In Situ Raman Investigation of TiO2 Nanotube Array-Based Ultraviolet Photodetectors: Effects of Nanotube Length
Abstract
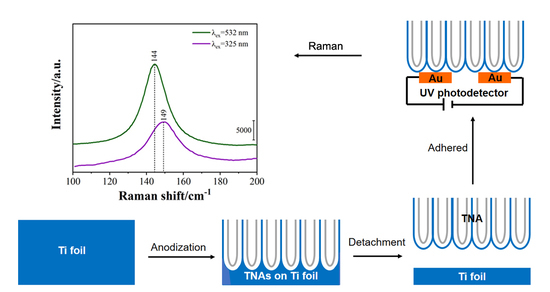
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. Chemicals
3.2. Instruments
3.3. Preparation of TiO2 Nanotube Arrays
3.4. Preparation of TiO2 Sol
3.5. Preparation of the TiO2 Nanotube Array-Based Ultraviolet Photodetector
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gong, D.; Grimes, C.A.; Varghese, O.K.; Hu, W.; Singh, R.S.; Chen, Z.; Dickey, E. Titanium oxide nanotube arrays prepared by anodic oxidation. J. Mater. Res. 2001, 16, 3331–3334. [Google Scholar] [CrossRef] [Green Version]
- Varghese, O.K.; Gong, D.; Paulose, M.; Ong, K.G.; Grimes, C. Hydrogen sensing using titania nanotubes. Sens. Actuators B Chem. 2003, 93, 338–344. [Google Scholar] [CrossRef]
- Nakagawa, H.; Yamamoto, N.; Okazaki, S.; Chinzei, T.; Asakura, S. A room-temperature operated hydrogen leak sensor. Sens. Actuators B Chem. 2003, 93, 468–474. [Google Scholar] [CrossRef]
- Grimes, C.A. Synthesis and application of highly ordered arrays of TiO2 nanotubes. J. Mater. Chem. 2007, 17, 1451–1457. [Google Scholar] [CrossRef]
- Allam, N.K.; Grimes, C.A. Effect of cathode material on the morphology and photoelectrochemical properties of vertically oriented TiO2 nanotube arrays. Sol. Energy Mater. Sol. Cells 2008, 92, 1468–1475. [Google Scholar] [CrossRef]
- Yang, L.; Yang, W.; Cai, Q. Well-dispersed Pt Au nanoparticles loaded into anodic titania nanotubes: A high antipoison and stable catalyst system for methanol oxidation in alkaline media. J. Phys. Chem. C 2007, 111, 16613–16617. [Google Scholar] [CrossRef]
- Grimes, C.A.; Ong, K.G.; Varghese, O.K.; Yang, X.; Mor, G.; Paulose, M.; Dickey, E.; Ruan, C.; Pishko, M.V.; Kendig, J.W.; et al. A Sentinel Sensor Network for Hydrogen Sensing. Sensors 2003, 3, 69–82. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Fu, W.; Yang, H.; Qi, Q.; Zeng, Y.; Zhang, T.; Ge, R.; Zou, G. Synthesis and characterization of TiO2 nanotubes for humidity sensing. Appl. Surf. Sci. 2008, 254, 5545–5547. [Google Scholar] [CrossRef]
- Hong, S.P.; Kim, S.; Kim, N.; Yoon, J.; Kim, C. A short review on electrochemically self-doped TiO2 nanotube arrays: Synthesis and applications. Korean J. Chem. Eng. 2019, 36, 1753–1766. [Google Scholar] [CrossRef]
- Zhou, X.; Liu, N.; Schmuki, P. Photocatalysis with TiO2 nanotubes:“colorful” reactivity and designing site-specific photocatalytic centers into TiO2 nanotubes. ACS Catal. 2017, 7, 3210–3235. [Google Scholar] [CrossRef] [Green Version]
- Fan, H.; Zhang, S.; Zhu, X. Nitrided TiO2 nanoparticles/nanotube arrays for better electrochemical properties. Chem. Phys. Lett. 2019, 730, 340–344. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, Y.; Guo, F.; Liu, J.; Yang, F. Kinetic analysis of the anodic growth of TiO2 nanotubes: Effects of voltage and temperature. J. Mater. Chem. C 2019, 7, 14098–14108. [Google Scholar] [CrossRef]
