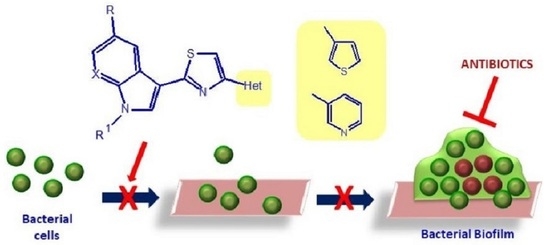
3.1.4. General Procedure for the Synthesis of Thiazole Derivatives (1a–n) and (2a–n)
A suspension of the proper thioamides 3a–c, 4a–d, 5a–d, 6d, 7a, 8a (2 mmol) and bromoacetyl derivatives 11, 12 (2 mmol) in anhydrous ethanol (8 mL) was refluxed for 30 min. After cooling, the obtained precipitate was filtered off, dried, and recrystallized from ethanol to give the desired thiazoles 1a–n and 2a–n.
Tert-Butyl-3-[4-(Thiophen-3-yl)-1,3-Thiazol-2-yl]-1H-Indole-1-Carboxylate (1a)
Yellow solid; yield: 79%; mp: 219–220 °C; IR cm−1: 1750 (CO); 1H NMR (200 MHz, DMSO-d6) δ: 1.68 (s, 9H, 3 x CH3), 7.40–7.53 (m, 2H, H-5′ and H-6′), 7.66–7.74 (m, 2H, H-4″ and H-5″), 8.00 (s, 1H, H-5), 8.10 (dd, 1H, J = 2.8, 1.3 Hz, H-2″), 8.16–8.20 (m, 1H, H-7′), 8.33 (s, 1H, H-2′), 8.47–8.52 (m, 1H, H-4′); 13C (50 MHz, DMSO-d6) δ: 27.6 (3 x q), 84.8 (s), 112.2 (d), 114.9 (d), 121.5 (d), 122.5 (d), 123.8 (d), 125.5 (d), 125.6 (d), 126.3 (d), 126.6 (s), 126.8 (s), 127.1 (d), 135.0 (s), 136.2 (s), 148.6 (s), 150.9 (s), 160.2 (s). Anal. Calcd for C20H18N2O2S2: C, 62.80; H, 4.74; N, 7.32%. Found: C, 62.59; H, 4.56; N, 7.21%.
1-Methyl-3-[4-(Thiophen-3-yl)-1,3-Thiazol-2-yl]-1H-Indole (1b)
Yellow solid; yield: 98%; mp: 248–249 °C; 1H NMR (200 MHz, DMSO-d6) δ: 3.89 (s, 3H, CH3), 7.23–7.36 (m, 2H, H-5′ and H-6′), 7.55–7.72 (m, 3H, H-4″, H-5″ and H-7′), 7.79 (s, 1H, H-5), 8.03 (dd, 1H, J = 2.8, 1.3 Hz, H-2″), 8.19 (s, 1H, H-2′), 8.31–8.35 (m, 1H, H-4′); 13C (50 MHz, DMSO-d6) δ: 32.9 (q), 109.1 (s), 110.0 (d), 110.6 (d), 120.6 (d), 121.1 (d), 122.2 (d), 122.5 (d), 124.5 (s), 126.3 (d), 127.0 (d), 130.8 (d), 136.2 (s), 137.0 (s), 149.8 (s), 162.5 (s). Anal. Calcd for C16H12N2S2: C, 64.83; H, 4.08; N, 9.45%. Found: C, 65.11; H, 3.91; N, 9.59%.
1-(2-Methoxyethyl)-3-[4-(Thiophen-3-yl)-1,3-Thiazol-2-yl]-1H-Indole (1c)
Orange solid; yield: 80%; mp: 169–170 °C; 1H NMR (200 MHz, DMSO-d6) δ: 3.24 (s, 3H, CH3), 3.72 (t, 2H, J = 5.1 Hz, CH2), 4.46 (t, 2H, J = 5.1 Hz, CH2), 7.23–7.33 (m, 2H, H-5′ and H-6′), 7.62–7.81 (m, 3H, H-4″, H-5″ and H-7′), 7.81 (s, 1H, H-5), 8.04 (dd, 1H, J = 2.8, 1.4 Hz, H-2″), 8.17 (s, 1H, H-2′), 8.30–8.34 (m, 1H, H-4′); 13C (50 MHz, DMSO-d6) δ: 45.7 (t), 58.1 (q), 70.6 (t), 109.3 (s), 110.1 (d), 110.9 (d), 120.5 (d), 121.1 (d), 122.3 (d), 122.5 (d), 124.6 (s), 126.3 (d), 127.0 (d), 130.3 (d), 136.1 (s), 136.5 (s), 149.7 (s), 162.4 (s). Anal. Calcd for C18H16N2OS2: C, 63.50; H, 4.74; N, 8.23%. Found: C, 63.26; H, 4.90; N, 8.02%.
