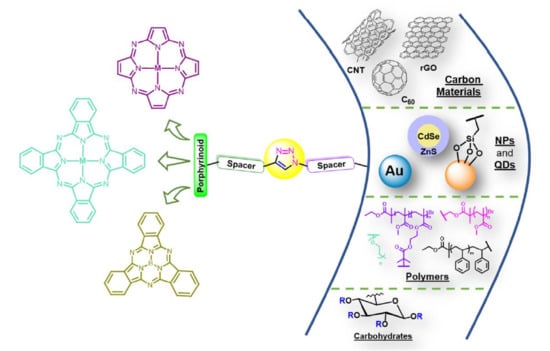
The CuAAC approach is a versatile and efficient strategy to synthesize new phthalocyanine derivatives and to incorporate phthalocyanine units on the surface of several nanomaterials, such as carbon materials (e.g., graphene oxide, carbon nanotubes), silica nanoparticles, gold nanoparticles, and quantum dots (e.g., CdSe/ZnS), as well as in polymers, biomolecules, and electrodes [
9,
10]. As discussed in the following sections, this approach has led to the development of improved and multifunctional systems based on Pcs with electrical and optical properties adequate for photovoltaic applications, sensing, photodynamic therapy, etc.
2.1. Phthalocyanines and New Carbon Nanomaterials
Innovative phthalocyanine carbon nanomaterials with improved electronic, chemical and mechanical properties have been obtained in recent decades. In this context, in 2016, Kadem et al. described the covalent attachment of the asymmetrical zinc(II) phthalocyanine
ZnPc1, functionalized with a terminal alkyne unit, to the surface of azido-substituted single-walled carbon nanotubes (SWCNTs) and to azido-substituted reduced graphene oxide (rGO), using CuSO
4·5H
2O as catalyst and sodium ascorbate (Na Asc) as reducing agent (
Scheme 1) [
11].
The obtained hybrids were characterized by using adequate techniques (e.g., FTIR, Raman spectroscopy, UV-Vis absorption). From absorption spectra and cyclic voltammetry studies it was found that the covalent attachment of ZnPc1 to SWCNTs or to rGO resulted in band gaps’ values lower than the ones found in free ZnPc1 (1.62 eV versus 1.77 eV) or in the hybrids obtained from its non-covalently attachment to SWCNTs or rGO (ca. 1.68 eV). The electron transfer process across the substituted ZnPc to the SWCNTs or rGO is favored by the presence of the triazole lone pair electrons. The studies have also shown an improvement in the electrical conductivity for both types of hybrids (covalently and non-covalently linked) when compared with the pure phthalocyanine film, being particularly relevant to the increase observed for the hybrid covalently linked to SWCNTs (218.6 mS/cm versus 11.4 mS/cm). The results obtained are contributions for the relevant area of photovoltaic applications.
In 2018, Yang and co-workers [
12], having in mind the synthesis of novel organic-inorganic hybrid materials with a high content of grafted phthalocyanine derivative on the surface of multi-walled carbon nanotubes (MWCNT), reported the synthesis of
ZnPc-MWCNT assemblies as illustrated in
Scheme 2. The hybrid was prepared in two steps that started with the reaction of an acetylene-functionalized MWCNT with 4-azidophthalonitrile in the presence of copper(I) iodide, 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU) and l-methyl-2-pyrrolidone (NMP). The second step consisted in the reaction of the formed phthalonitrile-modified MWCNT with 4-nitro-5-(
m-tolyloxy)phthalonitrile. The covalent conjugation of the phthalocyanine to the MWCNT was confirmed by FTIR, TEM, SEM, AFM, UV-Vis and fluorescence spectra. It was verified that the solubility of
ZnPc-MWCNT in tetrahydrofuran (THF) was significantly improved when compared with the one of the MWCNT.
