D. Physicochemical Properties of Dried Apple Slices: Impact of Osmo-Dehydration, Sonication, and Drying Methods. Molecules 2020, 25, 1078. https://doi.org/10.3390/molecules25051078
Cichowska-Bogusz J, Figiel A, Carbonell-Barrachina AA, Pasławska M, Witrowa-Rajchert D. Physicochemical Properties of Dried Apple Slices: Impact of Osmo-Dehydration, Sonication, and Drying Methods. Molecules. 2020; 25(5):1078. https://doi.org/10.3390/molecules25051078
Chicago/Turabian StyleCichowska-Bogusz, Joanna, Adam Figiel, Angel Antonio Carbonell-Barrachina, Marta Pasławska, and Dorota Witrowa-Rajchert. 2020. "Physicochemical Properties of Dried Apple Slices: Impact of Osmo-Dehydration, Sonication, and Drying Methods" Molecules 25, no. 5: 1078. https://doi.org/10.3390/molecules25051078





