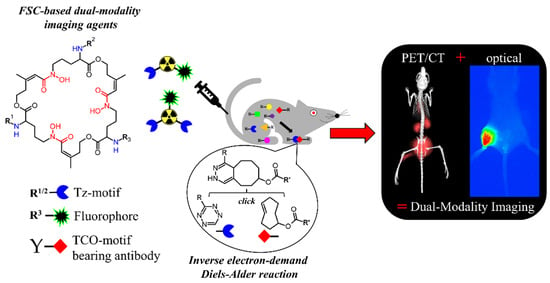
Hybrid Imaging Agents for Pretargeting Applications Based on Fusarinine C—Proof of Concept
Abstract
:1. Introduction
2. Results
2.1. Synthesis
2.2. Radiolabeling
2.3. In Vitro Characterization
2.4. Biodistribution Studies
2.5. Imaging Studies
3. Materials and Methods
3.1. Analytics
Mass Spectrometry
3.2. Synthesis
3.2.1. [Fe]fusarinine C ([Fe]FSC) and [Fe]N-Monoacetylfusarinine C ([Fe]MAFC)
- [Fe]FSC: analytical RP-HPLC tR = 6.95 min; MALDI TOF-MS: m/z [M + H]+ = 779.93 [C33H51FeN6O12; Mr = 779.63 (calculated)]
- [Fe]MAFC analytical RP-HPLC tR = 7.67 min; MALDI TOF-MS: m/z [M + H]+ = 822.04 [C35H53FeN6O13; Mr = 821.67 (calculated)]
3.2.2. Conjugation of PEGylated tetrazine (PEG5-Tz)
- [Fe]MAFC-PEG5-Tz: 12.5 mg [9.5 µmol, 71%], RP-HPLC tR = 10.2 min; MALDI TOF-MS: m/z [M + H]+ = 1312.21 [C58H84FeN11O20; Mr = 1311.19 (calculated)].
- [Fe]FSC-(PEG5-Tz)2: 4.76 mg [2.71 µmol, 33%], RP-HPLC tR = 11.4 min; MALDI TOF-MS: m/z [M + H]+ = 1759.03 [C79H113FeN16O26; Mr = 1758.68 (calculated)].
3.2.3. Synthesis of Monomeric FSC-based Tz Hybrid Imaging Agents
- SulfoCyanine5-[Fe]MAFC-PEG5-Tz: 0.61 mg [0.32 µmol, 36%], RP-HPLC tR = 10.6 min; MALDI TOF-MS: m/z [M + H]+ = 1936.99 [C90H120FeN13O27S2; Mr = 1935.96 (calculated)]
- SulfoCyanine7-[Fe]MAFC-PEG5-Tz: 0.85 mg [0.42 µmol, 49%], RP-HPLC tR = 11.2 min; MALDI TOF-MS: m/z [M + H]+ = 2002.85 [C95H126FeN13O27S2; Mr = 2002.06 (calculated)]
- IRDye800CW-[Fe]MAFC-PEG5-Tz: 1.11 mg [0.48 µmol, 55%], RP-HPLC tR = 9.5 min; MALDI TOF-MS: m/z [M + H]+ = 2297.02 [C104H136FeN13O34S4; Mr = 2296.36 (calculated)]
- SulfoCyanine5-MAFC-PEG5-Tz: 0.55 mg [0.29 µmol, 34%], gradient B (tR = 32.5 min); Analytical data: RP-HPLC tR = 10.8 min; MALDI TOF-MS: m/z [M + H]+ = 1883.75 [C90H123N13O27S2; Mr = 1883.14 (calculated)]
- SulfoCyanine7-MAFC-PEG5-Tz: 0.70 mg [0.36 µmol, 41%], gradient B (tR = 35.5 min); Analytical data: RP-HPLC tR = 11.4 min; MALDI TOF-MS: m/z [M + H]+ = 1949.70 [C95H129N13O27S2; Mr = 1949.24 (calculated)]
- IRDye800CW-MAFC-PEG5-Tz: 1.23 mg [0.55 µmol, 63%], gradient B (tR = 29.2 min); Analytical data: RP-HPLC tR = 9.7 min; MALDI TOF-MS: m/z [M + H]+ = 2244.26 [C104H139N13O34S4; Mr = 2243.54 (calculated)]
3.2.4. Synthesis of Dimeric FSC-based Tz Hybrid Imaging Agents
- SulfoCyanine5-FSC-(PEG5-Tz)2: 0.80 mg [0.34 µmol, 60%], gradient B (tR = 36.7 min); Analytical data: RP-HPLC tR = 11.61 min; MALDI TOF-MS: m/z [M + H]+ = 2332.75 [C111H153N18O33S2; Mr = 2331.63 (calculated)]
- IRDye800CW-FSC-(PEG5-Tz)2: 0.61 mg [0.22 µmol, 40%], gradient B (tR = 31.5 min); Analytical data: RP-HPLC tR = 11.10 min; MALDI TOF-MS: m/z [M + H]+ = 2693.40 [C125H169N18O40S4; Mr = 2692.03 (calculated)]
3.2.5. Modification of Rituximab (RTX)
3.3. Radiochemistry
Radio-ITLC
3.4. In Vitro Characterization
3.4.1. Distribution Coefficient (logD)
3.4.2. Protein Binding
3.4.3. Cell-Binding Studies
3.4.4. Fluorescence Microscopy
3.5. In Vivo Characterization
3.5.1. Biodistribution Studies
3.5.2. Imaging Studies
Pretargeting Model
PET/CT Imaging
Optical Imaging
3.6. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Youn, H.; Chung, J.K. Reporter gene imaging. Am. J. Roentgenol. 2013, 201, 206–214. [Google Scholar] [CrossRef] [PubMed]