In 2019, Mpeta and Nyokong reported that the reaction of reduced graphene oxide nanosheets bearing azido groups (GONS) with alkynyl-substituted cobalt(II) phthalocyanine derivatives (
CoPc2 and
CoPc3) allowed to obtain
CoPc2-GONS and
CoPc3-GONS assemblies (
Scheme 3) [
13]. The cycloaddition reaction was performed in
N,
N-dimethylformamide (DMF) in the presence of Cu(PPh
3)
3Br and trimethylamine. The electrocatalytic activity of the resulting triazole hybrids was evaluated in the oxidation of 2-mercaptoethanol, a compound with an important role in the metabolism and cellular homeostasis and used in corrosion inhibition processes. The study showed that the electrode obtained with
CoPc3-GONS (the alkyne group is separated from the phthalocyanine ring by an aliphatic group and a benzene ring) is a promising electrochemical sensor for 2-mercaptoethanol, showing the best catalytic activity when compared with the one with
CoPc2-GONS. Additionally, the same hybrid exhibited the highest sensitivity and a lower limit of detection than other sensors reported in the literature.
2.2. Phthalocyanine-Silica Nanoparticles
In recent years, silica nanoparticles (SiNPs) have received significant attention due to their chemical inertness, non-toxicity, optical transparency, and excellent thermal stability. SiNPs have been widely used in catalysis, chemical processes in industry, removal of metal ions, encapsulation of organic light-emitting diodes and also as nanocarriers for photosensitizers [
14].
The CuAAC reaction has been used for the modification of silica nanoparticles and in their subsequent conjugation with organic molecules, namely with Pcs. For example, Nyokong and co-workers described the synthesis of the mono-substituted zinc(II) phthalocyanines
ZnPc4a–
c bearing terminal alkynyl units and their conjugation with silica nanoparticles functionalized with azido groups (
Scheme 4) [
15]. The alkynyl-substituted phthalocyanines were prepared by reacting the adequate carboxyphenoxyphthalocyanines with hex-5-yn-1-ol in the presence of the coupling agents
N,
N’-dicyclohexylcarbodiimide (DCC) and 4-(dimethylamino)pyridine (DMAP); the conjugation with the azido-nanoparticles was performed in THF/H
2O, at room temperature, in the CuAAC conditions (
Scheme 4).
From the photophysical properties of the hybrids and of the starting materials, it was observed that the esterification of the zinc(II) phthalocyanines with hex-5-yn-1-ol increases the fluorescence quantum yields and the fluorescence is maintained after the grafting process. The triplet quantum yields of
ZnP4a–
c (
ca. 0.65) increased significantly when compared to those of the precursor phthalocyanine complexes (0.45), but decreased after the grafting to the SiNPs. On the other hand, the triplet lifetimes of all hybrids increased in comparison to the lifetimes of the non-immobilized ZnPcs and this behavior was justified by considering a possible protection provided by the polymeric silica nanoparticles to the phthalocyanines in the triplet state [
15].
In 2017, Wong and co-workers selected the CuAAC approach in different steps of the synthetic strategy to develop the triazole photoactive nanoparticles
ZnPc5c@MSN (
Scheme 5) [
16]. The approach involved the conjugation of mesoporous silica nanoparticles (MSN), functionalized with alkyne units, with the phthalocyanine dimer
ZnPc5c containing an azido terminal group. This dimer was obtained by reacting the previously described [
17] alkynyl
ZnPc5a with the acetal linker (obtained from 4-(2-bromoethoxy)benzaldehyde and 3-azidopropan-1-ol) under CuAAC conditions, followed by the reaction of the obtained
ZnPc5b with sodium azide. The alkyne-silica nanoparticles were prepared by reacting the amino mesoporous silica nanoparticles (obtained according with literature data [
18]) with 3-bromopropyne (
Scheme 5). The conjugation of
ZnPc5c with the alkyne-functionalized MSN was performed in DMF in the presence of CuI and Et
3N. The study showed that the fluorescence and the photosensitizing efficiency strongly quenched in the native triazole
ZnPc5c@MSN form was restored in acidic PBS (pH 5.5) or after its internalization in human colon adenocarcinoma HT29 cells. The release of
ZnPc units from the acetal linker under the acidic conditions justified the high intracellular fluorescence and the photocytotoxicity observed even in the in vivo studies. This behavior was not observed in analogue systems with a non-cleavable dimer, and that suggested that this nanosystem was a promising photosensitizing agent for PDT.