Tert-Butyl 5-Methoxy-3-[4-(Thiophen-3-yl)-1,3-Thiazol-2-yl]-1H-Indole-1-Carboxylate (1d)
Pink solid; yield: 89%; mp: 185–186 °C; IR cm−1: 1733 (CO); 1H NMR (200 MHz, DMSO-d6) δ: 1.67 (s, 9H, 3 x CH3), 3.90 (s, 3H, CH3), 7.09 (dd, 1H, J = 9.0, 2.6 Hz, H-6′), 7.66–7.73 (m, 2H, H-4″ and H-7′), 7.98 (s, 1H, H-5), 8.04–8.07 (m, 3H, H-2″, H-4′ and H-5″), 8.29 (s, 1H, H-2′); 13C (50 MHz, DMSO-d6) δ: 27.6 (3 x q), 55.3 (q), 84.7 (s), 103.8 (d), 112.2 (d), 114.2 (d), 114.7 (s), 115.8 (d), 122.4 (d), 126.1 (d), 126.2 (d), 127.2 (d), 127.6 (s), 129.6 (s), 136.2 (s), 148.6 (s), 150.8 (s), 156.2 (s), 160.4 (s). Anal. Calcd for C21H20N2O3S2: C, 61.14; H, 4.89; N, 6.79%. Found: C, 60.89; H, 5.03; N, 6.56%.
5-Methoxy-1-Methyl-3-[4-(Thiophen-3-yl)-1,3-Thiazol-2-yl]-1H-Indole (1e)
Orange solid; yield: 98%; mp: 232–233 °C; 1H NMR (200 MHz, DMSO-d6) δ: 3.86 (s, 3H, CH3), 3.87 (s, 3H, CH3), 6.95 (dd, 1H, J = 8.9, 2.5 Hz, H-6′), 7.48 (d, 1H, J = 8.9 Hz, H-7′), 7.66–7.69 (m, 2H, H-4″ and H-5″), 7.77 (s, 1H, H-5), 7.83 (d, 1H, J = 2.5 Hz, H-4′), 8.00 (dd, 1H, J = 2.7, 1.5 Hz, H-2″), 8.13 (s, 1H, H-2′); 13C (50 MHz, DMSO-d6) δ: 33.1 (q), 55.3 (q), 102.3 (d), 108.5 (s), 109.8 (d), 111.5 (d), 112.4 (d), 122.2 (d), 125.1 (s), 126.2 (d), 127.1 (d), 131.2 (d), 132.2 (s), 136.0 (s), 149.5 (s), 155.0 (s), 162.7 (s). Anal. Calcd for C17H14N2OS2: C, 62.55; H, 4.32; N, 8.58%. Found: C, 62.31; H, 4.15; N, 8.32%.
5-Methoxy-1-(2-Methoxyethyl)-3-[4-(Thiophen-3-yl)-1,3-Thiazol-2-yl]-1H-Indole (1f)
Yellow solid; yield: 91%; mp: 213–214 °C; 1H NMR (200 MHz, DMSO-d6) δ: 3.24 (s, 3H, CH3), 3.69 (t, 2H, J = 5.1 Hz, CH2), 3.87 (s, 3H, CH3), 4.41 (t, 2H, J = 5.1 Hz, CH2), 6.93 (dd, 1H, J = 8.9, 2.5 Hz, H-6′), 7.55 (d, 1H, J = 8.9 Hz, H-7′), 7.64–7.71 (m, 2H, H-4″ and H-5″), 7.78 (s, 1H, H-5), 7.83 (d, 1H, J = 2.5 Hz, H-4′), 8.00 (dd, 1H, J = 2.7, 1.5 Hz, H-2″), 8.10 (s, 1H, H-2′); 13C (50 MHz, DMSO-d6) δ: 45.9 (t), 55.3 (q), 58.1 (q), 70.7 (t), 102.3 (d), 108.8 (s), 109.8 (d), 111.8 (d), 112.3 (d), 121.2 (d), 125.1 (s), 126.2 (d), 127.1 (d), 130.6 (d), 131.6 (s), 136.1 (s), 149.5 (s), 154.9 (s), 162.6 (s). Anal. Calcd for C19H18N2O2S2: C, 61.60; H, 4.90; N, 7.56%. Found: C, 61.45; H, 4.79; N, 7.39%.
Tert-Butyl 5-Bromo-3-[4-(Thiophen-3-yl)-1,3-Thiazol-2-yl]-1H-Indole-1-Carboxylate (1g)
Pink solid; yield: 85%; mp: 174–175 °C; IR cm−1: 1739 (CO); 1H NMR (200 MHz, DMSO-d6) δ: 1.68 (s, 9H, 3 x CH3), 7.64 (dd, 1H, J = 8.9, 2.0 Hz, H-6′), 7.70–7.71 (m, 2H, H-4″ and H-5″), 8.01 (s, 1H, H-5), 8.04 (dd, 1H, J = 2.1, 1.3 Hz, H-2″), 8.11 (d, 1H, J = 8.9 Hz, H-7′), 8.38 (s, 1H, H-2′), 8.61 (d, 1H, J = 2.0 Hz, H-4′); 13C (50 MHz, DMSO-d6) δ: 27.6 (3 x q), 85.4 (s), 112.6 (d), 116.4 (s), 116.9 (d), 119.3 (d), 122.4 (d), 123.8 (d), 126.2 (d), 127.0 (d), 127.3 (s), 128.1 (d), 128.4 (s), 133.9 (s), 136.1 (s), 148.3 (s), 150.9 (s), 159.8 (s). Anal. Calcd for C20H17BrN2O2S2: C, 52.06; H, 3.71; N, 6.07%. Found: C, 51.85; H, 3.94; N, 6.22%.
