Mustard Seed (Brassica nigra) Extract Exhibits Antiproliferative Effect against Human Lung Cancer Cells through Differential Regulation of Apoptosis, Cell Cycle, Migration, and Invasion
Abstract
:1. Introduction
2. Results
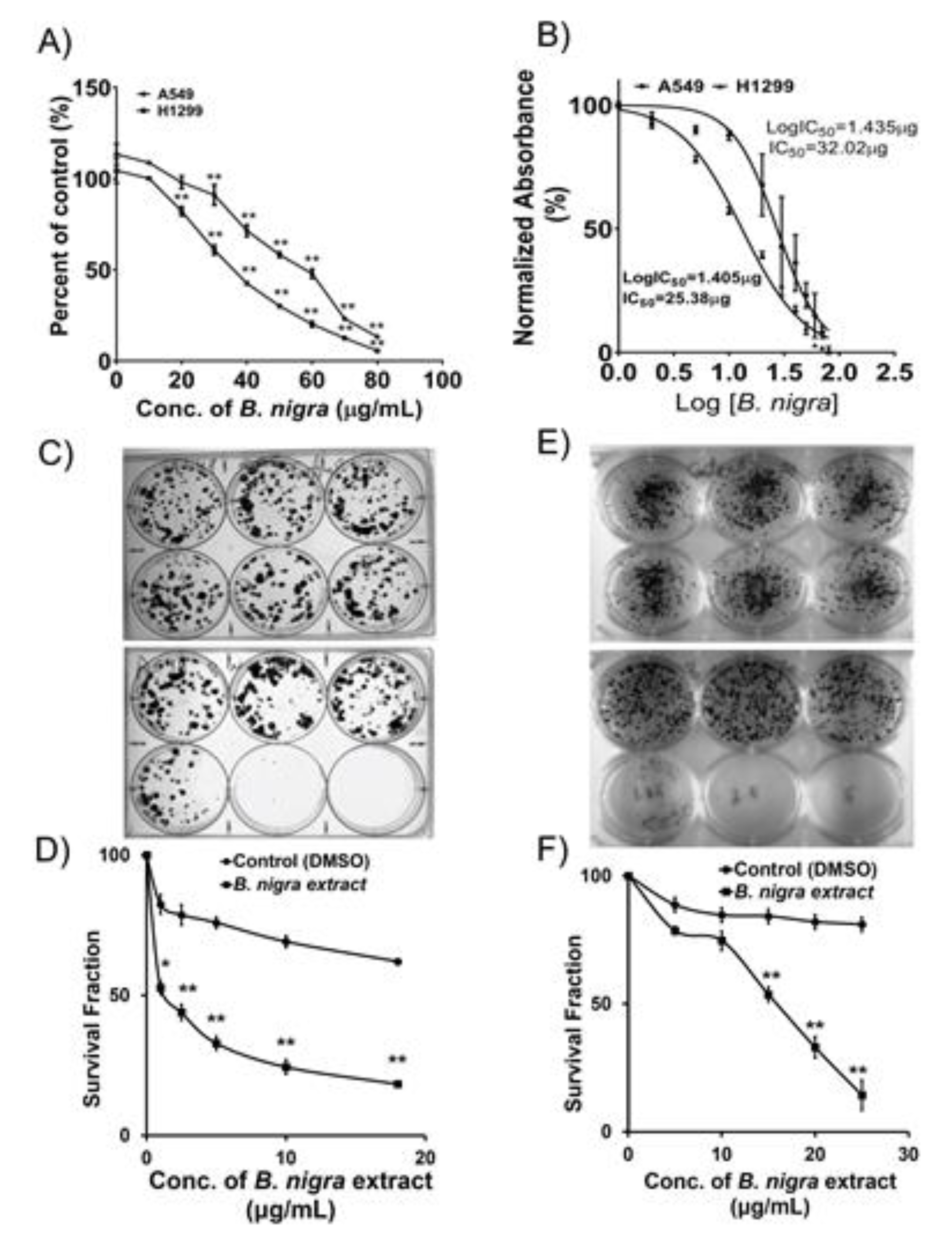
2.1. B. nigra Extract Exhibited Antiproliferative Activities against A549 and H1299 Cells
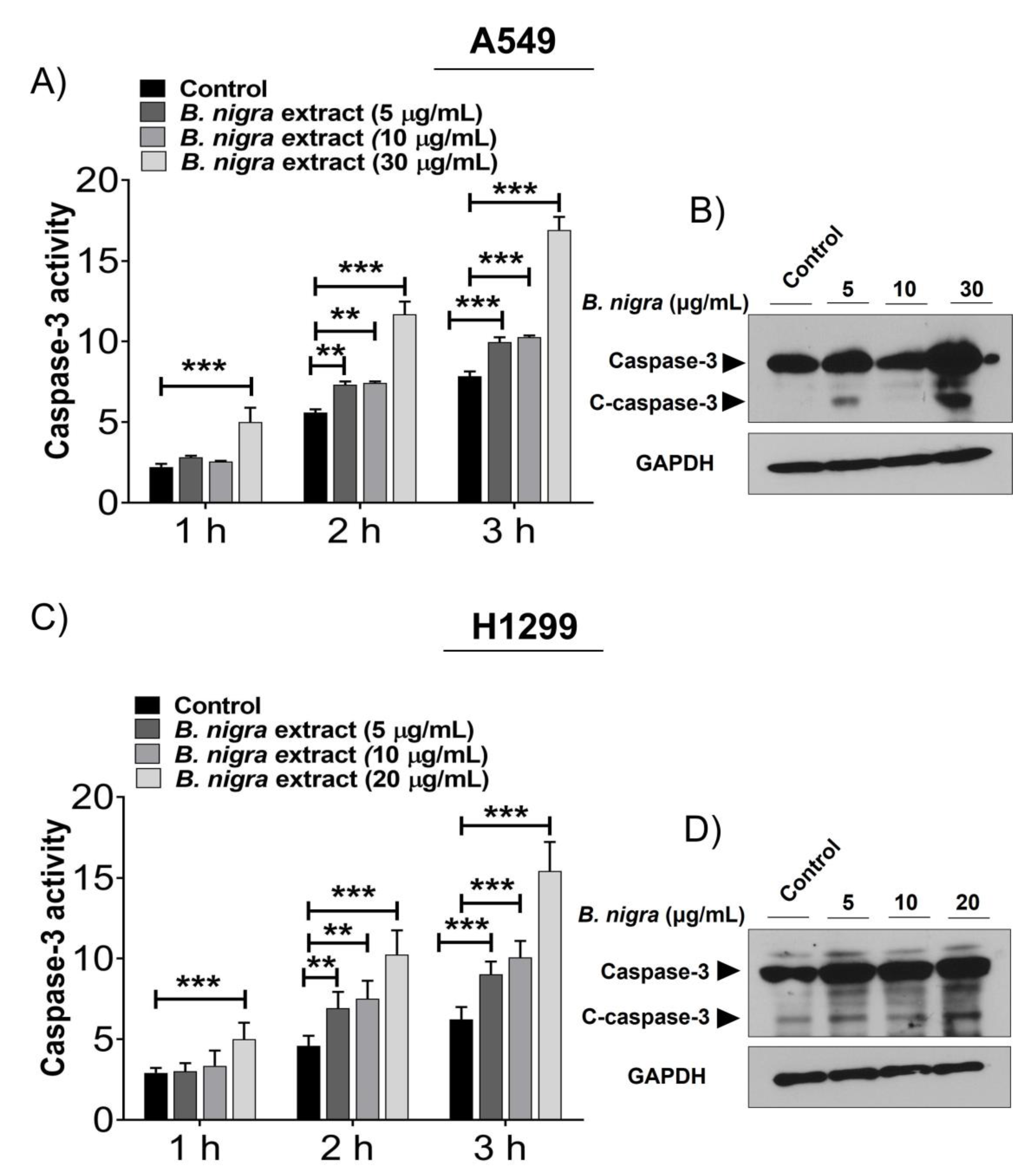
2.2. B. nigra Extract Induced Apoptosis through Promotion of Caspase-3 Activity.
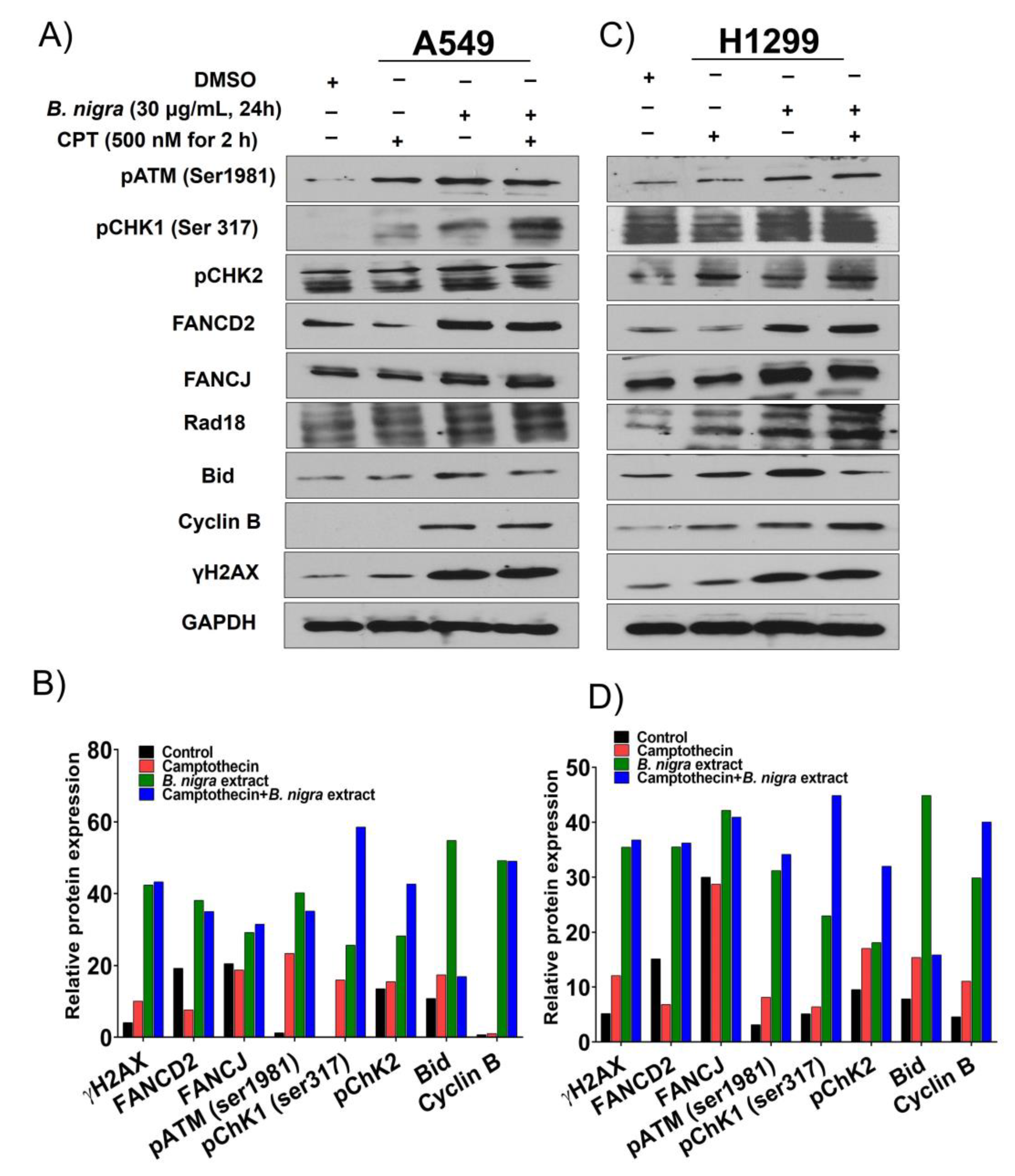
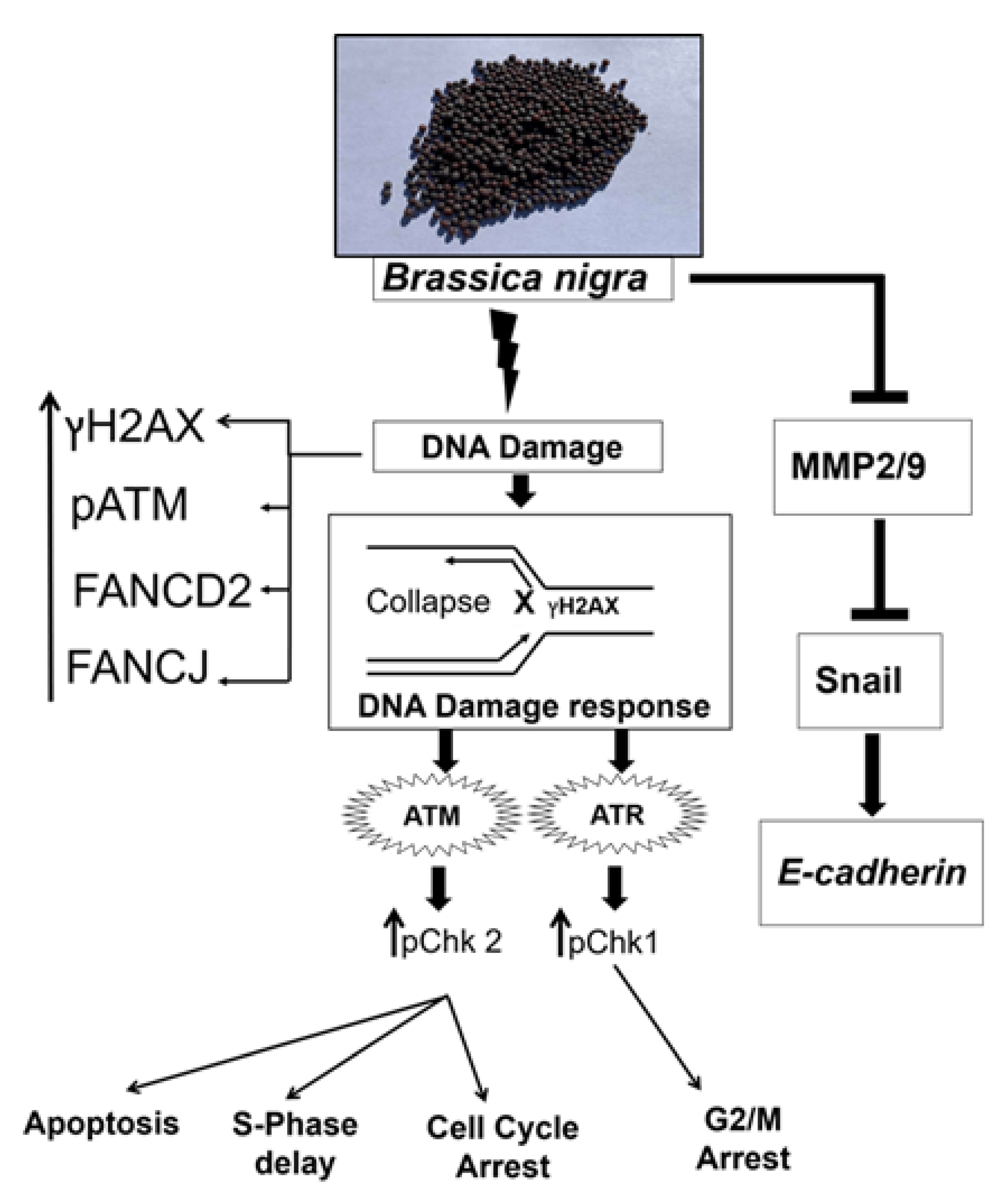
2.3. B. nigra Extract Induced Replication-Associated DNA Damage
2.4. B. nigra Extract Altered Cell Cycle Distribution
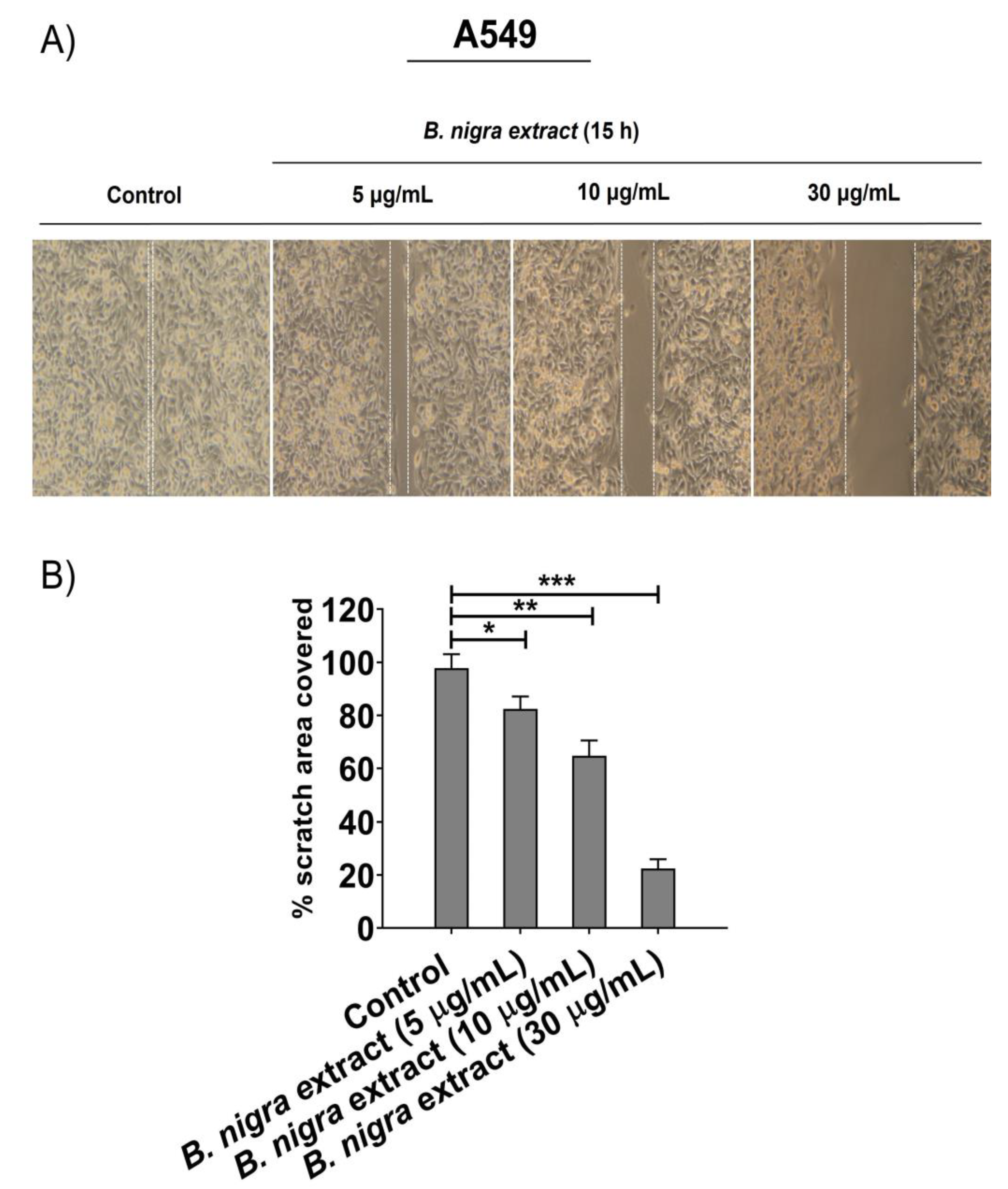
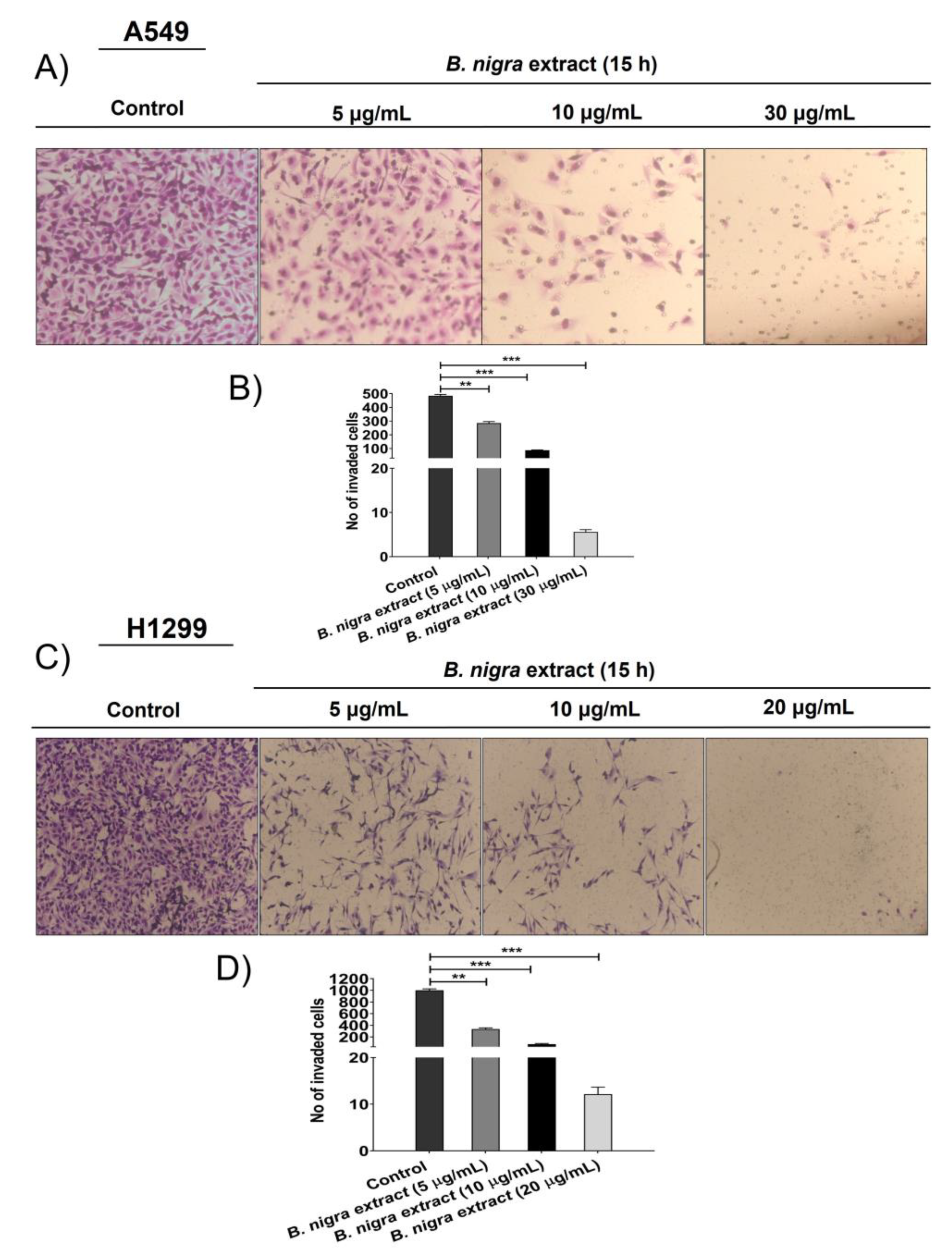
2.5. B. nigra extract Inhibited Migration and Invasion of A549 cells
2.6. B. nigra Altered the Expression of Epithelial-to-Mesenchymal Transition (EMT)-Associated Genes
3. Discussion
4. Materials and Methods
4.1. Cell Lines and Antibodies
4.2. Collection and Authentication of Plant Material
4.3. Preparation of B. nigra Ethanolic Extract
4.4. Cell Viability and Cytotoxicity Assay
4.5. Clonogenic Survival Assays
4.6. Apoptosis Assay
4.7. Western Blot
4.8. Cell Cycle Analysis
4.9. Migration Wound Healing Assay
4.10. Transwell Invasion Assay
4.11. In Situ Gelatinase Zymography
4.12. Gene Expression Studies
4.13. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Global Burden of Disease Cancer Collaboration; Fitzmaurice, C.; Abate, D.; Abbasi, N.; Abbastabar, H.; Abd-Allah, F.; Abdel-Rahman, O.; Abdelalim, A.; Abdoli, A.; Abdollahpour, I.; et al. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life-Years for 29 Cancer Groups, 1990 to 2017. JAMA Oncol. 2019, 5, 1749. [Google Scholar] [CrossRef] [Green Version]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2018. CA Cancer J. Clin. 2018, 68, 7–30. [Google Scholar] [CrossRef]
- Inamura, K. Lung Cancer: Understanding Its Molecular Pathology and the 2015 WHO Classification. Front. Oncol. 2017, 7, 193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spiro, S.G.; Silvestri, G.A. One hundred years of lung cancer. Am. J. Respir. Crit. Care Med. 2005, 172, 523–529. [Google Scholar] [CrossRef] [PubMed]