2.3. Phthalocyanine-Gold Nanoparticles
Gold nanoparticles (AuNPs) are one of the most significant and stable groups of metal nanoparticles with potential application in non-linear optical devices for protecting light-sensitive materials, amongst other applications [
19]. With the aim of improving the non-linear optics of Pcs, in 2017, Bankole and Nyokong selected the CuAAC approach to obtain conjugates of AuNPs with phthalocyanines [
20]. The nanocomposites
AuNPs-ZnPc6 and
AuNPs-InPc6 (
Scheme 6) were obtained using azide derivatized gold nanoparticles and the indium(III) and zinc(II) phthalocyanine complexes
InPc6 and
ZnPc6 bearing alkynyl units. The azide derivatized gold nanoparticles were obtained by partial ligand exchange of tetraoctylammonium bromide moieties with 3-azidopropylamine via N-Au bonds and the coupling of both components was performed in the presence of CuSO
4·5H
2O and sodium ascorbate (
Scheme 6) [
20]. Photophysical studies showed that the obtained nanocomposites had lower fluorescence quantum yields and lifetime values than those obtained for the unconjugated phthalocyanines; the quenching of the excited singlet states was justified as being due to the heavy atom effect.
Another gold nanomaterial that has been conjugated with phthalocyanines via the CuAAC reaction is the nanoporous gold (npAu). Recently, it has been shown that npAu can be used as a support for biomolecules in biosensors development studies, also as a catalyst for the oxidation of small molecules and as a material with plasmonic properties useful for optical sensing. This monolithic nanomaterial is known to exhibit a high surface area and so it can be easily functionalized with a thiol or disulfide groups in the context of self-assembled monolayers (SAMs) [
21].
In 2014, Wittstock and co-workers [
22] reported the development of a npAu composite (
npAu-ZnPc7) which demonstrated to have photocatalytic features; the synthesis was performed by coupling the Zn(II) complex of tetrakis(hex-5-ynyloxy)phthalocyanine (
ZnPc7) with the nanoporous gold material functionalized with azide units (
npAu-N3) (
Scheme 7). In the formation of the self-assembled monolayer (SAM) on the nanoporous gold material, the authors used 11-azidoundecane-1-thiol as the azide linker and octane-1-thiol as a shorter ‘spacer’ in order to avoid mutual steric hindrances between the active molecules. The cycloaddition reaction between both components was performed in a mixture of THF and water in the presence of Cu[TBTA]PF
6 and hydroquinone. The photocatalytic efficacy of the nanocomposite was evaluated in the oxidation of (
S)-(−)-citronellol. A conversion of 40% was reached while no reactivity was detected when the same photocatalyst was immobilized on the planar gold surface or when it was dissolved in the reaction solution. The authors have commented that this better performance is probably associated to the material nanoporosity and consequently to an increase in the surface area and also to the npAu electronic and optical properties namely the plasmonic effect.
In 2019, the same group used a similar SAM approach to immobilize
ZnPc7 on npAu functionalized with a short azide linker (azidohexylthiolate groups) aiming to improve the interaction between the ZnPc and the npAu surface [
23]. The catalytic efficacy of the resulting hybrid system was evaluated in the photooxidation of 1,3-diphenylisobenzofuran (DPBF), a well-known
1O
2 quencher, at different irradiation wavelength; the highest photocatalytic activity was observed at 700 nm. The hybrid showed higher photocatalytic activity than the separated components (
ZnPc7 in solution or the npAu). The synergism between the npAu surface plasmons and the ZnPc during the DPBF photooxidation was confirmed by the improvement of nearly an order of magnitude in the photooxidation activity only when both absorption sites were irradiated simultaneously.
2.5. Phthalocyanine-Carbohydrate Conjugates
Phthalocyanines (Pcs) are potential near infra-red photosensitizers to be used to treat bacterial infections and cancers. However, their clinical application is hindered by their poor solubility in biological fluids. In fact, the poor water solubility and aggregation of Pcs in that medium are typically high and recent research has been focused on the development of water-soluble phthalocyanines [
25]. In such way, carbohydrates are an attractive type of biomolecules that can be conjugated with phthalocyanines to improve their water solubility. Several examples based on the attachment of phthalocyanines to carbohydrates are known.