5-Bromo-1-Methyl-3-[4-(Thiophen-3-yl)-1,3-Thiazol-2-yl]-1H-Indole (1h)
Orange solid; yield: 90%; mp: 250–251 °C; 1H NMR (200 MHz, DMSO-d6) δ: 3.89 (s, 3H, CH3), 7.44 (dd, 1H, J = 8.7, 1.9 Hz, H-6′), 7.58 (d, 1H, J = 8.7 Hz, H-7′), 7.67–7.68 (m, 2H, H-4″ and H-5″), 7.81 (s, 1H, H-5), 7.99 (dd, 1H, J = 2.1, 1.3 Hz, H-2″), 8.24 (s, 1H, H-2′), 8.47 (d, 1H, J = 1.9 Hz, H-4′); 13C (50 MHz, DMSO-d6) δ: 33.1 (q), 108.8 (s), 110.3 (d), 112.8 (d), 113.8 (s), 122.1 (d), 122.8 (d), 125.0 (d), 126.1 (s), 126.2 (d), 127.1 (d), 132.0 (d), 135.8 (s), 136.4 (s), 150.3 (s), 161.8 (s). Anal. Calcd for C16H11BrN2S2: C, 51.20; H, 2.95; N, 7.46%. Found: C, 50.93; H, 2.90; N, 7.18%.
5-Bromo-1-(2-Methoxyethyl)-3-[4-(Thiophen-3-yl)-1,3-Thiazol-2-yl]-1H-Indole (1i)
Yellow solid; yield: 92%; mp: 226–227 °C; 1H NMR (200 MHz, DMSO-d6) δ: 3.24 (s, 3H, CH3), 3.72 (t, 2H, J = 5.0 Hz, CH2), 4.46 (t, 2H, J = 5.0 Hz, CH2), 7.42 (dd, 1H, J = 8.8, 2.0 Hz, H-6′), 7.65 (d, 1H, J = 8.8 Hz, H-7′), 7.63–7.68 (m, 2H, H-4″ and H-5″), 7.81 (s, 1H, H-5), 7.99 (dd, 1H, J = 2.1, 1.3 Hz, H-2″), 8.22 (s, 1H, H-2′), 8.46 (d, 1H, J = 2.0 Hz, H-4′); 13C (50 MHz, DMSO-d6) δ: 46.0 (t), 58.1 (q), 70.6 (t), 109.0 (s), 110.4 (d), 113.1 (d), 113.8 (s), 122.1 (d), 122.7 (d), 125.0 (d), 126.1 (s), 126.2 (d), 127.1 (d), 131.5 (d), 135.4 (s), 136.3 (s), 150.2 (s), 161.8 (s). Anal. Calcd for C18H15BrN2OS2: C, 51.55; H, 3.61; N, 6.68%. Found: C, 51.27; H, 3.77; N, 6.81%.
5-Fluoro-3-[4-(Thiophen-3-yl)-1,3-Thiazol-2-yl]-1H-Indole (1j)
Yellow solid; yield: 74%; mp: 209–210 °C; IR cm−1: 3270 (NH); 1H NMR (200 MHz, DMSO-d6) δ: 7.10 (td, 1H, J = 11.6, 9.2, 2.6 Hz, H-6′), 7.51 (dd, 1H, J = 11.6, 4.6 Hz, H-7′), 7.64–7.71 (m, 2H, H-4″ and H-5″), 7.79 (s, 1H, H-5), 8.01–8.08 (m, 2H, H-2″ and H-4′), 8.23 (d, 1H, J = 2.9 Hz, H-2′); 11.90 (bs, 1H, NH); 13C (50 MHz, DMSO-d6) δ: 105.3 (d, JC6′-F = 24.4 Hz), 110.0 (d), 110.4 (d, JC7′a-F = 4.6 Hz), 110.2 (d, JC4′F = 26.0 Hz), 113.4 (d, JC7′-F = 9.6 Hz), 122.2 (d), 124.5 (d, JC3′a-F = 10.8 Hz), 126.2 (d), 127.0 (d), 128.7 (d), 133.2 (s), 134.3 (s), 136.4 (s), 150.1 (s), 157.9 (d, JC5′-F = 235 Hz). Anal. Calcd for C15H9FN2S2: C, 59.98; H, 3.02; N, 9.33%. Found: C, 59.67; H, 2.93; N, 9.18%.
5-Fluoro-1-Methyl-3-[4-(Thiophen-3-yl)-1,3-Thiazol-2-yl]-1H-Indole (1k)
Yellow solid; yield: 95%; mp: 231–232 °C; 1H NMR (200 MHz, DMSO-d6) δ: 3.90 (s, 3H, CH3), 7.18 (td, 1H, J = 11.7, 9.2, 2.6 Hz, H-6′), 7.57–7.71 (m, 3H, H-4″, H-5″ and H-7′), 7.78 (s, 1H, H-5), 8.02–8.08 (m, 2H, H-2″ and H-4′), 8.25 (s, 1H, H-2′); 13C (50 MHz, DMSO-d6) δ: 33.2 (q), 105.5 (d, JC6′-F = 24.4 Hz), 109.3 (d, JC7′a-F = 4.6 Hz), 110.0 (d), 110.7 (d, JC4′F = 26.3 Hz), 113.5 (d, JC7′-F = 9.7 Hz), 122.6 (d), 124.8 (d, JC3′a-F = 10.8 Hz), 126.2 (d), 127.0 (d), 132.4 (d), 133.8 (s), 136.3 (s), 150.2 (s), 157.2 (d, JC5′-F = 234 Hz), 162.1 (s). Anal. Calcd for C16H11FN2S2: C, 61.12; H, 3.53; N, 8.91%. Found: C, 60.88; H, 3.47; N, 8.74%.