- Jemal, A.; Ma, J.; Rosenberg, P.S.; Siegel, R.; Anderson, W.F. Increasing lung cancer death rates among young women in southern and midwestern states. J. Clin. Oncol. 2012, 30, 2739–2744. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cardenal, F.; Nadal, E.; Jové, M.; Faivre-Finn, C. Concurrent systemic therapy with radiotherapy for the treatment of poor-risk patients with unresectable stage III non-small-cell lung cancer: A review of the literature. Ann. Oncol. 2014, 2, 278–288. [Google Scholar] [CrossRef]
- Kong, F.-M.S.; Zhao, J.; Wang, J.; Faivre-Finn, C. Radiation dose effect in locally advanced non-small cell lung cancer. J. Thorac. Dis. 2014, 6, 336–347. [Google Scholar]
- Das, A.K.; Bell, M.H.; Nirodi, C.S.; Story, M.D.; Minna, J.D. Radiogenomics predicting tumor responses to radiotherapy in lung cancer. Semin. Radiat. Oncol. 2010, 20, 149–155. [Google Scholar] [CrossRef] [Green Version]
- Thomson, C.A.; Dickinson, S.; Bowden, T. Cruciferous vegetables, isothiocyanates, indoles, and cancer prevention. In Nutrition and Health: Bioactive Compounds and Cancer; Milner, J.A., Romagnolo, D.F., Eds.; Springer Science+Business Media/Humana Press: Totowa, NJ, USA, 2010; pp. 535–566. [Google Scholar] [CrossRef]
- Beaver, L.M.; Lӧhr, C.V.; Clarke, J.D.; Glasser, S.T.; Watson, G.W.; Wong, C.P.; Zhang, Z.; Williams, D.E.; Dashwood, R.H.; Shannon, J.; et al. Broccoli Sprouts Delay Prostate Cancer Formation and Decrease Prostate Cancer Severity with a Concurrent Decrease in HDAC3 Protein Expression in Transgenic Adenocarcinoma of the Mouse Prostate (TRAMP) Mice. Curr. Dev. Nutr. 2017, 2, nzy002. [Google Scholar] [CrossRef]
- Clarkea, J.D.; Dashwood, R.H.; Ho, E. Multi-targeted prevention of cancer by sulforaphane. Cancer Lett. 2008, 269, 291–304. [Google Scholar] [CrossRef] [Green Version]