In 2014, Yilmaz and co-workers reported the synthesis of the glycosylated copper(II) phthalocyanine derivative
CuPc9. This compound was obtained from the CuAAC reaction of the alkynyl-substituted phthalocyanine
CuPc8 with 2-acetamido-2-deoxy-β-
D-glucopyranosyl azide
2 (
Scheme 9) [
26]. Phthalocyanine
CuPc8 was obtained by cyclotetramerization of dimethyl (3,4-dicyanophenyl)propargylmalonate, in pentan-1-ol and in the presence of DBU [
27]. The 1,3-dipolar cycloaddition took place in THF, at room temperature, in the presence of CuSO
4·5H
2O and sodium ascorbate. The photophysical characterization of
CuPc9 showed that the coordination of the phthalocyanine inner core with copper(II) was responsible for a drastic decrease in the photoluminescence intensity and in the quantum yield; the increase in the non-radiative processes and decreasing the fluorescence emission was justified by considering the energy transfer from the excited state of the ligand to the metal ion.
In the same year, Bächle and co-workers, aiming to obtain “third generation PSs” with adequate amphiphilicity and specific uptake by tumor cells due to sugar moieties, prepared the AB
3-saccharide zinc(II) phthalocyanines
ZnPc10b and
ZnPc11b using phthalonitrile and the glycoconjugated phthalonitriles
6 and
7; the latter ones were obtained from adequate alkyne-substituted phthalonitriles
4 and
5 and the protected 6-azido-glucopyranoside
3 (
Scheme 10) [
28]. The co-tetramerization of the glycoconjugated phthalonitrile precursors
6 and
7 with phthalonitrile in pentan-1-ol in the presence of zinc(II) chloride, afforded
ZnPc10a and
ZnPc11a, which after deprotection of the sugar units gave rise to
ZnPc10b and
ZnPc11b. The authors commented on the significance of the 2-methoxyethoxymethyl (MEM) group as the protecting group of the sugar units. That was due to the stability under harsh basic cyclotetramerization conditions and to the facile removal under mild acidic conditions. The conjugates exhibited well-defined UV-Vis spectra in DMSO (ε
max > 10
5 M
−1 cm
−1 and absorption maxima λ > 680 nm) but broadened and diminished Q-bands were observed in water and that was probably due to aggregation. Interestingly, the formation of
J-type aggregated forms seems to occur with
ZnPc10b since an unexpected bathochromic shifted in the Q band was observed.
Park and co-workers also reported the synthesis of the more hydrophilic A
3B-type azaphthalocyanine zinc(II) complexes
ZnPc14a and
ZnPc14b. These Pcs contain three bulky three-dimensional bornane groups and one or two β-
D-glucosyl or β-
D-galactosyl moieties [
29]. The authors have envisaged that the presence of the bornane groups could prevent the axial or peripheral approach between photosensitizers (PSs) without altering their optical properties. The coupling of the azaphthalocyanine complexes bearing one or two alkyne units (obtained from Z
nPc12a and
ZnPc12b after deprotection with TBAF) with the adequate azide carbohydrates was performed in the presence of CuI and PMDTA (
Scheme 11). The structures of the expected compounds were confirmed using adequate spectroscopic techniques and the photophysical studies confirmed that in DMSO all compounds were monomeric, but aggregation was detected in DMSO/water.
Liu and co-workers also used azide-substituted glucose derivatives to prepare the acetylated glucosyl phthalocyanines
ZnPc16a and
ZnPc16b and the glucosyl ones
ZnPc17a and
ZnPc17b using the propargylated phthalocyanine
ZnPc15 (
Scheme 12) [
30]. The cycloaddition reaction was done in both cases in the presence of CuSO
4·5H
2O and sodium ascorbate, at room temperature, affording the expected conjugates
ZnPc16 and
ZnPc17.
The biological studies showed that compounds ZnPc17a and ZnPc17b have a specific affinity to MCF-7 breast cancer cells when compared with HELF normal cells; furthermore, both compounds are localized in the lysosome and exhibit high photocytotoxicity towards MCF-7 cells.