5-Fluoro-1-(2-Methoxyethyl)-3-[4-(Thiophen-3-yl)-1,3-Thiazol-2-yl]-1H-Indole (1l)
Yellow solid; yield: 88%; mp: 200–201 °C; 1H NMR (200 MHz, DMSO-d6) δ: 3.25 (s, 3H, CH3), 3.72 (t, 2H, J = 5.0 Hz, CH2), 4.46 (t, 2H, J = 5.0 Hz, CH2), 7.16 (td, 1H, J = 11.8, 9.2, 2.6 Hz, H-6′), 7.64–7.71 (m, 3H, H-4″, H-5″ and H-7′), 7.80 (s, 1H, H-5), 8.01–8.07 (m, 2H, H-2″ and H-4′), 8.23 (s, 1H, H-2′); 13C (50 MHz, DMSO-d6) δ: 46.0 (t), 58.1 (q), 70.7 (t), 105.5 (d, JC6′-F = 25.0 Hz), 109.5 (d, JC7′a-F = 4.6 Hz), 110.1 (d), 110.7 (d, JC4′F = 26.1 Hz), 117.3 (d, JC7′-F = 9.8 Hz), 122.3 (d), 124.9 (d, JC3′a-F = 10.7 Hz), 126.2 (d), 127.0 (d), 131.8 (d), 133.3 (s), 136.3 (s), 150.2 (s), 158.1 (d, JC5′-F = 234 Hz), 162.0 (s). Anal. Calcd for C18H15FN2OS2: C, 60.31; H, 4.22; N, 7.82%. Found: C, 60.03; H, 4.05; N, 7.64%.
3-[4-(Thiophen-3-yl)-1,3-Thiazol-2-yl]-1H-Pyrrolo[2,3-b]Pyridine (1m)
Yellow solid; yield: 93%; mp: 268–269 °C; IR cm−1: 3273 (NH); 1H NMR (200 MHz, DMSO-d6) δ: 7.37 (dd, 1H, J = 7.9, 4.9 Hz, H-5′), 7.64–7.73 (m, 2H, H-4″ and H-5″), 7.84 (s, 1H, H-5), 8.09 (dd, 1H, J = 2.8, 1.3 Hz, H-2″), 8.34 (d, 1H, J = 2.0 Hz, H-2′), 8.41 (dd, 1H, J = 4.9, 1.5 Hz, H-6′), 8.80 (dd, 1H, J = 7.9, 1.5 Hz, H-4′), 12.50 (bs, 1H, NH); 13C (50 MHz, DMSO-d6) δ: 111.0 (s), 112.1 (d), 118.2 (d), 120.2 (s), 123.4 (d), 127.2 (d), 128.0 (d), 129.2 (d), 134.4 (d), 137.2 (s), 141.0 (d), 145.2 (s), 151.4 (s), 162.1 (s). Anal. Calcd for C14H9N3S2: C, 59.34; H, 3.20; N, 14.83%. Found: C, 59.03; H, 3.44; N, 15.06%.
1-Methyl-3-[4-(Thiophen-3-yl)-1,3-Thiazol-2-yl]-1H-Pyrrolo[2,3-b]Pyridine (1n)
Yellow solid; yield: 96%; mp: 254–255 °C; 1H NMR (200 MHz, DMSO-d6) δ: 3.92 (s, 3H, CH3), 7.34 (dd, 1H, J = 7.9, 4.7 Hz, H-5′), 7.64–7.73 (m, 2H, H-4″ and H-5″), 7.83 (s, 1H, H-5), 8.07 (dd, 1H, J = 2.8, 1.3 Hz, H-2″), 8.38 (s, 1H, H-2′), 8.42 (dd, 1H, J = 4.7, 1.6 Hz, H-6′), 8.72 (dd, 1H, J = 7.9, 1.6 Hz, H-4′); 13C (50 MHz, DMSO-d6) δ: 31.7 (q), 108.3 (s), 110.7 (d), 117.3 (d), 118.0 (s), 122.4 (d), 126.3 (d), 127.0 (d), 131.1 (2 x d), 136.3 (s), 142.2 (d), 146.0 (s), 150.4 (s), 161.3 (s). Anal. Calcd for C15H11N3S2: C, 60.58; H, 3.73; N, 14.13%. Found: C, C, 60.33; H, 3.59; N, 13.89%.