- Jakubikova, J.; Cervi, D.; Ooi, M.; Kim, K.; Nahar, S.; Klippel, S.; Cholujova, D.; Leiba, M.; Daley, J.F.; Delmore, J.; et al. Anti-tumor activity and signaling events triggered by the isothiocyanates, sulforaphane and phenethyl isothiocyanate, in multiple myeloma. Haematol 2011, 96, 1170–1179. [Google Scholar] [CrossRef] [PubMed]
- Foster, S.; Duke, J.A. A Field Guide to Medicinal Plants: Eastern and Central North America, 2nd ed.; Houghton Miffin Co.: Boston, MA, USA, 1990; p. 384. ISBN 0395467225. [Google Scholar]
- Ghani, A. Medicinal Plants of Bangladesh: Chemical Constituents & Uses, 2nd ed.; Asiatic Society of Bangladesh: Dhaka, Bangladesh, 2003; pp. 1–16. [Google Scholar]
- Evans, W.C. Trease and Evans Pharmacognosy, 15th ed.; Elsevier: New Delhi, India, 2007; p. 330. [Google Scholar]
- Kirtikar, K.R.; Basu, B.D. Indian Medicinal Plants, 2nd ed.; International Book Distributors: Dehradun, India, 2005; pp. 168–170. [Google Scholar]
- Kokate, C.K.; Purohit, A.P.; Gokhale, S.B. Pharmacognosy, 39th ed.; Nirali Prakashan: Pune, India, 2007; pp. 229–230, 293–294. [Google Scholar]
- Higdon, J.V.; Delage, B.; Williams, D.E.; Dashwood, R.H. Cruciferous vegetable and human cancer risk: Epidemiologic evidence and mechanistic basis. Pharmacol. Res. 2007, 55, 224–236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fowke, J.H. Head and neck cancer: A case for inhibition by isothiocyanates and indols from Cruciferous vegetables. Eur. J. Cancer Prev. 2007, 16, 348–356. [Google Scholar] [CrossRef] [PubMed]
- Juge, N.; Mithen, R.F.; Traka, M. Molecular basis for chemoprevention by sulforaphane: A comprehensive review. Cell. Mol. Life Sci. 2007, 64, 1105–1127. [Google Scholar] [CrossRef]
- Zhao, H.; Lin, J.; Grossman, H.B.; Hermandes, L.M.; Dinney, C.P.; Wu, X. Dietary isothiocyanates GSTM 1, GSTT 1, NAT 2 polymorphism and bladder cancer risk. Int. J. Cancer 2007, 120, 2208–2213. [Google Scholar] [CrossRef] [PubMed]
- Kamel, E.M.; Ahmed, S.A. Chemical constituents, Cytotoxic and Antibcterial activities of the aerial parts of Brassica nigra. Int. J. Bioassays 2013, 2, 1134–1138. [Google Scholar]