- Grootendorst, M.R.; Cariati, M.; Kothari, A.; Tuch, D.S.; Purushotham, A. Cerenkov luminescence imaging (CLI) for image-guided cancer surgery. Clin. Transl. Imaging 2016, 4, 353–366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Massari, R.; Ucci, A.; D’Elia, A.; Campisi, C.; Bertani, E.; Soluri, A. Directional probe for radio-guided surgery: A pilot study: A. Med. Phys. 2018, 45, 622–628. [Google Scholar] [CrossRef] [PubMed]
- Gibbs, S.L. Near infrared fluorescence for image-guided surgery. Quant. Imaging Med. Surg. 2012, 2, 177–187. [Google Scholar] [CrossRef] [PubMed]
- Te Velde, E.A.; Veerman, T.; Subramaniam, V.; Ruers, T. The use of fluorescent dyes and probes in surgical oncology. Eur. J. Surg. Oncol. 2010, 36, 6–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagaya, T.; Nakamura, Y.A.; Choyke, P.L.; Kobayashi, H. Fluorescence-Guided Surgery. Front. Oncol. 2017, 7. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wang, Z.; Zhao, T.; Li, Y.; Huang, G.; Sumer, B.D.; Gao, J. Optical molecular imaging for tumor detection and image-guided surgery. Biomaterials 2018, 157, 62–75. [Google Scholar] [CrossRef] [PubMed]
- Nahrendorf, M.; Keliher, E.; Marinelli, B.; Waterman, P.; Feruglio, P.F.; Fexon, L.; Pivovarov, M.; Swirski, F.K.; Pittet, M.J.; Vinegoni, C.; et al. Hybrid PET-optical imaging using targeted probes. Proc. Natl. Acad. Sci. USA 2010, 107, 7910–7915. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Azhdarinia, A.; Ghosh, P.; Ghosh, S.; Wilganowski, N.; Sevick-Muraca, E.M. Dual-labeling strategies for nuclear and fluorescence molecular imaging: A review and analysis. Mol. Imaging Biol. 2012, 14, 261–276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lütje, S.; Rijpkema, M.; Helfrich, W.; Oyen, W.J.G.; Boerman, O.C. Targeted Radionuclide and Fluorescence Dual-modality Imaging of Cancer: Preclinical Advances and Clinical Translation. Mol. Imaging Biol. 2014, 16, 747–755. [Google Scholar] [CrossRef] [PubMed]
- Welling, M.M.; Bunschoten, A.; Kuil, J.; Nelissen, R.G.H.H.; Beekman, F.J.; Buckle, T.; Van Leeuwen, F.W.B. Development of a Hybrid Tracer for SPECT and Optical Imaging of Bacterial Infections. Bioconjug. Chem. 2015, 26, 839–849. [Google Scholar] [CrossRef] [PubMed]
- Kang, C.M.; Koo, H.-J.; An, G.I.; Choe, Y.S.; Choi, J.Y.; Lee, K.-H.; Kim, B.-T. Hybrid PET/optical imaging of integrin αVβ3 receptor expression using a 64Cu-labeled streptavidin/biotin-based dimeric RGD peptide. EJNMMI Res. 2015, 5, 60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baranski, A.; Schäfer, M.; Bauder-Wüst, U.; Roscher, M.; Schmidt, J.; Stenau, E.; Simpfendörfer, T.; Teber, D.; Maier-Hein, L.; Hadaschik, B.; et al. PSMA-11 Derived Dual-labeled PSMA-Inhibitors for Preoperative PET Imaging and Precise Fluorescence-Guided Surgery of Prostate Cancer. J. Nucl. Med. 2018, 59, 639–645. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knall, A.