Tert-Butyl 3-[4-(Pyridin-3-yl)-1,3-Thiazol-2-yl]-1H-Indole-1-Carboxylate Hydrobromide (2a)
Pink solid; yield: 90%; mp: 171–172 °C; IR cm−1: 1737 (CO), 3420 (NH+); 1H NMR (200 MHz, DMSO-d6) δ: 1.69 (s, 9H, 3 x CH3), 7.43–7.54 (m, 2H, H-5′ and H-6′), 8.09 (dd, 1H, J = 8.1, 5.4 Hz, H-5″), 8.14–8.23 (m, 1H, H-7′), 8.44 (s, 1H, H-2′), 8.49–8.53 (m, 1H, H-4′), 8.62 (s, 1H, H-5), 8.88 (d, 1H, J = 5.4 Hz, H-6″), 9.08 (d, 1H, J = 8.1 Hz, H-4″), 9.53 (s, 1H, H-2″); 13C (50 MHz, DMSO-d6) δ: 27.6 (3xq), 85.0 (s), 114.3 (s), 115.0 (d), 118.1 (d), 121.5 (d), 124.0 (d), 125.6 (d), 126.3 (d), 126.5 (s), 127.2 (d), 132.4 (s), 134.9 (s), 140.1 (d), 141.3 (d), 141.6 (d), 148.4 (s), 148.5 (s), 161.8 (s). Anal. Calcd for C21H20BrN3O2S: C, 55.03; H, 4.40; N, 9.17%. Found: C, 54.78; H, 4.57; N, 8.98%.
1-Methyl-3-[4-(Pyridin-3-yl)-1,3-Thiazol-2-yl]-1H-Indole Hydrobromide (2b)
Orange solid; yield: 79%; mp: 266–267 °C; IR cm−1 3423 (NH+); 1H NMR (200 MHz, DMSO-d6) δ: 3.91 (s, 3H, CH3), 7.25–7.38 (m, 2H, H-5′ and H-6′), 7.57–7.61 (m, 1H, H-7′), 8.21 (dd, 1H, J = 8.2, 5.7 Hz, H-5″), 8.29 (s, 1H, H-2′), 8.35–8.40 (m, 1H, H-4′), 8.52 (s, 1H, H-5), 8.94 (d, 1H, J = 5.7 Hz, H-6″), 9.21 (d, 1H, J = 8.2 Hz, H-4″), 9.55 (d, 1H, J = 1.7 Hz, H-2″); 13C (50 MHz, DMSO-d6) δ: 32.9 (q); 108.8 (s), 110.7 (d), 116.2 (d), 120.6 (d), 121.3 (d), 122.7 (d), 124.4 (s), 127.5 (d), 131.4 (d), 133.1 (s), 137.0 (s), 139.0 (d), 140.3 (d), 142.1 (d), 147.5 (s), 163.9 (s). Anal. Calcd for: C17H14BrN3S: C, 54.85; H, 3.79; N, 11.29%. Found: C, 54.64; H, 4.00; N, 11.47%.
1-(2-Methoxyethyl)-3-[4-(Pyridin-3-yl)-1,3-Thiazol-2-yl]-1H-Indole Hydrobromide (2c)
Orange solid; yield: 73%; mp: 190–191 °C; IR cm−1 3425 (NH+); 1H NMR (200 MHz, DMSO-d6) δ: 3.24 (s, 3H, CH3), 3.73 (t, 2H, J = 5.0 Hz, CH2), 4.47 (t, 2H, J = 5.0 Hz, CH2), 7.25–7.35 (m, 2H, H-5′ and H-6′), 7.64–7.68 (m, 1H, H-7′), 8.23 (dd, 1H, J = 8.2, 5.8 Hz, H-5″), 8.27 (s, 1H, H-2′), 8.33–8.37 (m, 1H, H-4′), 8.56 (s, 1H, H-5), 8.82 (d, 1H, J = 5.8 Hz, H-6″), 9.24 (d, 1H, J = 8.2 Hz, H-4″), 9.58 (d, 1H, J = 1.5 Hz, H-2″); 13C (50 MHz, DMSO-d6) δ: 45.8 (t), 50.1 (q), 70.6 (t), 109.1 (s), 111.0 (d), 116.3 (d), 120.6 (d), 121.3 (d), 122.7 (d), 124.5 (s), 127.6 (d), 131.0 (d), 133.1 (s), 136.6 (s), 139.1 (d), 140.4 (d), 142.3 (d), 147.5 (s), 163.9 (s). Anal. Calcd for: C19H18BrN3OS: C, 54.81; H, 4.36; N, 10.09%. Found: C, 54.60; H, 4.21; N, 9.92%.
Tert-Butyl 5-Methoxy-3-[4-(Pyridin-3-yl)-1,3-Thiazol-2-yl]-1H-Indole-1-Carboxylate Hydrobromide (2d)
Orange solid; yield: 88%; mp: 154–155 °C; IR cm−1: 1734 (CO), 3422 (NH+); 1H NMR (200 MHz, DMSO-d6) δ: 1.68 (s, 9H, 3 x CH3), 3.91 (s, 3H, CH3), 7.11 (dd, 1H, J = 9.1, 2.6 Hz, H-6′), 7.82 (d, 1H, J = 2.6 Hz, H-4′), 8.05 (d, 1H, J = 9.1 Hz, H-7′), 8.13 (dd, 1H, J = 8.2, 5.6 Hz, H-5″), 8.38 (s, 1H, H-2′), 8.63 (s, 1H, H-5), 8.91 (d, 1H, J = 5.6 Hz, H-6″), 9.07 (d, 1H, J = 8.2 Hz, H-4″), 9.50 (d, 1H, J = 2.0 Hz, H-2″); 13C (50 MHz, DMSO-d6) δ: 27.6 (3 x q), 55.4 (q), 84.9 (s), 103.8 (d), 114.0 (d), 115.8 (d), 118.0 (d), 126.9 (d), 127.2 (d), 129.5 (s), 132.4 (s), 140.0 (d), 141.2 (d), 141.3 (s), 141.5 (d), 148.2 (s), 148.5 (s), 149.7 (s), 156.2 (s), 161.9 (s). Anal. Calcd for: C22H22BrN3O3S: C, 54.10; H, 4.54; N, 8.60%. Found: C, 54.35; H, 4.30; N, 8.46%.