- Uhl, M.; Laky, B.; Lhoste, E.; Kassie, F.; Kundi, M.; Knasmuller, S. Effects of Mustard Sprouts and Allylisothiocyanate on benzo(a)pyrene-induced DNA damage in human derived cells: A Model Study with the Single Cell Gel Electrophoresis/Hep G2 Assay. Teratog. Carcinog. Mutagen. 2003, 1, S273–S282. [Google Scholar] [CrossRef]
- Tripathi, K.; Hussein, U.K.; Anupalli, R.; Barnett, R.; Bachaboina, L.; Scalici, J.; Rocconi, R.P.; Owen, L.B.; Piazza, G.A.; Palle, K. Allyl isothiocyanate induces replication-associated DNA damage response in NSCLC cells and sensitizes to ionizing radiation. Oncotarget 2015, 6, 5237–5252. [Google Scholar] [CrossRef]
- Bhattacharya, A.; Li, Y.; Wade, K.L.; Paonessa, J.D.; Fahey, J.W.; Zhang, Y. Allyl isothiocyanate-rich mustard seed powder inhibits bladder cancer growth and muscle invasion. Carcinogenesis 2010, 12, 2105–2110. [Google Scholar] [CrossRef]
- Sávioa, A.L.V.; Silvab, G.N.; Salvadori, D.M.F. Inhibition of bladder cancer cell proliferation by allyl isothiocyanate (mustard essential oil). Mutat. Res. 2015, 771, 29–35. [Google Scholar] [CrossRef] [Green Version]
- Yuan, H.; Zhu, M.; Guo, W.; Jin, L.; Chen, W.; Brunk, U.T.; Zhao, M. Mustard seeds (Sinapis Alba Linn) attenuate azoxymethane-induced colon carcinogenesis. Redox Rep. 2011, 16, 38–44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, M.; Yuan, H.; Guo, W.; Li, X.; Jin, L.; Brunk, U.T.; Han, J.; Zhao, M.; Lu, Y. Dietary Mustard Seeds (Sinapis alba Linn) Suppress 1,2-Dimethylhydrazine-Induced Immuno-Imbalance and Colonic Carcinogenesis in Rats. Nut. Cancer 2012, 64, 464–472. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; D’Souza, S.S.; Tickoo, S.; Salimath, B.P.; Singh, H.B. Antiangiogenic and Proapoptotic Activities of Allyl Isothiocyanate Inhibit Ascites Tumor Growth in Vivo. Integ. Cancer Ther. 2009, 8, 75–87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jie, M.; Cheung, W.M.; Yu, V.; Zhou, Y.; Tong, P.H.; Ho, J.W.S. Anti-Proliferative Activities of Sinigrin on Carcinogen Induced Hepatotoxicity in Rats. PLoS ONE 2014, 9, e110145. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.R.; Kang, Y.J.; Choi, H.Y.; Kang, G.H.; Kim, J.H.; Kim, B.W.; Han, Y.S.; Nah, S.Y.; Paik, H.D.; Park, Y.S.; et al. Induction of apoptotic cell death by synthetic naringenin derivatives in human lung epithelial carcinoma A549 cells. Biol. Pharm. Bull. 2007, 30, 2394–2398. [Google Scholar] [CrossRef] [Green Version]