A silicon(IV) phthalocyanine bearing two axial sugar units
SiPc20 was synthesized as illustrated in
Scheme 13. Using a base-catalyzed ligand exchange approach, the silicon(IV) phthalocyanine dichloride
SiPc18 in the presence of hex-5-yn-1-ol afforded the silicon dialkyne
SiPc19 (
Scheme 13) [
31]. The last step involved the CuAAC reaction of the dialkynyl
SiPc19 and azido-2,3,4,6-tetra-
O-acetyl-β-
D-glucopyranose (GlcN
3). This reaction was performed in DMF in the presence of copper(I) iodide and
N,
N-diisopropylethylamine (DIPEA) affording the
SiPc20 in 31% yield. Other sugar-azide derivatives, including those with galactosyl, xylosyl and mannosyl units, have also been used by the same group.
In 2019, Ziegler and co-workers reported the synthetic access to the four galactoconjugated zinc(II) phthalocyanines
ZnPc21–24 (
Figure 2) using phthalonitrile and the adequate galactophthalonitrile derivative or just the galactophthalonitrile derivatives [
32]. In the access to the galactophthalonitriles the approach was similar to the one previously reported by the same group but now using the 6-azido-1,2:3,4-di-
O-isopropylidene-α-
D-galactopyranose and the adequate bis-alkynyl phthalonitriles
6 and
7 (see
Scheme 10). These glycoconjugated zinc(II) phthalocyanines in DMSO showed Q bands in the red visible light region (679–737 nm) with molar absorption coefficients (ε
max) higher than 10
5 M
–1 cm
–1. From the spectroscopic behavior in solution it was concluded that the synthesized phthalocyanines form
J-aggregates.
Also in 2019, Uruma and co-workers reported the synthesis of the glucosylated phthalocyanine
ZnPc27 and its photodynamic action towards QR-32 and QRsP-11 cell lines (
Scheme 14) [
33]. The authors selected glucose functionalized with a propargyl unit and the zinc(II) phthalocyanine
ZnPc25 bearing an azido alkoxy group as reagents.
ZnPc26 has been obtained and after deprotection with sodium methoxide in methanol gave rise to
ZnPc27. In the photocytotoxicity assays,
ZnPc27 caused apoptosis in QR-32 and QRsP-11 cell lines of rodent; a stronger photocytotoxicity against QR-32 cells than to QRsP-11 cells has been observed.
2.6. CuAAC Reactions Involving Phthalocyanines and Polymers
As previously mentioned, the solubility of phthalocyanines in various solvents is critical for their application in different fields. In recent years the possibility to organize phthalocyanines by using polymeric systems appeared to be an effective approach.
Torres and co-workers described the immobilization of a Pc on a polymer by using the CuAAC reaction (
Scheme 15) [
34]. The authors used the helical rigid rod alkynyl-terminated polyisocyanide
L,L-PIAAPE and the azide phthalocyanine
ZnPc28; the reaction was performed in dichloromethane in the presence of copper(I) bromide and an excess of PMDETA at room temperature. The expected polymer
L,L-PIAAPE-ZnPc28 was obtained in 84% yield. From UV-Vis, fluorescence, and circular dichroism studies, it was confirmed that the phthalocyanine attachment did not affect the rod-like helical arrangement of the starting polymer.
Haddleton and co-workers combined the Mitsunobu reaction and the CuAAC approach to prepare the octasubstituted copper(II) phthalocyanines
CuPc30 with two different chain lengths of monomethyl ether polyethylene glycol (mPEG, 750–2000 Da) (
Scheme 16) [
35]. The alkyne-terminated phthalonitrile
9 was prepared from 4,5-bis(4-hydroxyphenoxy)phthalonitrile
8 using commercially available hex-5-yn-1-ol, triphenyl phosphine and diisopropyl azodicarboxylate (DIAD) according to the Mitsunobu conditions, giving rise to the “clickable” metal free
Pc29 after the cyclotetramerization step. Its conjugation with the two different azido monomethyl ether polyethylene glycol chains was performed in the presence of CuI and DIPEA affording
CuPc30.
The PEGylated copper phthalocyanine polymers obtained proved to be highly soluble in a range of organic solvents (e.g., chloroform, dichloromethane, THF) with no evidence of aggregation. In water, the presence of dimeric species was detected by UV-Vis spectra with a minor contribution of monomeric species. From the DSC studies it was observed that the PEGylated CuPc30 with the shorter chain (n = 16) exhibited a significant difference in thermal behavior when compared with commercial mPEGs and the authors have suggested that tunable thermal properties can be achieved with these systems by incorporating other mPEGs with different chain lengths.
