Insecticidal Toxicities of Three Main Constituents Derived from Trachyspermum ammi (L.) Sprague ex Turrill Fruits against the Small Hive Beetles, Aethina tumida Murray
Abstract
:1. Introduction
2. Results
2.1. Characterization of the Essential Oil
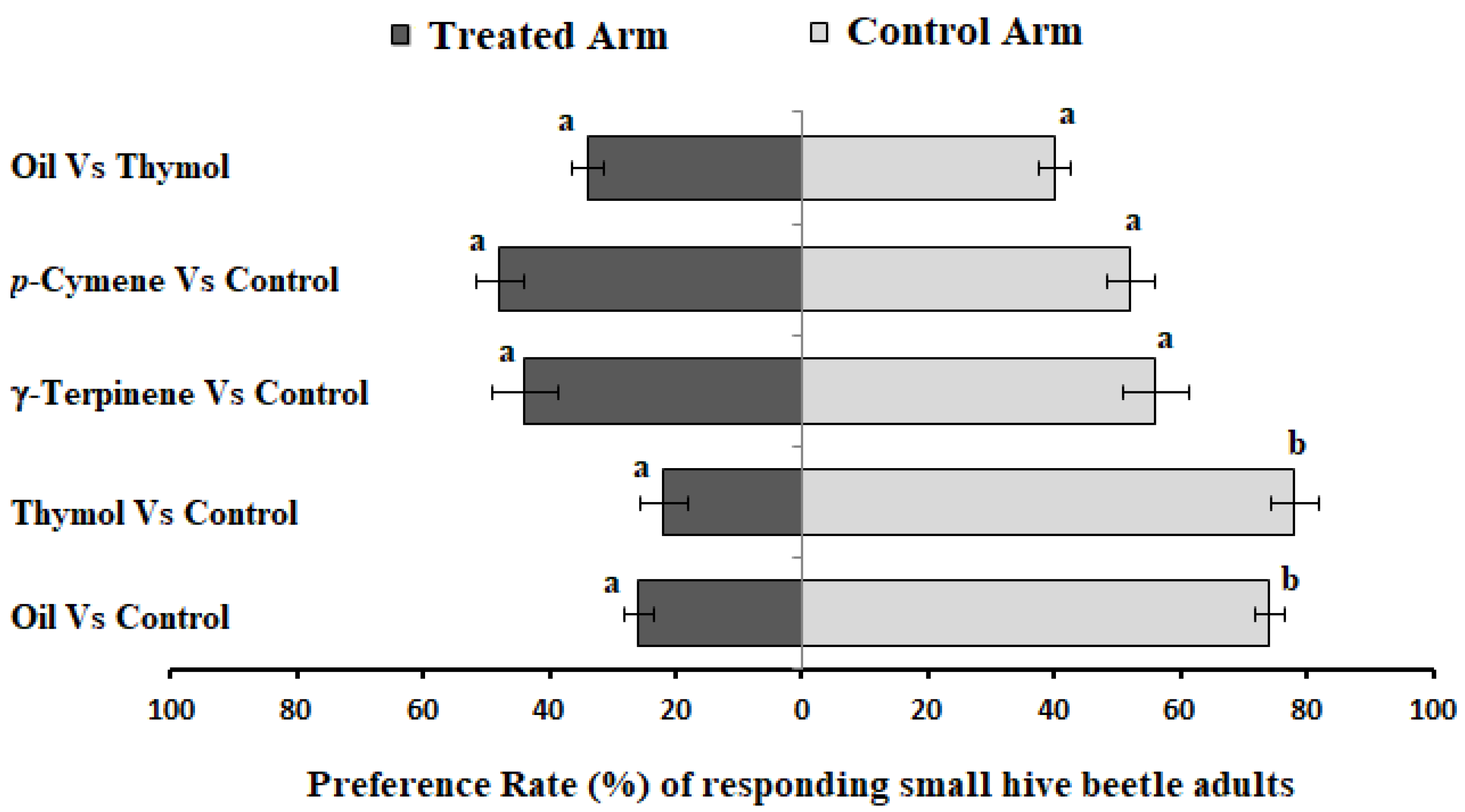
2.2. Olfactory Orientation Responses of Adult SHBs
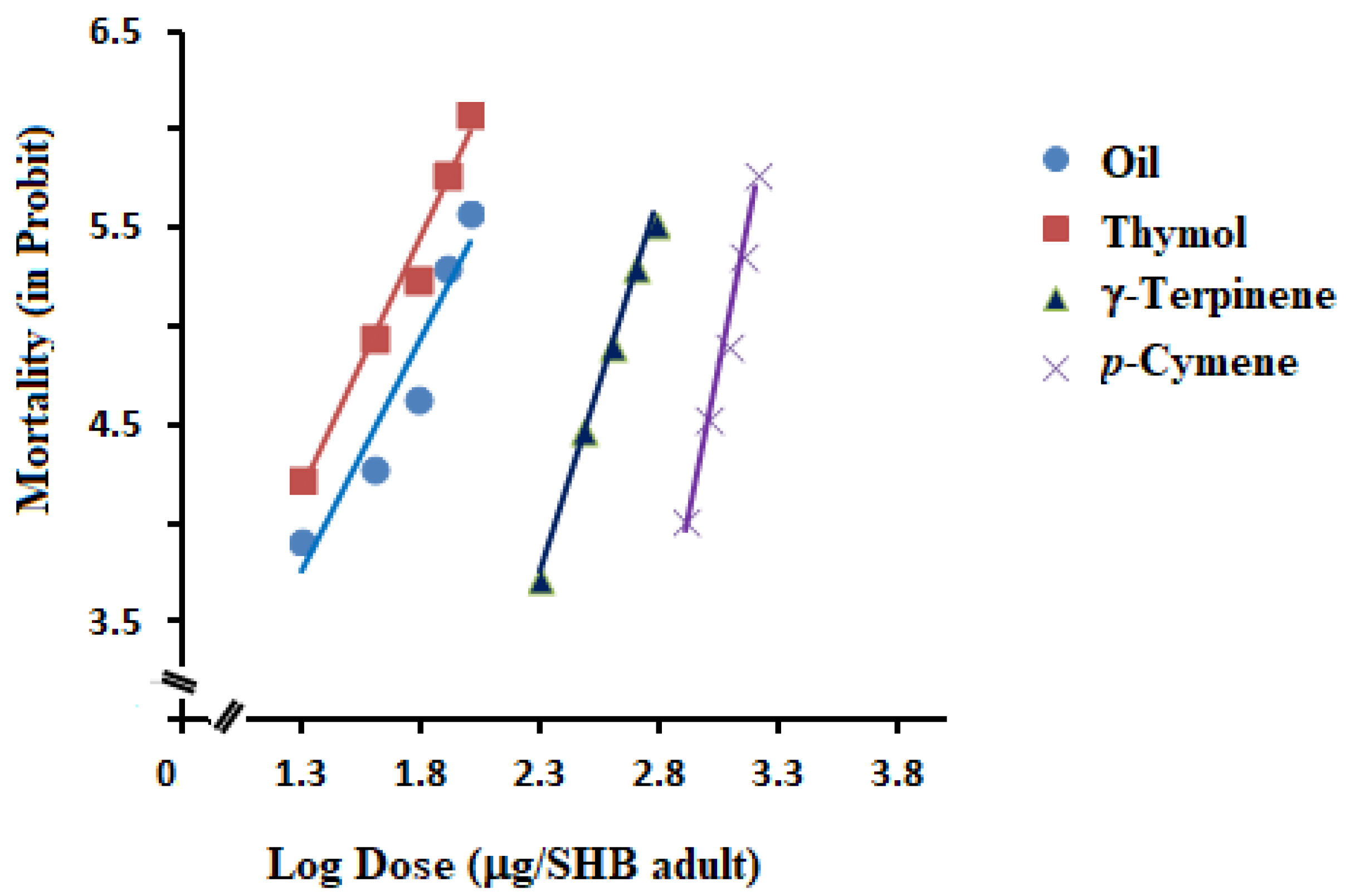
2.3. Contact Toxicity of Essential Oil and Its Main Constituents
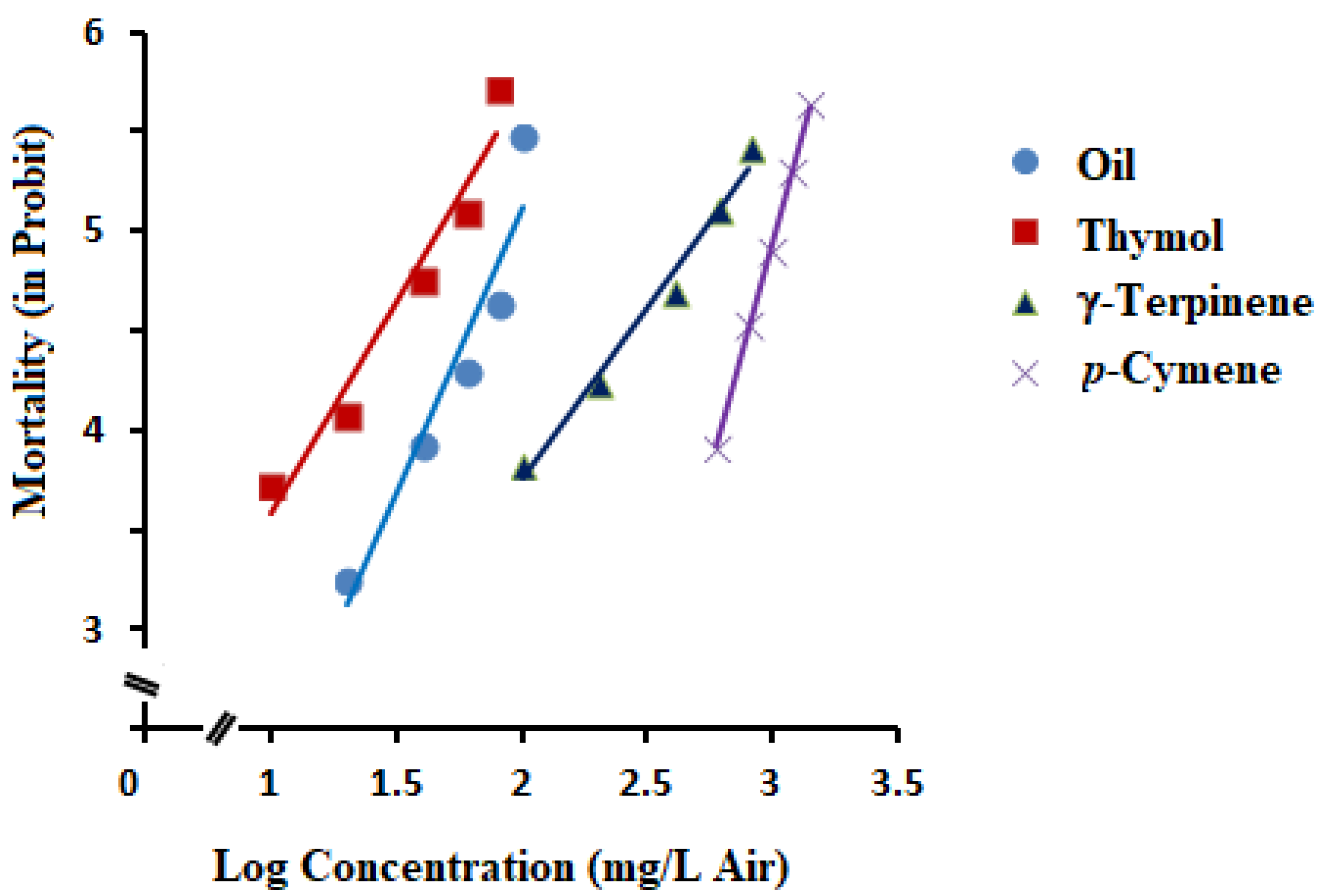
2.4. Fumigant Toxicity of Essential Oil and Its Main Constituents
3. Discussion
4. Materials and Methods
4.1. Materials
4.1.1. Plant Material
4.1.2. Insects
4.1.3. Chemicals
4.1.4. GC-MS Analysis
4.2. Methods
4.2.1. Essential Oil Extraction
4.2.2. Olfactory Orientation Assay of SHBs
4.2.3. Contact Toxicity Assay
4.2.4. Fumigant Toxicity Assay
4.2.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kearns, C.A.; Inouye, D.W.; Waser, N.M. Endangered mutualisms: The conservation of plant-pollinator interactions. Annu. Rev. Ecol. Syst. 1998, 29, 83–112. [Google Scholar] [CrossRef]