- Zheng, Z.; Zhuge, F.; Wang, Y.; Zhang, J.; Gan, L.; Zhou, X.; Li, H.; Zhai, T. Decorating Perovskite Quantum Dots in TiO2 Nanotubes Array for Broadband Response Photodetector. Adv. Funct. Mater. 2017, 27, 1703115. [Google Scholar] [CrossRef]
- Motola, M.; Čaplovičová, M.; Krbal, M.; Sopha, H.; Thirunavukkarasu, G.K.; Gregor, M.; Plesch, G.; Macak, J.M. Ti3+ doped anodic single-wall TiO2 nanotubes as highly efficient photocatalyst. Electrochim. Acta 2020, 331, 135374. [Google Scholar] [CrossRef]
- Marien, C.B.D.; Cottineau, T.; Robert, D.; Drogui, P. TiO2 Nanotube arrays: Influence of tube length on the photocatalytic degradation of Paraquat. Appl. Catal. B Environ. 2016, 194, 1–6. [Google Scholar] [CrossRef]
- Ouyang, W.; Teng, F.; Fang, X. High Performance BiOCl Nanosheets/TiO2 Nanotube Arrays Heterojunction UV Photodetector: The Influences of Self-Induced Inner Electric Fields in the BiOCl Nanosheets. Adv. Funct. Mater. 2018, 28, 1707178. [Google Scholar] [CrossRef]
- Ge, M.Z.; Cao, C.Y.; Huang, J.Y.; Li, S.; Zhang, S.-N.; Deng, S.; Li, Q.; Zhang, K.-Q.; Lai, Y. Synthesis, modification, and photo/photoelectrocatalytic degradation applications of TiO2 nanotube arrays: A review. Nanotechnol. Rev. 2016, 5, 75–112. [Google Scholar] [CrossRef]
- Zheng, L.; Hu, K.; Teng, F.; Fang, X. Novel UV-Visible Photodetector in Photovoltaic Mode with Fast Response and Ultrahigh Photosensitivity Employing Se/TiO2 Nanotubes Heterojunction. Small 2016, 13, 1602448. [Google Scholar] [CrossRef]
- Chen, D.; Wei, L.; Meng, L.; Wang, D.; Chen, Y.; Tian, Y.-F.; Yan, S.; Mei, L.; Jiao, J. High-Performance Self-Powered UV Detector Based on SnO2-TiO2 Nanomace Arrays. Nanoscale Res. Lett. 2018, 13, 92. [Google Scholar] [CrossRef] [Green Version]
- Hosseini, Z.S.; Shasti, M.; Sani, S.R.; MortezaAli, A. Photo-detector diode based on thermally oxidized TiO2 nanostructures/p-Si heterojunction. J. Appl. Phys. 2016, 119, 14503. [Google Scholar] [CrossRef]
- Li, S.; Deng, T.; Zhang, Y.; Li, Y.; Yin, W.; Chen, Q.; Liu, Z. Solar-blind ultraviolet detection based on TiO2 nanoparticles decorated graphene field-effect transistors. Nanophotonics 2019, 8, 899–908. [Google Scholar] [CrossRef]
- Zou, J.; Zhang, Q.; Huang, K.; Marzari, N. Ultraviolet Photodetectors Based on Anodic TiO2 Nanotube Arrays. J. Phys. Chem. C 2010, 114, 10725–10729. [Google Scholar] [CrossRef]
- Jahromi, H.D.; Sheikhi, M.H.; Yousefi, M.H. A numerical approach for analyzing quantum dot infrared photodetectors’ parameters. Opt. Laser Technol. 2012, 44, 572–577. [Google Scholar] [CrossRef]
