A Cyclodextrin-Based Controlled Release System in the Simulation of In Vitro Small Intestine
Abstract
:1. Introduction
2. Results and Discussion
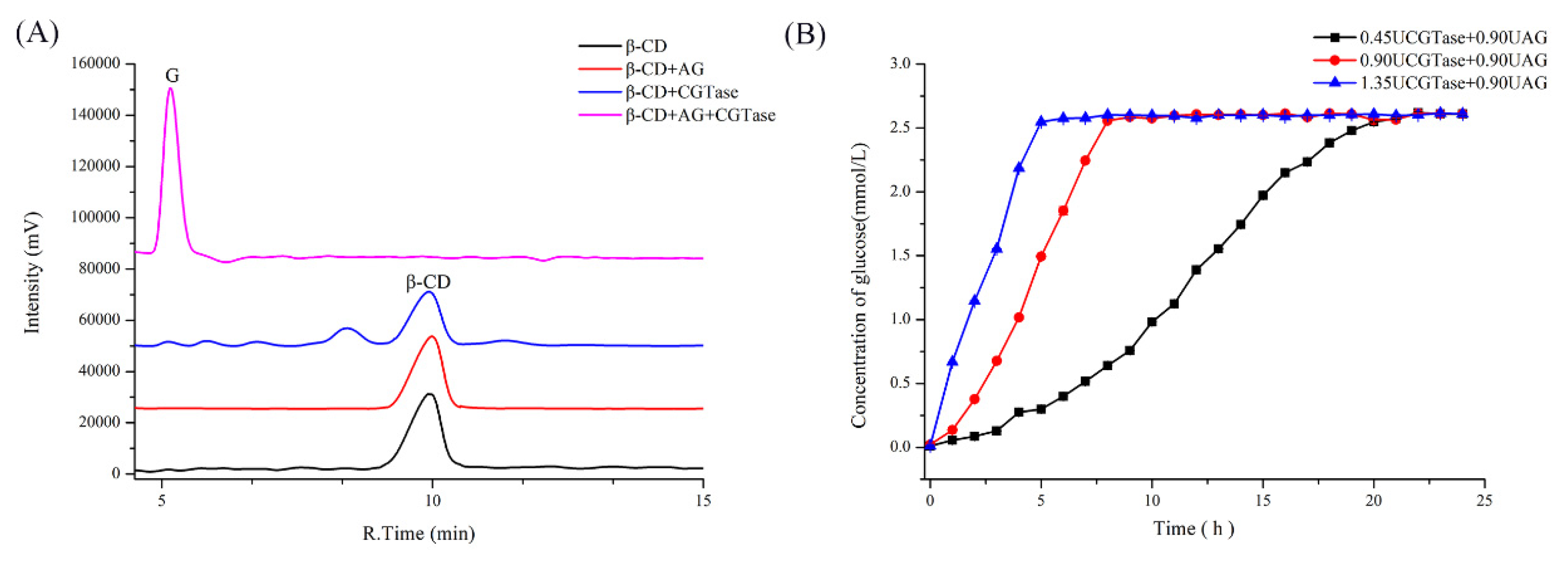
2.1. Substrate Specificity of Single and Dual Enzyme
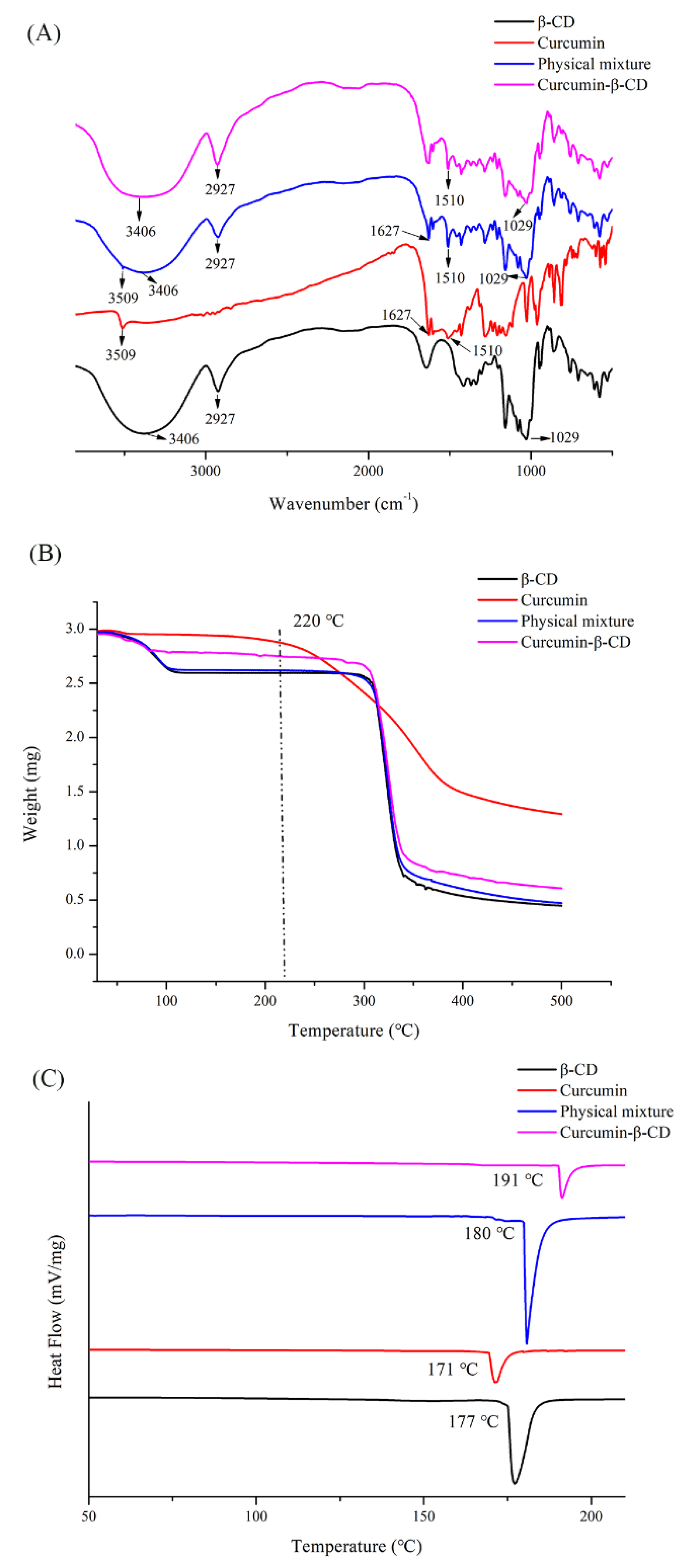
2.2. Preparation and Characterization of the Vanillin-/Curcumin-β-CD Complexes
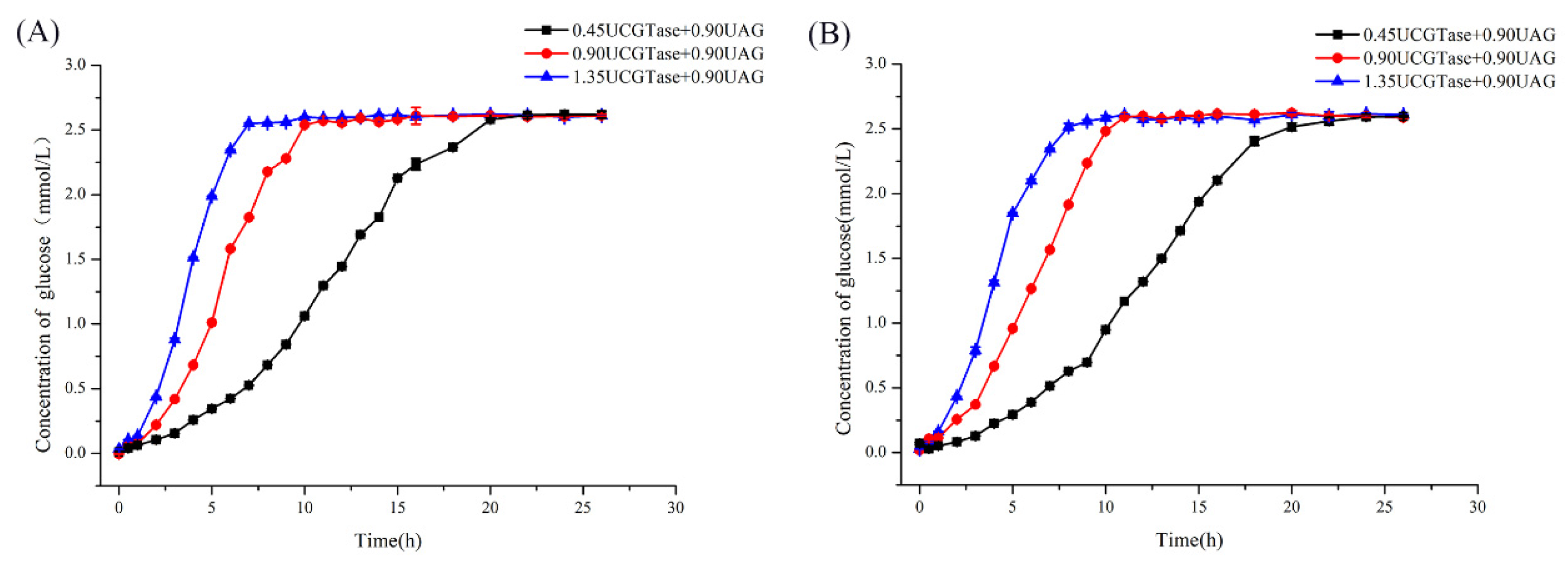
2.3. Qualitative and Kinetic Analysis of CD-Complex Degradation by Dual-Enzyme
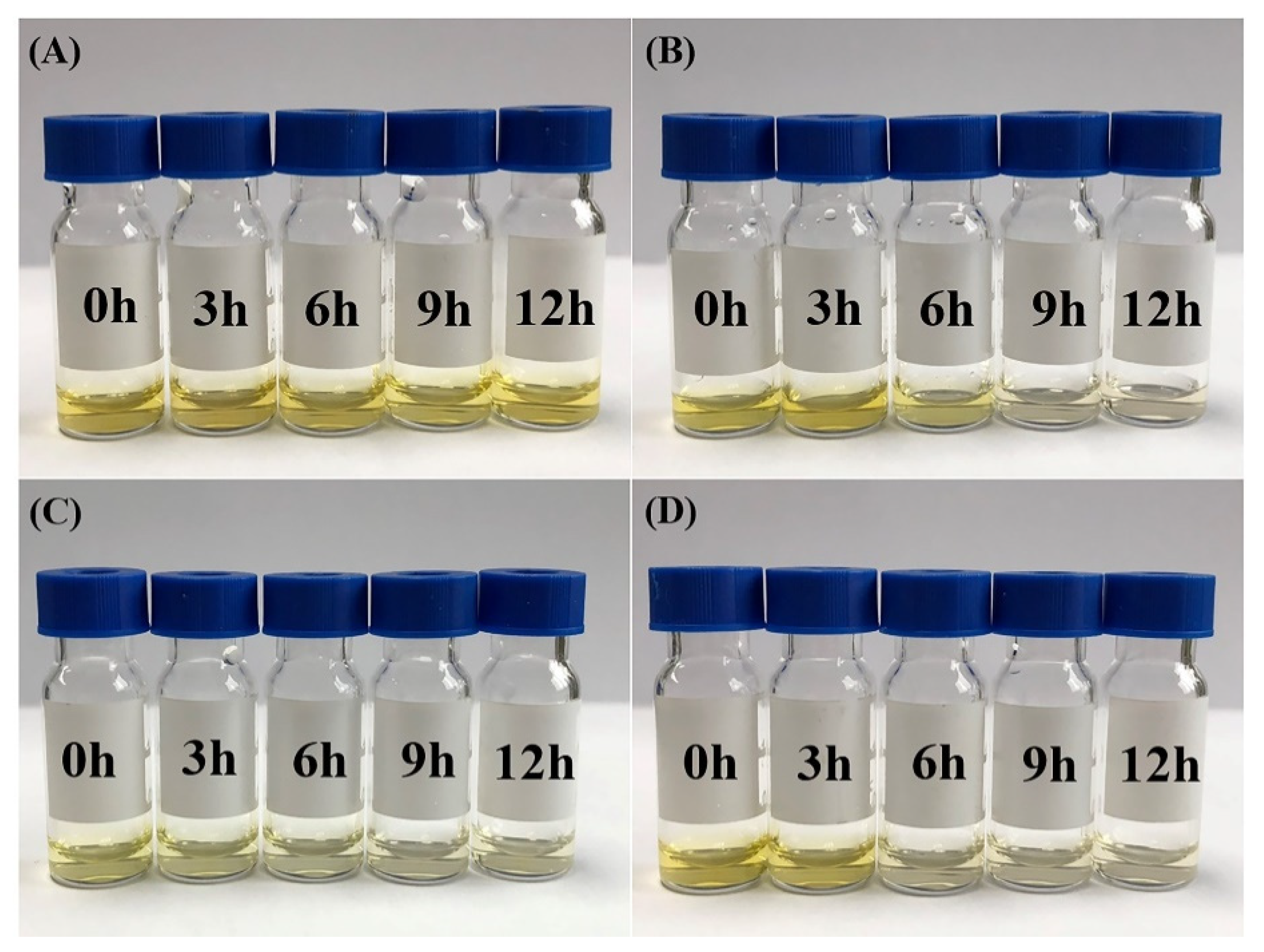
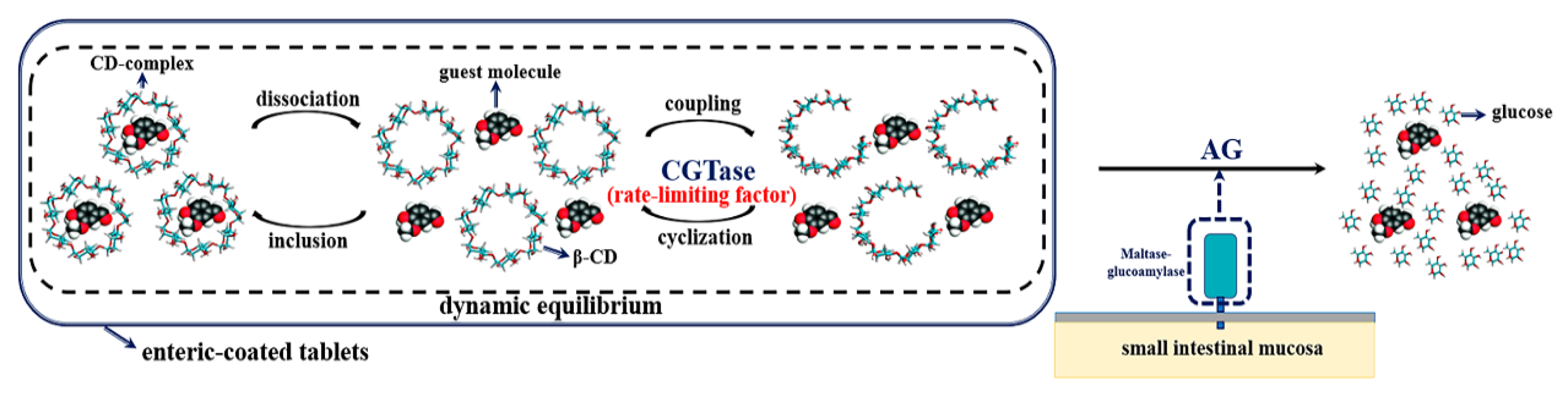
2.4. Controlled Release of Guest Molecules in the CD-Complex System
2.5. Mimic Pathway of the CD-Based Controlled Release System in the Small Intestine
3. Materials and Methods
3.1. Materials
3.2. Methods
3.2.1. Enzyme Assays
3.2.2. β-CD Hydrolysis Assays
3.2.3. Vanillin/Curcumin Release Assays
3.2.4. Preparation of Inclusion Complexes
3.2.5. Fourier Transform Infrared (FT-IR) Spectroscopy
3.2.6. Differential Scanning Calorimetry (DSC)
3.2.7. Thermogravimetric Analysis (TGA)
3.2.8. High Performance Liquid Chromatography (HPLC)
3.2.9. Kinetics of the Release of β-CD Inclusion Complex
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Langer, R.; Kost, J. Controlled release technology: Bioengineering aspects. Trends Biotechnol. 1984, 2, 22. [Google Scholar] [CrossRef]