- Palle, K.; Vaziri, C. Rad18 E3 ubiquitin ligase activity mediates Fanconi anemia pathway activation and cell survival following DNA Topoisomerase 1 inhibition. Cell Cycle 2011, 10, 1625–1638. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez, F.J.; Lewis-Tuffin, L.J.; Anastasiadis, P.Z. E-Cadherin’s dark side: Possible role in tumor progression. Biochim. Biophys. Acta 2012, 1826, 23–31. [Google Scholar] [CrossRef] [Green Version]
- Abbaoui, B.; Lucas, C.R.; Riedl, K.M.; Clinton, S.K.; Mortazavi, A. Cruciferous Vegetables, Isothiocyanates, and Bladder Cancer Prevention. Mol. Nutr Food Res. 2018, 62, 1800079. [Google Scholar] [CrossRef]
- Zhang, N.Q.; Ho, S.C.; Mo, X.F.; Lin, F.Y.; Huang, W.Q.; Luo, H.; Huang, J.; Zhang, C.X. Glucosinolate and isothiocyanate intakes are inversely associated with breast cancer risk: A case–control study in China. Br. J. Nutr. 2018, 119, 957–964. [Google Scholar] [CrossRef] [Green Version]
- Talalay, P.; Fahey, J.W. Phytochemicals from cruciferous plants protect against cancer by modulating carcinogen metabolism. J. Nutr. 2001, 131, 3027S–3033S. [Google Scholar] [CrossRef]
- Abdull Raziz, A.F.; Noor, N.M. Cruciferous vegetables: Dietary phytochemicals for cancer prevention. Asian Pac. J. Cancer Prev. 2013, 14, 1565–1570. [Google Scholar] [CrossRef] [Green Version]
- Kiasalari, Z.; Roghani, M.; Khalili, M.; Rahmati, B.; Baluchnejadmojarad, T. Antiepileptogenic effect of curcumin on kainite-induced model of temporal lobe epilepsy. Pharm. Biol. 2013, 51, 1572–1578. [Google Scholar] [CrossRef] [PubMed]
- Sujatha, R.; Srinivas, L. Modulation of lipid peroxidation by dietary components. Toxicol In vitro 1995, 9, 231–236. [Google Scholar] [CrossRef]
- Anand, P.; Murali, Y.K.; Tandon, V.; Murthy, P.S.; Chandra, R. Insulinotropic effect of aqueous extract of Brassica nigra improves glucose homeostasis in streptozotocin induced diabetic rats. Exp. Clin. Endocrinol. Diabetes 2009, 117, 251–256. [Google Scholar] [CrossRef] [PubMed]