5-Methoxy-1-Methyl-3-[4-(Pyridin-3-yl)-1,3-Thiazol-2-yl]-1H-Indole Hydrobromide (2e)
Orange solid; yield: 96%; mp: 210–211 °C; IR cm−1 3429 (NH+); 1H NMR (200 MHz, DMSO-d6) δ: 3.87 (s, 3H, CH3), 3.90 (s, 3H, CH3), 6.99 (dd, 1H, J = 8.9, 2.4 Hz, H-6′), 7.63 (d, 1H, J = 8.9 Hz, H-7′), 7.83 (d, 1H, J = 2.4 Hz, H-4′), 8.19–8.25 (m, 2H, H-2′ and H-5″), 8.50 (s, 1H, H-5), 8.94 (d, 1H, J = 5.3 Hz, H-6″), 9.20 (d, 1H, J = 8.3 Hz, H-4″), 9.51 (s, 1H, H-2″); 13C (50 MHz, DMSO-d6) δ: 33.1 (q), 55.4 (q), 102.5 (d), 108.4 (s), 111.6 (d), 112.3 (d), 115.9 (d), 125.0 (s), 127.5 (d), 131.7 (d), 132.2 (s), 133.1 (s), 139.1 (d), 140.4 (d), 141.9 (d), 147.4 (s), 155.1 (s), 164.2 (s). Anal. Calcd for: C18H16BrN3OS: C, 53.74; H, 4.01; N, 10.44%. Found: C, 53.50; H, 3.83; N, 10.70%.
5-Methoxy-1-(2-Methoxyethyl)-3-[4-(Pyridin-3-yl)-1,3-Thiazol-2-yl]-1H-Indole Hydrobromide (2f)
Orange solid; yield: 89%; mp: 122–123 °C; IR cm−1 3426 (NH+); 1H NMR (200 MHz, DMSO-d6) δ: 3.24 (s, 3H, CH3), 3.71 (t, 2H, J = 5.0 Hz, CH2), 3.89 (s, 3H, CH3), 4.42 (t, 2H, J = 5.0 Hz, CH2), 6.95 (dd, 1H, J = 8.9, 2.5 Hz, H-6′), 7.56 (d, 1H, J = 8.9 Hz, H-7′), 7.81 (d, 1H, J = 2.5 Hz, H-4′), 8.18–8.22 (m, 2H, H-2′ and H-5″), 8.50 (s, 1H, H-5), 8.93 (d, 1H, J = 5.2 Hz, H-6″), 9.15 (d, 1H, J = 8.4 Hz, H-4″), 9.51 (d, 1H, J = 1.3 Hz, H-2″); 13C (50 MHz, DMSO-d6) δ: 46.0 (t), 55.4 (q), 58.1 (q), 70.6 (t), 102.5 (d), 108.7 (s), 111.9 (d), 112.2 (d), 115.8 (d), 125.1 (s), 127.3 (d), 131.1 (d), 131.7 (s), 133.0 (s), 139.5 (d), 140.8 (d), 141.6 (d), 147.6 (s), 155.0 (s), 164.1 (s). Anal. Calcd for: C20H20BrN3O2S: C, 53.82; H, 4.52; N, 9.41%. Found: C, 53.65; H, 4.47; N, 9.66%.
Tert-Butyl 5-Bromo-3-[4-(Pyridin-3-yl)-1,3-Thiazol-2-yl]-1H-Indole-1-Carboxylate Hydrobromide (2g)
Yellow solid; yield: 78%; mp: 185–186 °C; IR cm−1: 1749 (CO), 3421 (NH+); 1H NMR (200 MHz, DMSO-d6) δ: 1.68 (s, 9H, CH3); 7.63 (dd, 1H, J = 8.9, 2.0 Hz, H-6′), 8.01–8.13 (m, 2H, H-5″ and H-7′), 8.45 (s, 1H, 2′), 8.57 (d, 1H, J = 2.0 Hz, H-4′), 8.60 (s, 1H, H-5), 8.89 (d, 1H, J = 5.5 Hz, H-6″), 8.99 (d, 1H, J = 8.3 Hz, H-4″), 9.46 (d, 1H, J = 1.8 Hz, H-2″); 13C (50 MHz, DMSO-d6) δ: 27.6 (3 x q), 85.5 (s), 113.4 (s), 116.5 (d), 116.8 (s), 118.2 (d), 123.5 (d), 127.1 (d), 127.6 (d), 128.0 (s), 128.1 (d), 132.2 (s), 133.7 (s), 140.3 (d), 140.9 (d), 141.9 (d), 148.1 (s), 148.5 (s), 161.3 (s). Anal. Calcd for: C21H19Br2N3O2S: C, 46.95; H, 3.56; N, 7.82%. Found: C, 47.18; H, 3.41; N, 7.56%.