- Ge, J.; Neofytou, E.; Cahill, T.J.; Beygui, R.E.; Zare, R.N. Drug Release from Electric-Field-Responsive Nanoparticles. ACS Nano 2011, 6, 227–233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Augustine, R.; Kalva, N.; Kim, H.A.; Zhang, Y.; Kim, I. pH-Responsive Polypeptide-Based Smart Nano-Carriers for Theranostic Applications. Molecules 2019, 24, 2961. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clear, N.J.; Milton, A.; Humphrey, M.; Henry, B.T.; Wulff, M.; Nichols, D.J.; Anziano, R.J.; Wilding, I. Evaluation of the Intelisite capsule to deliver theophylline and frusemide tablets to the small intestine and colon. Eur. J. Pharm. Sci. 2001, 13, 375–384. [Google Scholar] [CrossRef]
- Sadeghi, A.; Avadi, M.; Ejtemaimehr, S.; Abashzadeh, S.; Partoazar, A.; Dorkoosh, F.; Faghihi, M.; Rafiee-Tehrani, M.; Junginger, H. Development of a Gas Empowered Drug Delivery system for peptide delivery in the small intestine. J. Control. Release 2009, 134, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Takenaka, H.; Kawashima, Y.; Lin, S.-Y. Preparation of enteric-coated microcapsules for tableting by spray-drying technique and In Vitro simulation of drug release from the tablet in GI tract. J. Pharm. Sci. 1980, 69, 1388–1392. [Google Scholar] [CrossRef]
- Rujivipat, S.; Bodmeier, R. Improved drug delivery to the lower intestinal tract with tablets compression-coated with enteric/nonenteric polymer powder blends. Eur. J. Pharm. Biopharm. 2010, 76, 486–492. [Google Scholar] [CrossRef]
- Alvarez-Fuentes, J.; Arevalo, M.F.; González-Rodríguez, M.L.; Cirri, M.; Mura, P. Development of Enteric-coated Timed-release Matrix Tablets for Colon Targeting. J. Drug Target. 2004, 12, 607–612. [Google Scholar] [CrossRef]