- Tseng, E.; Kamath, A.; Morris, M.E. Effect of organic isothiocyanates on the P-glycoprotein and MRP1-mediated transport of daunomycin and vinblastine. Pharm. Res. 2002, 19, 1509–1515. [Google Scholar] [CrossRef] [PubMed]
- Gómez de Saravia, S.G.; Gaylarde, C.C. The antimicrobial activity of an aqueous extract of Brassica nigra. Biodeterior. Biodegrad. 1998, 41, 145–148. [Google Scholar] [CrossRef]
- Kwak, Y.; Lee, J.; Ju, J. Anti-cancer activities of brassica juncea leaves in vitro. EXCLI J. 2016, 15, 699–710. [Google Scholar]
- Savio, A.L.; da Silva, G.N.; de Camargo, E.A.; Salvadori, D.M. Cell cycle kinetics, apoptosis rates, DNA damage and TP53 geneexpression in bladder cancer cells treated with allyl isothiocyanate (mustard essential oil). Mutat. Res. 2014, 762, 40–46. [Google Scholar] [CrossRef]
- Moldovan, G.L.; D’Andrea, A.D. How the fanconi anemia pathway guards the genome. Annu. Rev. Genet. 2009, 43, 223–249. [Google Scholar] [CrossRef] [Green Version]
- Koster, D.A.; Palle, K.; Bot, E.S.M.; Bjornsti, M.A.; Dekker, N.H. Antitumour drugs impede DNA uncoiling by topoisomerase I. Nature 2007, 448, 213–217. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The Next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [Green Version]
- Shan, Y.; Zhang, L.; Bao, Y.; Li, B.; He, C.; Gao, M.; Feng, X.; Xu, W.; Zhang, X.; Wang, S. Epithelial-mesenchymal transition, a novel target of sulforaphane via COX-2/MMP2,9/Snail, ZEB1 and miR-200c/ZEB1 pathways in human bladder cancer cells. J. Nutr. Biochem. 2013, 24, 1062–1069. [Google Scholar] [CrossRef]
- Bae, J.S.; Noh, S.J.; Kim, K.M.; Park, S.H.; Hussein, U.K.; Park, H.S.; Park, B.H.; Ha, S.H.; Lee, H.; Chung, M.J.; et al. SIRT6 is involved in the progression of ovarian carcinomas via beta-catenin-mediated epithelial to mesenchymal transition. Front. Oncol. 2018, 8, 538. [Google Scholar] [CrossRef] [Green Version]