In 2014, Şen and co-workers reported that the conjugation of the unsymmetrically substituted zinc(II) phthalocyanine
ZnPc31, with three
tert-butyl groups and a terminal alkynyl moiety with the azido-terminated polystyrene
PS-N3 and poly(
tert-butyl acrylate)
PtBA-N3 afforded the polymers
PS-ZnPc31 and
PtBA-ZnPc31 (
Scheme 17) [
36].
ZnPc31 was obtained through the statistical condensation of 4-
tert-butylphthalonitrile and 4-(pent-4-ynyloxy)phthalonitrile in the presence of zinc(II) acetate and the polymers were obtained using the atom transfer radical polymerization (ATRP) approach in the presence of PMDETA/CuBr, followed by the reaction of the obtained bromo end-functionalized polymers with NaN
3.
The electrochemical studies allowed to conclude that conjugation of the ZnPc31 to the polymers increased the chemical stability of the complex but decreased the electrochemical reversibility during redox reactions. However, the presence of polymer did not affect the spectroelectrochemical and electrocolorimetric responses of ZnPc31.
A double click reaction was used to incorporate phthalocyanine
ZnPc31 and 4-ethynyl-
N,
N-dimethylaniline in the azido-functional poly(methyl methacrylate-
co-2-(2-bromoisobutyryloxy)ethyl methacrylate) copolymer (PMMEM) (
Scheme 18) [
37]. The obtained copolymer
PMMEM-ZnPc31-EDMA was crosslinked in the presence of the liquid crystal mesogen 4’-(octyloxy)-4-biphenylcarbonitrile by ultraviolet irradiation using benzophenone as initiator and ethylene glycol dimethacrylate as difunctional crosslinker. The resulting polymer-dispersed liquid crystal film was characterized using differential scanning calorimetry and polarized optical microscopy.
Aimi and co-workers selected
ZnPc32 to be assembled to the azide-terminated poly(methyl methacrylate) (
N3-PMMA) and to the azide-terminated block copolymer (
N3-BCP) to afford the hybrid systems
PMMA-ZnPc33 and
BCP-ZnPc33 (
Scheme 19) with potential applications in optoelectronic devices [
38]. Phthalocyanine
ZnPc33 was obtained from the reaction of 6-chlorohex-1-yne with the hydroxyphthalocyanine
ZnPc32; this template resulted from the tetracyclization of 4,5-bis(dodecyloxy)phthalonitrile with 4-benzyloxyphthalonitrile in the presence of ZnCl
2, followed by cleavage of the benzyl group with H
2/Pd/C. The final CuAAC reaction was performed in the presence of CuI and DIPEA in THF. The resulting Pc-containing polymer films exhibited a cylindrical morphology in which the Pc units showed π-π interactions inside the confined PMMA cylinders. The authors commented that these results can be explored in the development of organic photovoltaic (OPV) devices and organic field effect transistors (OFETs).
McGrath and co-workers described the synthesis and the antimicrobial activity of the two dendritic water-soluble zinc(II) phthalocyanines
ZnPc34a,
b where the triethylene glycol (TEG) moieties where introduced by coupling the azide
10 bearing three TEG [
39] units with the adequate alkynyl-substituted zinc phthalocyanines in the presence of CuI and DIPEA (
Figure 3) [
40].
The aggregation studies performed with ZnPc34b have shown that it is significantly less aggregated in aqueous media than the peripherally substituted isomer ZnPc34a and its Q band is about 80 nm red-shifted when compared with that of ZnPc34a. Both ZnPcs showed no dark toxicity against bacteria and yeast, at 10 μM concentration, but upon irradiation (400–850 nm) both demonstrated to be phototoxic to Acinetobacter baumannii (99.9999% cell inactivation with ZnPc34b) and to Pseudomonas aeruginosa (90% cell inactivation).
The azide functional methoxypoly(ethyleneglycol) (
mPEG-N3) (PEG average M
n = 25700) was selected by Dincer and co-workers to prepare the symmetrical and asymmetrical PEGylated zinc(II) phthalocyanines
ZnPc35 and
ZnPc36 (
Figure 4) using the adequate tetra and mono terminal alkynyl substituted ZnPc [
41]. The coupling between both components was performed using CuBr and PMDETA. The results showed that the introduction of the four PEG units improved the photophysicochemical properties of
ZnPc35 when compared with those of its precursor and with
ZnPc36, namely in terms of water solubility, ability to generate
1O
2 and stability. As a result of such properties they can be considered as potential PDT candidates.