- Liu, F.; Lizio, R.; Meier, C.; Petereit, H.-U.; Blakey, P.; Basit, A.W. A novel concept in enteric coating: A double-coating system providing rapid drug release in the proximal small intestine. J. Control. Release 2009, 133, 119–124. [Google Scholar] [CrossRef]
- Kang, J.-H.; Hwang, J.-Y.; Seo, J.-W.; Kim, H.-H.; Shin, U.S. Small intestine- and colon-specific smart oral drug delivery system with controlled release characteristic. Mater. Sci. Eng. C 2018, 91, 247–254. [Google Scholar] [CrossRef]
- Chen, J.; Qin, X.; Zhong, S.; Chen, S.; Su, W.; Liu, Y. Characterization of Curcumin/Cyclodextrin Polymer Inclusion Complex and Investigation on Its Antioxidant and Antiproliferative Activities. Molecules 2018, 23, 1179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ren, X.; Yue, S.; Xiang, H.; Xie, M. Inclusion complexes of eucalyptus essential oil with β-cyclodextrin: preparation, characterization and controlled release. J. Porous Mater. 2018, 25, 1577–1586. [Google Scholar] [CrossRef]
- Kuplennik, N.; Sosnik, A. Enhanced Nanoencapsulation of Sepiapterin within PEG-PCL Nanoparticles by Complexation with Triacetyl-Beta Cyclodextrin. Molecules 2019, 24, 2715. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.H.; Yun, J.; Xing, Y.G.; Xiao, Y.; Tang, Y. Complexation of Cinnamon Essential Oil by β-Cyclodextrin and its Release Characteristics at High Temperature. Adv. Mater. Res. 2010, 146, 619–622. [Google Scholar] [CrossRef]