- Klein, A.M.; Vassiere, B.E.; Cane, J.H.; Steffan-Dewenter, I.; Cunningham, S.A.; Kremen, C.; Tscharntke, T. Importance of crop pollinators in changing landscapes for world crops. Proc. R. Soc. Lond. Ser. B Biol. Sci. 2007, 274, 303–313. [Google Scholar] [CrossRef] [Green Version]
- Smith, K.M.; Loh, E.H.; Rostal, M.K.; Zambrana-Torrelio, C.M.; Mendiola, L.; Daszak, P. Pathogens, pests, and economics: Drivers of honey bee colony declines and losses. Ecohealth 2013, 10, 434–445. [Google Scholar] [CrossRef]
- Neumann, P.; Pettis, J.; Schafer, M.O. Quo vadis Aethina tumida? Biology and control of small hive beetles. Apidologie 2016, 47, 427–466. [Google Scholar] [CrossRef] [Green Version]
- Lundie, A.E. The small hive beetle, Aethina tumida. In Scientific Bulletin 220; Union of South Africa, Department of Agriculture and Forestry: Pretoria, South Africa, 1940; p. 30. [Google Scholar]
- Dekebo, A.; Hong, S.; Jung, C. Attractiveness of the Small Hive Beetle (Aethina tumida) to Volatiles from Honey bee (Apis mellifera) and Beehive Materials. J. Apic. 2017, 32, 315–326. [Google Scholar] [CrossRef]