In 2017, Zapotoczny and co-workers reported the synthesis of a novel azide-functionalized silicon phthalocyanine
SiPc39 (
Scheme 20) [
42]. This Pc was then grafted with polymer brushes containing acetylene pendant groups via the CuAAC approach, using CuBr and PMDETA. The synthetic methodology to
SiPc39 was similar to the one mentioned in
Scheme 13, but it involved the base-catalyzed ligand exchange of the chlorine atoms in silicon(IV) phthalocyanine dichloride
SiPc37 with 3-chloropropyldimethylmethoxysilane and then reaction with NaN
3. The polymer brushes with acetylene side groups were obtained by surface-initiated photoiniferter-mediated polymerization. FTIR, quartz crystal microbalance, and atomic force microscopy were used to confirm the coupling of
SiPc39 with the brushes. The photophysical properties of the phthalocyanine
SiPc39 were not affected after the mentioned conjugation taking place, as evidenced by UV-Vis absorption and emission spectroscopy.
In 2017, Gül and co-workers selected the zinc phthalocyanine
ZnPc40 with three
tert-butylphenoxy groups and one 4-ethynylbenzyloxy moiety to be coupled to azido polystyrene PS-N
3 under CuAAC conditions (CuBr/PMDETA) (
Scheme 21) [
43]. The A
3B-type Pc
ZnPc40 was obtained through the condensation of 4-(4-ethynylbenzyloxy)phthalonitrile and 4-(4-
tert-butylphenoxy)phthalonitrile in 2-dimethylaminoethanol (DMAE) and the polymer was obtained from the azidation of the bromo-terminated polystyrene (PS-Br).
2.7. Phthalocyanines for the Development of New Electrodes
In the last decades, different methods and materials have been used to develop novel electrochemical sensors. According to the literature data, the modification of electrodes and their conjugation with metallophthalocyanines via the CuAAC approach did improve the stability of the sensors. In this section, it will be presented some examples of electrodes containing phthalocyanines able to recognize different analytes, such as metal ions (Hg(II), Pb(II), Cu(II)), hydrazine and hydrogen peroxide. In general, the approaches used Pcs with alkynyl units that were grafted into electrodes bearing the azido component (
Scheme 22).
In 2016, Nyokong and co-workers reported the immobilization of
MnPc41 bearing terminal alkynyl units (hex-5-ynyloxy) [
44] in glassy carbon electrodes (GCE) modified with the required azidobenzene component (
Scheme 22B) [
45]. The graft of the azido component in GCE was performed by electrodeposition of the azidobenzene diazonium salt (scanning from +0.5 V to −1 V for five cycles) (
Scheme 22A) and the click reaction was performed by immersing the grafted electrode into a solution of DMF containing
MnPc41, Cu(PPh
3)
3Br and triethylamine. The authors observed that the novel electrode was an efficient and robust sensor for hydrazine, showing a sensitivity of 27.38 µA mM
−1 and a LOD of 15.4 × 10
−12 mol dm
−3.
Soon afterwards, the same group extended the study to electrodes prepared with the same Pc but coordinated with Co(II) and Ni(II) ions (
Scheme 22) and in the case of hydrazine they found LOD values of 6.09 µM and 8.69 µM for
CoPc41-GCE and
NiPc41-GCE respectively, while the sensitivity was 51.32 µA mM
−1 and 111.2 µA mM
−1 [
46].
In the same year, the group selected
Pc42 with (propargyloxy)phenoxy units and its Co(II) and Mn(III) complexes to anchor in gold electrodes functionalized with the phenylazide component for the electrocatalytic detection of hydrogen peroxide (
Figure 5) [
47]. From the electrocatalytic studies a sensitivity value of 0.134 µA mM
−1 cm
−2 was obtained for the electrode clicked with
MnPc42 and 0.242 µA mM
−1cm
−2 for the electrode clicked with
CoPc42.