- Dana, G.; Osnat, D.; Alexander, V.; Lijun, W.; Jallal, G.; Sandy, C.; Andreas, M. Ultrasound-mediated targeted drug delivery with a novel cyclodextrin-based drug carrier by mechanical and thermal mechanisms. J. Control. Release 2013, 170, 316–324. [Google Scholar]
- Horiuchi, Y.; Abe, K.; Hirayama, F.; Uekama, K. Release control of theophylline by β-cyclodextrin derivatives: hybridizing effect of hydrophilic, hydrophobic and ionizable β-cyclodextrin complexes. J. Control. Release 1991, 15, 177–183. [Google Scholar] [CrossRef]
- Ikeda, Y.; Kimura, K.; Hirayama, F.; Arima, H.; Uekama, K. Controlled release of a water-soluble drug, captopril, by a combination of hydrophilic and hydrophobic cyclodextrin derivatives. J. Control. Release 2000, 66, 271–280. [Google Scholar] [CrossRef]
- Rastegari, B.; Karbalaei-Heidari, H.R.; Zeinali, S.; Sheardown, H. The enzyme-sensitive release of prodigiosin grafted β-cyclodextrin and chitosan magnetic nanoparticles as an anticancer drug delivery system: Synthesis, characterization and cytotoxicity studies. Colloids Surfaces B 2017, 158, 589–601. [Google Scholar] [CrossRef]
- Dufresne, M.-H.; Marouf, E.; Kränzlin, Y.; Gauthier, M.A.; Leroux, J.-C. Lipase Is Essential for the Study of in Vitro Release Kinetics from Organogels. Mol. Pharm. 2012, 9, 1803–1811. [Google Scholar] [CrossRef]
- Rodríguez, S.D.; Bernik, D. Flavor release by enzymatic hydrolysis of starch samples containing vanillin–amylose inclusion complexes. LWT 2014, 59, 635–640. [Google Scholar] [CrossRef]