5-Bromo-1-Methyl-3-[4-(Pyridin-3-yl)-1,3-Thiazol-2-yl]-1H-Indole Hydrobromide (2h)
Orange solid; yield: 94%; mp: 279–280 °C; IR cm−1: 3420 (NH+); 1H NMR (200 MHz, DMSO-d6) δ: 3.89 (s, 3H, CH3), 7.41 (dd, 1H, J = 8.7, 1.9 Hz, H-6′), 7.56 (d, 1H, J = 8.7 Hz, H-7′), 8.18 (dd, 1H, J = 8.2, 5.6 Hz, H-5″), 8.31 (s, 1H, H-2′), 8.43 (d, 1H, J = 1.9 Hz, H-4′), 8.49 (s, 1H, H-5), 8.92 (d, 1H, J = 5.6 Hz, H-6″), 9.09 (d, 1H, J = 8.2 Hz, H-4″), 9.47 (d, 1H, J = 1.8 Hz, H-2″); 13C (50 MHz, DMSO-d6) δ: 33.2 (q), 108.4 (s), 112.9 (d), 114.1 (s), 116.2 (d), 122.7 (d), 125.2 (d), 125.9 (s), 127.3 (d), 132.7 (d), 132.8 (s), 135.8 (s), 139.6 (d), 141.0 (d), 141.4 (d), 147.8 (s), 163.4 (s). Anal. Calcd for: C17H13Br2N3S: C, 45.26; H, 2.90; N, 9.31%. Found: C, 45.44; H, 2.65; N, 9.47%.
5-Bromo-1-(2-Methoxyethyl)-3-[4-(Pyridin-3-yl)-1,3-Thiazol-2-yl]-1H-Indole Hydrobromide (2i)
Red solid; yield: 87%; mp: 196–197 °C; IR cm−1: 3427 (NH+); 1H NMR (200 MHz, DMSO-d6) δ: 3.23 (s, 3H, CH3), 3.72 (t, 2H, J = 5.0 Hz, CH2), 4.46 (t, 2H, J = 5.0 Hz, CH2), 7.42 (dd, 1H, J = 8.8, 2.0 Hz, H-6′), 7.65 (d, 1H, J = 8.8 Hz, H-7′), 8.18 (dd, 1H, J = 8.2, 5.6 Hz, H-5″), 8.32 (s, 1H, H-2′), 8.44 (d, 1H, J = 2.0 Hz, H-4′), 8.51 (s, 1H, H-5), 8.92 (d, 1H, J = 5.6 Hz, H-6″), 9.10 (d, 1H, J = 8.2 Hz, H-4″), 9.49 (d, 1H, J = 1.7 Hz, H-2″); 13C (50 MHz, DMSO-d6) δ: 46.0 (t), 58.1 (q), 70.6 (t), 108.7 (s), 113.2 (d), 114.0 (s), 116.3 (d), 122.7 (d), 125.2 (d), 126.0 (s), 127.2 (d), 132.2 (d), 132.7 (s), 135.5 (s), 139.8 (d), 141.2 (d), 141.3 (d), 148.0 (s), 163.3 (s). Anal. Calcd for: C19H17Br2N3OS: C, 46.08; H, 3.46; N, 8.48%. Found: C, 46.33; H, 3.60; N, 8.66%.
5-Fluoro-3-[4-(Pyridin-3-yl)-1,3-Thiazol-2-yl]-1H-Indole Hydrobromide (2j)
Yellow solid; yield: 90%; mp: 284–285 °C; IR cm−1: 3159 (NH), 3428 (NH+); 1H NMR (200 MHz, DMSO-d6) δ: 7.13 (td, 1H, J = 11.6, 9.3, 2.5 Hz, H-6′), 7.54 (dd, 1H, J = 9.3, 4.6 Hz, H-7′), 8.06–9.19 (m, 2H, H-4′ and H-5″), 8.34 (d, 1H, J = 2.9 Hz, H-2′), 8.48 (s, 1H, H-5), 8.90 (d, 1H, J = 4.8 Hz, H-6″), 9.14 (d, 1H, J = 8.4 Hz, H-4″), 9.54 (d, 1H, J = 1.7 Hz, H-2″), 12.02 (bs, 1H, NH); 13C (50 MHz, DMSO-d6): 105.4 (d, JC6′-F = 24.2 Hz), 110.0 (d, JC’7a-F = 4.6 Hz), 110.9 (d, JC4′-F = 26.2 Hz), 113.5 (d, JC7′-F = 10.2 Hz), 116.2 (d), 124.4 (d, JC3′a-F = 10.9 Hz), 127.4 (d), 129.4 (d), 133.0 (s), 133.2 (s), 139.4 (d), 140.6 (d), 141.9 (d), 147.6 (s), 158.1 (d, JC5′-F = 234 Hz), 164.1 (s). Anal. Calcd for: C16H11BrFN3S: C, 51.08; H, 2.95; N, 11.17%. Found: C, 51.36; H, 3.09; N, 11.41%.