-C.; Slugovc, C. Inverse electron demand Diels-Alder (iEDDA)-initiated conjugation: A (high) potential click chemistry scheme. Chem. Soc. Rev. 2013, 42, 5131–5142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karver, M.R.; Weissleder, R.; Hilderbrand, S.A. Synthesis and evaluation of a series of 1,2,4,5-tetrazines for bioorthogonal conjugation. Bioconjug. Chem. 2011, 22, 2263–2270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeglis, B.M.; Davis, C.B.; Abdel-Atti, D.; Carlin, S.D.; Chen, A.; Aggeler, R.; Agnew, B.J.; Lewis, J.S. Chemoenzymatic strategy for the synthesis of site-specifically labeled immunoconjugates for multimodal PET and optical imaging. Bioconjug. Chem. 2014, 25, 2123–2128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adumeau, P.; Carnazza, K.E.; Brand, C.; Carlin, S.D.; Reiner, T.; Agnew, B.J.; Lewis, J.S.; Zeglis, B.M. A pretargeted approach for the multimodal PET/NIRF imaging of colorectal cancer. Theranostics 2016, 6, 2267–2277. [Google Scholar] [CrossRef] [PubMed]
- Summer, D.; Grossrubatscher, L.; Petrik, M.; Michalcikova, T.; Novy, Z.; Rangger, C.; Klingler, M.; Haas, H.; Kaeopookum, P.; Von Guggenberg, E.; et al. Developing Targeted Hybrid Imaging Probes by Chelator Scaffolding. Bioconjug. Chem. 2017, 28, 1722–1733. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Summer, D.; Mayr, S.; Petrik, M.; Rangger, C.; Schoeler, K.; Vieider, L.; Matuszczak, B.; Decristoforo, C. Pretargeted Imaging with Gallium-68 — Improving the Binding Capability by Increasing the Number of Tetrazine Motifs. Pharmaceuticals 2018, 11, 102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yazdani, A.; Bilton, H.; Vito, A.; Genady, A.R.; Rathmann, S.M.; Ahmad, Z.; Janzen, N.; Czorny, S.; Zeglis, B.M.; Francesconi, L.C.; et al. A Bone-Seeking trans-Cyclooctene for Pretargeting and Bioorthogonal Chemistry: A Proof of Concept Study Using 99mTc- and 177Lu-Labeled Tetrazines. J. Med. Chem. 2016, 59, 9381–9389. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Not available. |






| 68Ga-Labeled Conjugate | Distribution Coefficient | Protein Binding (%) | ||
|---|---|---|---|---|
| logD (pH 7.4) | 1 h | 2 h | 4 h | |
| SulfoCy5-MAFC-PEG5-Tz | −2.85 ± 0.08 | 37.0 ± 0.3 | 37.4 ± 2.3 | 37.6 ± 1.2 |
| SulfoCy7-MAFC-PEG5-Tz | −1.92 ± 0.05 | 36.9 ± 0.8 | 40.4 ± 1.5 | 41.6 ± 2.7 |
| IRDye800CW-MAFC-PEG5-Tz | −2.40 ± 0.05 | 65.7 ± 1.3 | 67.3 ± 1.4 | 67.7 ± 0.2 |
| SulfoCy5-FSC-(PEG5-Tz)2 | −2.29 ± 0.10 | 35.7 ± 0.7 | 39.1 ± 0.4 | 40.8 ± 0.6 |
| IRDye800CW-FSC-(PEG5-Tz)2 | −2.46 ± 0.09 | 47.3 ± 0.2 | 50.3 ± 0.3 | 54.9 ± 1.1 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Summer, D.; Petrik, M.; Mayr, S.; Hermann, M.; Kaeopookum, P.; Pfister, J.; Klingler, M.; Rangger, C.; Haas, H.; Decristoforo, C. Hybrid Imaging Agents for Pretargeting Applications Based on Fusarinine C—Proof of Concept. Molecules 2020, 25, 2123. https://doi.org/10.3390/molecules25092123
Summer D, Petrik M, Mayr S, Hermann M, Kaeopookum P, Pfister J, Klingler M, Rangger C, Haas H, Decristoforo C. Hybrid Imaging Agents for Pretargeting Applications Based on Fusarinine C—Proof of Concept. Molecules. 2020; 25(9):2123. https://doi.org/10.3390/molecules25092123
Chicago/Turabian StyleSummer, Dominik, Milos Petrik, Sonja Mayr, Martin Hermann, Piriya Kaeopookum, Joachim Pfister, Maximilian Klingler, Christine Rangger, Hubertus Haas, and Clemens Decristoforo. 2020. "Hybrid Imaging Agents for Pretargeting Applications Based on Fusarinine C—Proof of Concept" Molecules 25, no. 9: 2123. https://doi.org/10.3390/molecules25092123