- Yoon, H.-S.; Lim, S.-T. Utilization of Enzyme-resistant Starch to Control Theophylline Release from Tablets. Starch 2009, 61, 154–160. [Google Scholar] [CrossRef]
- Alpers, D. Carbohydrates: Digestion, Absorption, and Metabolism. In Encyclopedia of Food Sciences and Nutrition; Elsevier: Amsterdam, The Netherlands, 2003; pp. 881–887. [Google Scholar]
- Divakar, S. Structure of β-cyclodextrin-vanillin inclusion complex. J. Agric. Food Chem. 1990, 38, 940–944. [Google Scholar] [CrossRef]
- Kayaci, F.; Uyar, T. Solid Inclusion Complexes of Vanillin with Cyclodextrins: Their Formation, Characterization, and High-Temperature Stability. J. Agric. Food Chem. 2011, 59, 11772–11778. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Deng, L.; Yang, S. Synthesis and characterization of β-cyclodextrin inclusion complex containing di(8-hydroxyquinoline)magnesium. Spectrochim. Acta Part A 2008, 70, 878–883. [Google Scholar] [CrossRef]
- Karathanos, V.T.; Mourtzinos, I.; Yannakopoulou, K.; Andrikopoulos, N.K. Study of the solubility, antioxidant activity and structure of inclusion complex of vanillin with β-cyclodextrin. Food Chem. 2007, 101, 652–658. [Google Scholar] [CrossRef]
- Tønnesen, H.H.; Másson, M.; Loftsson, T. Studies of curcumin and curcuminoids. XXVII. Cyclodextrin complexation: solubility, chemical and photochemical stability. Int. J. Pharm. 2002, 244, 127–135. [Google Scholar] [CrossRef]