- Gödel, K.C.; Steiner, U. Thin film synthesis of SbSI micro-crystals for self-powered photodetectors with rapid time response. Nanoscale 2016, 8, 15920–15925. [Google Scholar] [CrossRef] [PubMed]
- Soci, C.; Zhang, A.; Bao, X.-Y.; Kin, H.; Lo, Y.; Wang, D. Nanowire Photodetectors. J. Nanosci. Nanotechnol. 2010, 10, 1430–1449. [Google Scholar] [CrossRef] [Green Version]
- Raman, C.V.; Krishnan, K.S. A new type of secondary radiation. Nature 1928, 121, 501–502. [Google Scholar] [CrossRef]
- Baddour-Hadjean, R.; Pereira-Ramos, J.P. Raman Microspectrometry Applied to the Study of Electrode Materials for Lithium Batteries. Chem. Rev. 2010, 110, 1278–1319. [Google Scholar] [CrossRef]
- Ma, L.; Zhang, T.; Song, R.; Guo, L. In-situ Raman study of relation between microstructure and photoactivity of CdS@TiO2 core-shell nanostructures. Int. J. Hydrogen Energy 2018, 43, 13778–13787. [Google Scholar] [CrossRef]
- Dong, Z.; Xiao, F.; Zhao, A.; Liu, L.; Sham, T.-K.; Song, Y. Pressure induced structural transformations of anatase TiO2 nanotubes probed by Raman spectroscopy and synchrotron X-ray diffraction. RSC Adv. 2016, 6, 76142–76150. [Google Scholar] [CrossRef]
- Butler, H.J.; Ashton, L.; Bird, B.; Cinque, G.; Curtis, K.; Dorney, J.; Esmonde-White, K.; Fullwood, N.J.; Gardner, B.; Martin-Hirsch, P.L.; et al. Using Raman spectroscopy to characterize biological materials. Nat. Protoc. 2016, 11, 664–687. [Google Scholar] [CrossRef] [Green Version]
- Shipp, D.W.; Sinjab, F.; Notingher, I. Raman spectroscopy: Techniques and applications in the life sciences. Adv. Opt. Photon. 2017, 9, 315–428. [Google Scholar] [CrossRef] [Green Version]
- Panneerselvam, R.; Liu, G.K.; Wang, Y.H.; Liu, J.-Y.; Ding, S.-Y.; Li, J.-F.; Wu, D.-Y.; Tian, Z. Surface-enhanced Raman spectroscopy: Bottlenecks and future directions. Chem. Commun. 2018, 54, 10–25. [Google Scholar] [CrossRef]
- Hardwick, L.J.; Holzapfel, M.; Novák, P.; Dupont, L.; Baudrin, E. Electrochemical lithium insertion into anatase-type TiO2: An in situ Raman microscopy investigation. Electrochim. Acta 2007, 52, 5357–5367. [Google Scholar] [CrossRef]
- Zhong, D.; Jiang, Q.; Huang, B.; Zhang, W.-H.; Li, C. Synthesis and characterization of anatase TiO2 nanosheet arrays on FTO substrate. J. Energy Chem. 2015, 24, 626–631. [Google Scholar] [CrossRef]
- Yao, F.U.; Wang, H.C. Preparation of transparent TiO2 nanocrystalline film for UV sensor. Chin. Sci. Bull. 2006, 51, 1657–1661. [Google Scholar]
- Mazza, T.; Barborini, E.; Piseri, P.; Milani, P.; Cattaneo, D.; Bassi, A.L.; Bottani, C.E.; Ducati, C. Raman spectroscopy characterization of TiO2 rutile nanocrystals. Phys. Rev. B Condens. Matter. 2007, 75, 045416-1–045416-5. [Google Scholar] [CrossRef]
- Campion, A.; Kambhampati, P. Surface-enhanced Raman scattering. Chem. Soc. Rev. 1998, 27, 241–250. [Google Scholar] [CrossRef]