In another contribution, the authors reported the immobilization of
CoPc43 with 4-ethynylbenzyloxy units (
Figure 5) in glassy carbon electrodes modified with azide molecules and found that the platform is a good probe for hydrazine, with good catalytic rate constant (8.45 × 10
3 M
−1 s
−1) and low LOD (3.28 μM) [
48].
The same group also reported that the asymmetrical alkyne cobalt(II) phthalocyanine
CoPc44 after being immobilized in glassy carbon electrode (GCE) modified with the azido component, showed favorable sensitivity and lower LOD for Hg(II), Cu(II), Pb(II), and Cd(II) ions [
49]. Detection limits of 81.94, 327.71, 55.87 and 347.06 nM and sensitivity of 866.23 ± 5.48, 215.82 ± 2.16, 1979.48 ± 11.47 and 204.50 ± 1.10 μA/mM were found for Hg(II), Cu(II), Pb(II), and Cd(II), respectively.
2.8. Click Chemistry as a Tool for New Architectures Based on Phthalocyanines
Nyokong and co-workers have studied the non-linear optical (NLO) properties of
Pcs combining the effects of NO
2 and triazole linkers in their peripheral positions [
50]. The synthetic access to the 4-nitrophenyl-1,2,3-triazole Pc
ZnPc45 involved the cyclotetramerization of the clickable phthalonitrile
13 which was obtained from 4-nitrophenylazide (
11) and 4-(prop-2-ynyloxy)phthalonitrile (
12) (
Scheme 23). The click reaction was carried out in THF/H
2O using CuSO
4·5H
2O as a catalyst and sodium ascorbate as the reducing agent; the cyclotetramerization of the phthalonitrile was performed in the presence of zinc acetate and DMAE. The NLO studies showed that
ZnPc45 presented better optical limiting behavior than the tetra-substituted alkynyl zinc(II) phthalocyanine selected as a potential precursor of
ZnPc45; an improvement in triplet quantum yields and lifetimes was also observed.
Campidelli and co-workers explored the CuAAC approach to obtain the fullerene-phthalocyanine
C60-(ZnPc46)2 and fullerene-porphyrin-phthalocyanine triads
C60-ZnP-ZnPc46 (
Figure 6) and evaluated their self-assembly properties [
51,
52]. The azide dendron
(ZnPc46)2-N3 was also obtained using the CuAAC approach and was coupled to the alkynyl fullerene
14 after its deprotection with tetrabutylammonium fluoride with Cu(MeCN)
4PF
6 and 2,6-lutidine (
Scheme 24). The study showed that only weak interactions were detected for this system and the electrochemical assays suggested a better interaction of the C
60 moiety with the ZnP-ZnPc dendron. Additionally, the AFM and SEM analysis showed that these conjugates, when deposited on Si/SiO
2 surfaces, were organized in nanofibrils of ca. 4 to 8 nm of diameter.
Gürol and co-workers prepared the asymmetric zinc(II) phthalocyanines
ZnPc47a,
b with six hexylthia chains on peripheral positions and one 6-azido-hexylthia chain either on peripheral (a) or non-peripheral (b) positions to be assembled to the alkyne-functionalized triphenylene core
15 in the presence of CuI (
Figure 7) [
53]. The obtained triazole derivatives
ZnPc48a,
b were tested to detect volatile organic compounds after being deposited on the surface of acoustic wave transducers via the electrospraying method. The sensorial response for acetone, ethanol, hexane, isoprene, chloroform and toluene, which can be used as biomarkers of lung cancer, was determined. Compound
ZnPc48a showed a higher sensitivity for toluene and ethanol vapors. The obtained results confirmed the possibility to use Pc-based surface acoustic wave sensors for medical diagnosis.
Recently, Makhseed and co-workers described the synthesis of the zinc(
II) phthalocyanine
ZnPc49a and of its azaphthalocyanine analogue
ZnPc49b with multivalent propargyl moieties as new building blocks that prevent the macrocyclic planar cores from self-associating in solution or in the solid state [
54]. The authors investigated the utility of these platforms in the CuAAC reaction with benzyl azide, using CuI and DIPEA in refluxing chloroform (
Scheme 25). The photophysical characterization of the adducts
ZnPc50 in DMF allowed to conclude that they were exclusively in the monomeric form and the photophysical parameters were not affected by the formation of aggregates.