- Stella, V. Mechanisms of drug release from cyclodextrin complexes. Adv. Drug Deliv. Rev. 1999, 36, 3–16. [Google Scholar] [CrossRef]
- Sharma, R.A.; Gescher, A.; Steward, W. Curcumin: The story so far. Eur. J. Cancer 2005, 41, 1955–1968. [Google Scholar] [CrossRef]
- Radu, C.D.; Parteni, O.; Ochiuz, L. Applications of cyclodextrins in medical textiles — review. J. Control. Release 2016, 224, 146–157. [Google Scholar] [CrossRef]
- Yim, C.T.; Zhu, X.X.; Brown, G.R. Kinetics of Inclusion Reactions of β-Cyclodextrin with Several Dihydroxycholate Ions Studied by NMR Spectroscopy. J. Phys. Chem. B 1999, 103, 597–602. [Google Scholar] [CrossRef]
- Goh, K.M.; Mahadi, N.M.; Hassan, O.; Rahman, R.N.Z.R.A.; Illias, R.M. A predominant β-CGTase G1 engineered to elucidate the relationship between protein structure and product specificity. J. Mol. Catal. B Enzym. 2009, 57, 270–277. [Google Scholar] [CrossRef]
- Vikmon, M. Rapid and Simple Spectrophotometric Method for Determination of Micro-Amounts of Cyclodextrins. In Proceedings of the Proceedings of the First International Symposium on Cyclodextrins; Springer: Berlin, Germany, 1982; pp. 69–74. [Google Scholar]
- Rani, A.; Das, M.; Satyanarayana, S. Preparation and characterization of amyloglucosidase adsorbed on activated charcoal. J. Mol. Catal. B 2000, 10, 471–476. [Google Scholar] [CrossRef]
- Ji, H.; Wang, Y.; Bai, Y.; Li, X.; Qiu, L.; Jin, Z. Application of cyclodextrinase in non-complexant production of γ-cyclodextrin. Biotechnol. Prog. 2019, e2930. [Google Scholar] [CrossRef]