- Dekebo, A.; Jung, C. Attractive response of small hive beetle, Aethina tumida Murray (Coleoptera: Nitidulidae) to some of major volatile extract from hive materials and workers of Apis mellifera. J. Asia Pac. Entomol. 2019. under review. [Google Scholar]
- Lee, S.; Hong, K.; Cho, Y.S.; Choi, Y.S.; Yoo, M.; Lee, S. Review of the subgenus Aethina Erichson s. str. (Coleoptera: Nitidulidae: Nitidulinae) in Korea, reporting recent invasion of small hive beetle, Aethina tumida. J. Asia Pac. Entomol. 2017, 20, 553–558. [Google Scholar]
- Mohamadzade Namin, S.; Koh, Y.H.; Osabutey, A.F.; Jung, C. Invasion pathway of the honeybee pest, small hive beetle, Aethina tumida (Coleoptera: Nitidulidae) in the Republic of Korea inferred by mitochondrial DNA sequence analysis. J. Asia Pac. Entomol. 2019, 22, 963–968. [Google Scholar] [CrossRef]
- Elzen, P.J.; Baxter, J.R.; Westervelt, D.; Randall, C.; Delaplane, K.S.; Cutts, L.; Wilson, W.T. Field control and biology studies of a new pest species, Aethina tumida Murray (Coleoptera, Nitidulidae), attacking European honey bees in the Western Hemisphere. Apidologie 1999, 30, 361–366. [Google Scholar] [CrossRef] [Green Version]
- Hood, W.M. Overview of the small hive beetle Aethina tumida in North America. Bee World 2000, 81, 129–137. [Google Scholar] [CrossRef]
- De Guzman, L.I.; Frake, A.M.; Rinderer, T.E.; Arbogast, R.T. Effect Of Height and Color on the Efficiency of Pole Traps for Aethina tumida (Coleoptera: Nitidulidae). J. Econ. Entomol. 2011, 104, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.M.; Jung, C. Susceptibility of Soil Insecticides to small Hive Beetle, Aethina tumida Murray (Coleoptera: Nitidulidae), an Invasive Alien Pest of Honeybee. J. Apic. 2018, 33, 149–155. [Google Scholar]
- Alewu, B.; Nosiri, C.; Pesticides and Human Health. Pesticides in the Modern World—Effects of Pesticides Exposure. InTech, 2011; pp. 231–250. Available online: http://www.intechopen.com/books/pesticides-in-the-modern-world-effects-of-pesticides-exposure/pesticide-and-human-health (accessed on 10 August 2019).