- Li, H.Y.; Wang, J.S.; Huang, K.L.; Sun, G.S.; Zhou, M.L. In situ preparation of multi-layer TiO2 nanotube array thin films by anodic oxidation metho. Mater. Lett. 2011, 65, 1188–1190. [Google Scholar] [CrossRef]
- Liang, F.; Zhang, J.; Zheng, L.; Tsang, C.-K.; Li, H.; Shu, S.; Cheng, H.; Li, Y.Y. Selective electrodeposition of Ni into the intertubular voids of anodic TiO2 nanotubes for improved photocatalytic properties. J. Mater. Res. 2012, 28, 405–410. [Google Scholar] [CrossRef]
- Li, Y.; Ma, Q.; Han, J.; Ji, L.; Wang, J.; Chen, J.; Wang, Y. Controllable preparation, growth mechanism and the properties research of TiO2 nanotube arrays. Appl. Surf. Sci. 2014, 297, 103–108. [Google Scholar] [CrossRef]
- Yang, L.; Jiang, X.; Ruan, W.; Zhao, B.; Xu, W.; Lombardi, J.R. Observation of Enhanced Raman Scattering for Molecules Adsorbed on TiO2 Nanoparticles: Charge-Transfer Contribution. J. Phys. Chem. C 2008, 112, 20095–20098. [Google Scholar] [CrossRef]
- Zhang, X.; Yu, Z.; Ji, W.; Sui, H.; Cong, Q.; Wang, X.; Zhao, B. Charge-Transfer Effect on Surface-Enhanced Raman Scattering (SERS) in an Ordered Ag NPs/4-Mercaptobenzoic Acid/TiO2 System. J. Phys. Chem. C 2015, 119, 22439–22444. [Google Scholar] [CrossRef]
- Ho, C.H.; Lee, S. SERS and DFT investigation of the adsorption behavior of 4-mercaptobenzoic acid on silver colloids. Colloids Surf. A Physicochem. Eng. Asp. 2015, 474, 29–35. [Google Scholar] [CrossRef]
Sample Availability: Not available. |







| Raman Band | Assignment |
|---|---|
| 144 cm−1 | Eg(1) (Anatase TiO2) |
| 199 cm−1 | Eg(2) (Anatase TiO2) |
| 395 cm−1 | B1g(1) (Anatase TiO2) |
| 514 cm−1 | A1g/B1g(2) (Anatase TiO2) |
| 635 cm−1 | Eg(3) (Anatase TiO2) |
| 1073 cm−1 | in-plane ring breathing + C−S stretching (4-MBA) |
| 1148 cm−1 | C−H deformation (4-MBA) |
| 1176 cm−1 | C−H deformation (4-MBA) |
| 1407 cm−1 | COO- stretching (4-MBA) |
| 1592 cm−1 | totally symmetric C−C stretching (4-MBA) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, Y.; Shi, X.; Xia, P.; Li, S.; Lv, M.; Wang, Y.; Mao, Z. In Situ Raman Investigation of TiO2 Nanotube Array-Based Ultraviolet Photodetectors: Effects of Nanotube Length. Molecules 2020, 25, 1854. https://doi.org/10.3390/molecules25081854
Ren Y, Shi X, Xia P, Li S, Lv M, Wang Y, Mao Z. In Situ Raman Investigation of TiO2 Nanotube Array-Based Ultraviolet Photodetectors: Effects of Nanotube Length. Molecules. 2020; 25(8):1854. https://doi.org/10.3390/molecules25081854
Chicago/Turabian StyleRen, Yanyu, Xiumin Shi, Pengcheng Xia, Shuang Li, Mingyang Lv, Yunxin Wang, and Zhu Mao. 2020. "In Situ Raman Investigation of TiO2 Nanotube Array-Based Ultraviolet Photodetectors: Effects of Nanotube Length" Molecules 25, no. 8: 1854. https://doi.org/10.3390/molecules25081854