- Wan, H.; Ni, Y.; Li, D. Preparation, characterization and evaluation of an inclusion complex of steviolbioside with γ-cyclodextrin. Food Biosci. 2018, 26, 65–72. [Google Scholar] [CrossRef]
- Ji, H.; Bai, Y.; Li, X.; Wang, J.; Xu, X.; Jin, Z. Preparation of malto-oligosaccharides with specific degree of polymerization by a novel cyclodextrinase from Palaeococcus pacificus. Carbohydr. Polym. 2019, 210, 64–72. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |


| Km (mg/mL) | Vmax (mg/(mL × min)) | kcat (min−1) | kcat/Km (mL × min−1 × mg−1) | |
|---|---|---|---|---|
| β-CD | 1.39 | 1.30 | 4.48 | 3.22 |
| Vanillin-β-CD inclusion complex | 1.79 | 0.92 | 3.17 | 1.77 |
| Curcumin-β-CD inclusion complex | 1.75 | 0.88 | 3.04 | 1.74 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, D.; Xia, L.; Ji, H.; Jin, Z.; Bai, Y. A Cyclodextrin-Based Controlled Release System in the Simulation of In Vitro Small Intestine. Molecules 2020, 25, 1212. https://doi.org/10.3390/molecules25051212
Zheng D, Xia L, Ji H, Jin Z, Bai Y. A Cyclodextrin-Based Controlled Release System in the Simulation of In Vitro Small Intestine. Molecules. 2020; 25(5):1212. https://doi.org/10.3390/molecules25051212
Chicago/Turabian StyleZheng, Danni, Liuxi Xia, Hangyan Ji, Zhengyu Jin, and Yuxiang Bai. 2020. "A Cyclodextrin-Based Controlled Release System in the Simulation of In Vitro Small Intestine" Molecules 25, no. 5: 1212. https://doi.org/10.3390/molecules25051212