- WHO. Public Health Impact of Pesticides Used in Agriculture; WHO: London, UK, 1990. [Google Scholar]
- Koul, O.; Walia, S.; Dhaliwal, G.S. Essential oils as green pesticides: Potential and constraints. Biopestic. Int. 2008, 4, 63–84. [Google Scholar]
- Degaga, E.G. Grain Health Protectant Activity of Essential Oils against Infestation and Damage of Haricot Bean by Zabrotes subfasciatus (Boheman). Am. J. Exp. Agric. 2015, 9, 1–7. [Google Scholar] [CrossRef]
- Chaubey, M.K. Study of insecticidal properties of Trachyspermum ammi and Mentha arvensis essential oils against Sitophilus zeamais L. (Coleoptera: Curculionidae). Curr. Life Sci. 2018, 4, 10–17. [Google Scholar]
- Rizzo, R.; Verde, G.L.; Sinacori, M.; Maggi, F.; Cappellacci, L.; Petrelli, R.; Vittori, S.; Morshedloo, M.R.; Fofie, G.B.Y.; Benelli, G. Developing green insecticides to manage olive fruit flies? Ingestion toxicity of four essential oils in protein baits on Bactrocera oleae. Ind. Crop. Prod. 2020, 143, 111884. [Google Scholar] [CrossRef]
- Borugă, O.; Jianu, C.; Mişcă, C.; Goleţ, I.; Gruia, A.T.; Horhat, F.G. Thymus vulgaris essential oil: Chemical composition and antimicrobial activity. J. Med. Life 2014, 7, 56–60. [Google Scholar]
- Masoudi, S.; Rustaiyan, A.; Ameri, N.; Monfared, A.; Komeelizadeh, H.; Kamalinejad, M.; Jami-Roodi, J. Volatile oils of Carum copticum (L.) C.B. Clarke in Benth. et Hook and Semenovia tragioides (Boiss.) Manden from Iran. J. Essent. Oil Res. 2002, 14, 288–289. [Google Scholar] [CrossRef]
- Konakchiev, A.; Tsankova, E. The essential oils of Satureja montana ssp. kitaibelli Wierzb. and Satureja pilosa var. pilosa Velen from Bulgaria. J. Essent. Oil Res. 2002, 14, 120–121. [Google Scholar]
- Marchese, A.; Orhan, I.E.; Daglia, M.; Barbieri, R.; Di Lorenzo, A.; Nabavi, S.F.; Gortzi, O.; Izadi, M.; Nabavi, S.M. Antibacterial and antifungal activities of thymol: A brief review of the literature. Food Chem. 2016, 210, 402–414. [Google Scholar] [CrossRef]
- Calderone, N.W.; Wilson, W.T.; Spivak, M. Plant extracts used for control of the parasitic mites Varroa jacobsoni (Acari: Varroidae) and Acarapis woodi (Acari: Tarsonemidae) in colonies of Apis mellifera (Hymenoptera: Apidae). J. Econ. Entomol. 1997, 90, 1080–1086. [Google Scholar] [CrossRef]
- Tsao, R.; Yu, Q. Nematicidal activity of monoterpenoid compounds against economically important nematodes in agriculture. J. Essent. Oil Res. 2000, 12, 350–354. [Google Scholar] [CrossRef]
- El-Gengaihi, S.E.; Amer, S.A.A.; Mohamed, S.M. Biological activity of thyme oil and thymol against Tetranychus urticae Koch. Anz Schädl.kd. Pflanzenschutz Umwellschutz 1996, 69, 157–159. [Google Scholar] [CrossRef]