- Kim, K.M.; Hussein, U.K.; Park, S.H.; Kang, M.A.; Moon, Y.J.; Zhang, Z.; Song, Y.; Park, H.S.; Bae, J.S.; Park, B.H.; et al. FAM83H is involved in stabilization of β-catenin and progression of osteosarcomas. J. Exp. Clin. Cancer Res. 2019, 38, 267. [Google Scholar] [CrossRef] [Green Version]
- Jiang, X.; Liu, Y.; Ma, L.; Ji, R.; Qu, Y.; Xin, Y.; Lv, G. Chemopreventive activity of sulforaphane. Drug Des. Dev. Ther. 2018, 12, 2905–2913. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, G.; Yan, Y.; Zhou, Y.; Duan, Y.; Zeng, S.; Wang, X.; Lin, W.; Ou, C.; Zhou, J.; Xu, Z. Sulforaphane: Expected to Become a Novel Anti-tumor Compound. Oncol. Res. 2020, 28. [Google Scholar] [CrossRef] [PubMed]
- Weng, J.R.; Omar, H.A.; Kulp, S.K.; Chen, C.S. Pharmacological exploitation of indole-3-carbinol to develop potent antitumor agents. Mini Rev. Med. Chem. 2010, 5, 398–404. [Google Scholar] [CrossRef] [PubMed]
- Katz, E.; Nisani, S.; Chamovitz, D.A. Indole-3-carbinol: A plant hormone combatting cancer. F1000Research 2018, 7, 689. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y. Allyl isothiocyanate as a cancer chemopreventive phytochemical. Mol. Nutr. Food Res. 2010, 1, 127–135. [Google Scholar] [CrossRef] [Green Version]
- Chua, L.S.; Latiff, N.A.; Mohamed, M. Reflux extraction and cleanup process by column chromatography for high yield of andrographolide enriched extract. J. Appl. Res. Med. Arom Plants 2016, 2, 64–70. [Google Scholar] [CrossRef]
- Schultheiss, C.; Laggai, S.; Czepukojc, B.; Hussein, U.; List, M.; Barghash, A.; Tierling, S.; Hosseini, K.; Golob-Schwarzl, N.; Pokorny, J.; et al. The long non-coding RNA H19 suppresses carcinogenesis and chemoresistance in hepatocellular carcinoma. Cell Stress 2017, 1, 37–54. [Google Scholar] [CrossRef] [Green Version]
- Kulhanek-Heinze, S.; Gerbes, A.L.; Gerwig, T.; Vollmar, A.M.; Kiemer, A.K. Protein kinase A dependent signalling mediates antiapoptotic effects of the atrial natriuretic peptide in ischemic livers. J. Hepatol. 2004, 41, 414–420. [Google Scholar] [CrossRef] [PubMed]