- Pandey, S.K.; Upadhyay, S.; Tripathi, A.K. Insecticidal and repellent activities of thymol from the essential oil of Trachyspermum ammi (Linn) Sprague seeds against Anopheles stephensi. Parasitol. Res. 2009, 105, 507–512. [Google Scholar] [CrossRef]
- Bairwa, R.; Sodha, R.S.; Rajawat, B.S. Trachyspermum ammi. Pharmacogn. Rev. 2012, 6, 56–60. [Google Scholar] [CrossRef]
- Singh, G.; Maurya, S.; Catalan, C.; De Lampasona, M.P. Chemical constituents, antifungal and antioxidative effects of ajwain essential oil and its acetone extract. J. Agric. Food Chem. 2004, 52, 3292–3296. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry, 4th ed.; Allured Publ. Corp: Carol Stream, IL, USA, 2007. [Google Scholar]
- Babushok, V.I.; Linstrom, P.J.; Zenkevich, I.G. Retention Indices for Frequently Reported Compounds of Plant Essential Oils. J. Phys. Chem. Ref. Data 2011, 40, 1–47. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Zhong, S.; Qin, Y.; Zhang, S.; Gao, Z.; Dang, Z.; Pan, W. Identification of plant chemicals attracting and repelling whiteflies. Arthropod Plant Inte. 2014, 8, 183–190. [Google Scholar] [CrossRef]
- Badawy, M.E.I.; El-Arami, S.A.A.; Abdelgaleil, S.A.M. Acaricidal and quantitative structure activity relationship of monoterpenes against the two-spotted spider mite, Tetranychus urticae. Exp. Appl. Acarol. 2010, 52, 261–274. [Google Scholar] [CrossRef]
- Seo, S.M.; Kim, J.; Lee, S.G.; Shin, C.H.; Shin, S.C.; Park, I.K. Fumigant antitermitic activity of plant essential oils and components from Ajowan (Trachyspermum ammi), Allspice (Pimenta dioica), caraway (Carum carvi), dill (Anethum graveolens), Geranium (Pelargonium graveolens), and Litsea (Litsea cubeba) oils against Japanese termite (Reticulitermes speratus Kolbe). J. Agric. Food Chem. 2009, 57, 6596–6602. [Google Scholar]
- Pavela, R. Antifeedant and Larvicidal Effects of Some Phenolic Components of Essential Oils Lasp Lines of Introduction Against Spodoptera littoralis (Boisd.). J. Essent. Oil Bear. Plants 2011, 14, 266–273. [Google Scholar] [CrossRef]
- Lee, S.; Tsao, R.; Peterson, C.; Coats, J.R. Insecticidal activity of monoterpenoids to western corn root worm (Coleoptera: Chrysomelidae), two spotted spidermite (Acari: Tetranychidae) and Housefly (Diptera: Muscidae). J. Econ. Entomol. 1997, 90, 883–892. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hummelbrunner, A.L.; Isman, M.B. Acute, sublethal, antifeedant and synergistic effects of monoterpenoid essential oil compounds on the tobacco cutworm (Lepidoptera: Noctuidae). J. Agric. Food Chem. 2001, 49, 715–720. [Google Scholar] [CrossRef] [PubMed]
- Franzios, G.; Mirotsou, M.; Hatziapostolou, E.; Kral, J.; Scouras, Z.G.; Mauragani-Tsipidou, P. Insecticidal and genotoxic activities of mint essential oils. J. Agric. Food Chem. 1997, 45, 2690–2694. [Google Scholar] [CrossRef]
- Traboulsi, A.F.; Taoubi, K.; El-Haj, S.; Bessiere, J.M.; Rammal, S. Insecticidal properties of essential plant oils against the mosquito Culex pipiens molesters (Diptera: Culicidae). Pest Manag. Sci. 2002, 58, 491–495. [Google Scholar] [CrossRef]
- Dahlgren, L.; Johnson, R.M.; Siegfried, B.D.; Ellis, M.D. Comparative Toxicity of Acaricides to Honey Bee (Hymenoptera: Apidae) Workers and Queens. J. Econ. Entomol. 2012, 105, 1895–1902. [Google Scholar] [CrossRef]
- Imdorf, A.; Bogdanov, S.; Kilchenmann, V.; Maquelin, C. Apilife VAR: A new varroacide with thymol as the main ingredient. Bee World 1995, 76, 77–83. [Google Scholar] [CrossRef]
- Kanga, L.; Somorin, A. Susceptibility of the small hive beetle, Aethina tumida (Coleoptera: Nitidulidae), to insecticides and insect growth regulators. Apidologie 2011, 43, 95–102. [Google Scholar] [CrossRef] [Green Version]
- Imdorf, A.; Kilchenmann, V.; Bogdanov, S.; Bachofen, B.; Beretta, C. Toxizität von Thymol, Campher, Menthol und Eucalyptol auf Varroa jacobsoni (Oud.) und Apis mellifera L. im Labortest. Apidologie 1995, 26, 27–31. [Google Scholar] [CrossRef] [Green Version]
- Poulose, A.J.; Croteau, R. Biosynthesis of aromatic monoterpenes conversion of γ-terpinene to p-cymene and thymol in Thymus vuigaris L. Arch. Biochem. Biophys. 1978, 187, 307–314. [Google Scholar] [CrossRef]
- Priestley, C.M.; Williamson, E.M.; Wafford, K.A.; Satelle, D.B. Thymol, a constituent of thyme essential oil, is a positive allosteric modulator of human GABAA receptors and a homooligomeric GABA receptor from Drosophila melanogaster. Br. J. Pharmacol. 2003, 140, 1363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bloomquist, J.R. Chloride channels as tools for developing selective insecticides. Arch. Insect Biochem. Physiol. 2003, 54, 145–156. [Google Scholar] [CrossRef] [PubMed]
- Schmelzer, G.H. Trachyspermum ammi (L.) Sprague ex Turrill. In Plant Resources of South-East Asia-Medicinal and Poisonous Plants 2: No 12(2); van Valkenburg, J.L.C.H., Bunyapraphatsara, N., Eds.; Backhuys Publisher: Leiden, The Netherlands, 2001; pp. 552–555. Available online: https://www.researchgate.net/profile/Johan_Van_Valkenburg/publication/40218702_Medicinal_and_poisonous_plants_2/links/58f7272aaca2723d16a9a95f/Medicinal-and-poisonous-plants-2.pdf (accessed on 20 October 2019).
- European Pharmacopoeia, Council of Europe, 3rd ed.; Council of Europe: Strasbourg, France, 1997.