- Molinie, N.; Gautreau, A. Directional Collective Migration in Wound Healing Assays. Methods Mol. Biol. 2018, 1749, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Frankowski, H.; Gu, Y.-H.; Heo, J.H.; Milner, R.; del Zoppo, G.J. Use of gel zymography to examine matrix metalloproteinase (Gelatinase) expression in Brain tissue or in primary glial culture. Methods Mol. Biol. 2012, 814, 221–233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Sample Availability: Not available. |


| Gene | Primer Sequence | Product Size | Accession Number | |
|---|---|---|---|---|
| E-cadherin | Forward | TGGGCCAGGAAATCACATCC | 863 | NM_001317185.2 |
| Reverse | TGCAACGTCGTTACGAGTCA | |||
| SNAL1 (Snail) | Forward | CGAGTGGTTCTTCTGCGCTA | 160 | NM_005985.3 |
| Reverse | GGGCTGCTGGAAGGTAAACT | |||
| MMP2 | Forward | CGGCCGCAGTGACGGAA | 212 | NM_004530.4 |
| Reverse | CATCCTGGGACAGACGGAAGTTCTT | |||
| MMP9 | Forward | GACGCAGACATCGTCATCCA | 200 | NM_004994.2 |
| Reverse | GCCGCGCCATCTGCGTTTCCAAA | |||
| GAPDH | Forward | AACAGCGACACCCACTCCTC | 258 | NM_001256799.1 |
| Reverse | GGAGGGGAGATTCAGTGTGGT | |||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmed, A.G.; Hussein, U.K.; Ahmed, A.E.; Kim, K.M.; Mahmoud, H.M.; Hammouda, O.; Jang, K.Y.; Bishayee, A. Mustard Seed (Brassica nigra) Extract Exhibits Antiproliferative Effect against Human Lung Cancer Cells through Differential Regulation of Apoptosis, Cell Cycle, Migration, and Invasion. Molecules 2020, 25, 2069. https://doi.org/10.3390/molecules25092069
Ahmed AG, Hussein UK, Ahmed AE, Kim KM, Mahmoud HM, Hammouda O, Jang KY, Bishayee A. Mustard Seed (Brassica nigra) Extract Exhibits Antiproliferative Effect against Human Lung Cancer Cells through Differential Regulation of Apoptosis, Cell Cycle, Migration, and Invasion. Molecules. 2020; 25(9):2069. https://doi.org/10.3390/molecules25092069
Chicago/Turabian StyleAhmed, Asmaa Gamal, Usama Khamis Hussein, Amr E. Ahmed, Kyoung Min Kim, Hamada M. Mahmoud, Ola Hammouda, Kyu Yun Jang, and Anupam Bishayee. 2020. "Mustard Seed (Brassica nigra) Extract Exhibits Antiproliferative Effect against Human Lung Cancer Cells through Differential Regulation of Apoptosis, Cell Cycle, Migration, and Invasion" Molecules 25, no. 9: 2069. https://doi.org/10.3390/molecules25092069