- Park, C.G.; Shin, E.; Kim, J. Insecticidal activities of essential oils, Gaultheria fragrantissima and Illicium verum, their components and analogs against Callosobruchus chinensis adults. J. Asia Pac. Entomol. 2016, 19, 269–273. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |
| No. | Components a | RI b | RI c | Percentage Composition | Methods of Identification |
|---|---|---|---|---|---|
| 1 | α-Thujene | 926.3 | 927.8 | 0.79 | MS, RI |
| 2 | α-Pinene | 931.9 | 936.1 | 0.58 | MS, RI |
| 3 | β-Pinene | 974.8 | 977.7 | 4.56 | MS, RI |
| 4 | β-Myrcene | 991.8 | 989.2 | 1.07 | MS, RI |
| 5 | 3-Carene | 1008.6 | 1011.3 | 0.12 | MS, RI |
| 6 | α-Terpinene | 1015.8 | 1017.1 | 0.55 | MS, RI |
| 7 | p-Cymene | 1028.1 | 1024.3 | 27.92 | MS, RI |
| 8 | β-Phellandrene | 1031.9 | 1030.0 | 0.94 | MS, RI |
| 9 | γ-Terpinene | 1062.7 | 1059.7 | 32.72 | MS, RI |
| 10 | (Z)-Sabinene hydrate | 1069.5 | 1066.5 | 0.14 | MS, RI |
| 11 | (E)-Sabinene hydrate | 1096.0 | 1098.1 | 0.17 | MS, RI |
| 12 | Linalool | 1099.6 | 1099.0 | 1.21 | MS, RI |
| 13 | Undecane | 1102.1 | 1100.0 | 0.12 | MS, RI |
| 14 | (Z)-Verbenol | 1149.5 | 1144.4 | 0.13 | RI |
| 15 | p-Mentha-1,5-dien-8-ol | 1166.0 | 1166.6 | 1.21 | MS, RI |
| 16 | Terpinen-4-ol | 1176.0 | 1177.1 | 0.85 | MS, RI |
| 17 | α-Terpineol | 1191.7 | 1189.7 | 0.28 | MS, RI |
| 18 | (Z)-2,3-Epoxydecane | 1264.2 | - | 0.15 | MS |
| 19 | Thymol | 1295.8 | 1290.1 | 24.36 | MS, RI |
| 20 | Carvacrol | 1302.3 | 1300.4 | 0.51 | MS, RI |
| 21 | Thymol acetate | 1358.7 | 1356.4 | 0.20 | MS |
| 22 | Carvacrol acetate | 1372.2 | 1373.1 | 0.10 | MS |
| Total | 98.68% | ||||
| Test Samples | Probit Analysis | ||||||
|---|---|---|---|---|---|---|---|
| N | LD50 (µg/adult) | 95% CL | Slope ± SE | Intercept | X2 | Df | |
| Essential oil | 300 | 66.64 | 57.12–80.21 | 2.50 ± 0.75 | 0.45 | 20.432 | 48 |
| Thymol | 300 | 41.79 | 34.74–48.62 | 2.58 ± 0.72 | 0.82 | 16.856 | 48 |
| γ-Terpinene | 300 | 424.02 | 382.72–476.13 | 3.77 ± 1.09 | −4.91 | 19.250 | 48 |
| p-Cymene | 300 | 1208.71 | 1129.97–1297.72 | 5.80 ± 1.66 | −12.86 | 23.644 | 48 |
| Probit Analysis | |||||||
|---|---|---|---|---|---|---|---|
| Test Samples | N | LC50 (mg/L air) | 95% CL | Slope ± SE | Intercept | X2 | Df |
| Essential oil | 300 | 89.03 | 76.75–110.93 | 3.01 ± 0.94 | −0.86 | 31.800 | 48 |
| Thymol | 300 | 52.66 | 43.62–66.78 | 2.14 ± 0.60 | 1.31 | 26.497 | 48 |
| γ-Terpinene | 300 | 522.11 | 418.55–700.35 | 1.77 ± 0.56 | 0.20 | 18.409 | 48 |
| p-Cymene | 300 | 1027.69 | 945.24–1126.55 | 4.61 ± 1.36 | −8.89 | 19.859 | 48 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bisrat, D.; Jung, C. Insecticidal Toxicities of Three Main Constituents Derived from Trachyspermum ammi (L.) Sprague ex Turrill Fruits against the Small Hive Beetles, Aethina tumida Murray. Molecules 2020, 25, 1100. https://doi.org/10.3390/molecules25051100
Bisrat D, Jung C. Insecticidal Toxicities of Three Main Constituents Derived from Trachyspermum ammi (L.) Sprague ex Turrill Fruits against the Small Hive Beetles, Aethina tumida Murray. Molecules. 2020; 25(5):1100. https://doi.org/10.3390/molecules25051100
Chicago/Turabian StyleBisrat, Daniel, and Chuleui Jung. 2020. "Insecticidal Toxicities of Three Main Constituents Derived from Trachyspermum ammi (L.) Sprague ex Turrill Fruits against the Small Hive Beetles, Aethina tumida Murray" Molecules 25, no. 5: 1100. https://doi.org/10.3390/molecules25051100