5-Fluoro-1-Methyl-3-[4-(Pyridin-3-yl)-1,3-Thiazol-2-yl]-1H-Indole Hydrobromide (2k)
Orange solid; yield: 92%; mp: 277–278 °C; IR cm−1: 3420 (NH+); 1H NMR (200 MHz, DMSO-d6) δ: 3.89 (s, 3H, CH3), 7.13 (td, 1H, J = 11.7, 9.2, 2.6 Hz, H-6′), 7.60 (dd, 1H, J = 9.2, 4.4 Hz, H-7′), 8.05 (dd, 1H, J = 11.7, 2.6 Hz, H-4′), 8.16 (dd, 1H, J = 8.2, 5.6 Hz, H-5″), 8.32 (s, 1H, H-2′), 8.48 (s, 1H, H-5), 8.91 (d, 1H, J = 5.6 Hz, H-6″), 9.19 (d, 1H, J = 8.2 Hz, H-4″), 9.52 (d, 1H, J = 1.6 Hz, H-2″); 13C (50 MHz, DMSO-d6): 33.2 (q), 105.6 (d, JC6′-F = 24.9 Hz), 110.0 (d, JC’7a-F = 4.6 Hz), 110.9 (d, JC4′-F = 25.7 Hz), 112.1 (d, JC7′-F = 10.3 Hz), 115.9 (d), 124.7 (d, JC3′a-F = 11.0 Hz), 127.2 (d), 132.8 (s), 133.0 (d), 133.8 (s), 139.7 (d), 141.0 (d), 141.5 (d), 147.8 (s), 158.3 (d, JC5′-F = 236 Hz), 163.6 (s). Anal. Calcd for: C17H13BrFN3S: C, 52.32; H, 3.36; N, 10.77%. Found: C, 52.14; H, 3.48; N, 10.53%.
5-Fluoro-1-(2-Methoxyethyl)-3-[4-(Pyridin-3-yl)-1,3-Thiazol-2-yl]-1H-Indole Hydrobromide (2l)
Orange solid; yield: 88%; mp: 202 °C; IR cm−1: 3421 (NH+); 1H NMR (200 MHz, DMSO-d6) δ: 3.24 (s, 3H, CH3), 3.72 (t, 2H, J = 5.1 Hz, CH2), 4.46 (t, 2H, J = 5.1 Hz, CH2), 7.17 (td, 1H, J = 11.7, 9.2, 2.6 Hz, H-6′), 7.69 (dd, 1H, J = 9.2, 4.5 Hz, H-7′), 8.07 (dd, 1H, J = 11.7, 2.6 Hz, H-4′), 8.16 (dd, 1H, J = 8.2, 5.6 Hz, H-5″), 8.33 (s, 1H, H-2′), 8.50 (s, 1H, H-5), 8.91 (d, 1H, J = 5.6 Hz, H-6″), 9.14 (d, 1H, J = 8.2 Hz, H-4″), 9.54 (d, 1H, J = 1.7 Hz, H-2″); 13C (50 MHz, DMSO-d6): 46.1 (t), 58.1 (q), 70.6 (t), 105.6 (d, JC6′-F = 25.0 Hz), 109.2 (d, JC’7a-F = 4.7 Hz), 110.9 (d, JC4′-F = 26.3 Hz), 112.4 (d, JC7′-F = 9.8 Hz), 116.0 (d), 124.8 (d, JC3′a-F = 10.8 Hz), 127.2 (d), 132.5 (d), 132.8 (s), 133.4 (s), 139.9 (d), 141.2 (d), 141.4 (d), 147.9 (s), 162.1 (d, JC5′-F = 234 Hz), 163.6 (s). Anal. Calcd for: C19H17BrFN3OS: C, 52.54; H, 3.95; N, 9.67. Found: C, 52.23; H, 3.77; N, 9.81.
3-[4-(Pyridin-3-yl)-1,3-Thiazol-2-yl]-1H-Pyrrolo[2,3-b]Pyridine Hydrobromide (2m)
Yellow solid; yield: 95%; mp: 324–325 °C; IR cm−1: 3227 (NH), 3423 (NH+); 1H NMR (200 MHz, DMSO-d6) δ: 7.46 (dd, 1H, J = 7.9, 5.1 Hz, H-5′), 8.23 (dd, J = 8.2, 5.7 Hz, 1H, H-5″), 8.47–8.49 (m, 2H, H-2′ and H-6′), 8.63 (s, 1H, H-5), 8.94–8.95 (m, 2H, H-4′ and H-6″), 9.27 (d, 1H, J = 8.2 Hz, H-4″), 9.63 (d, 1H, J = 1.4 Hz, H-2″), 12.50 (bs, 1H, NH); 13C (50 MHz, DMSO-d6): 109.3 (s), 117.3 (2 x d), 118.4 (s), 127.5 (d), 128.8 (d), 132.3 (d), 132.9 (s), 139.2 (d), 140.5 (d), 141.3 (d), 142.4 (d), 145.4 (s), 147.7 (s), 165.0 (s). Anal. Calcd for: C15H11BrN4S: C, 50.15; H, 3.09; N, 15.60%. Found: C, 50.42; H, 3.20; N, 15.83%.
1-Methyl-3-[4-(Pyridin-3-yl)-1,3-Thiazol-2-yl]-1H-Pyrrolo[2,3-b]Pyridine Hydrobromide (2n)
Yellow solid; yield: 99%; mp: 280–282 °C; IR cm−1: 3425 (NH+); 1H NMR (200 MHz, DMSO-d6) δ: 3.92 (s, 3H, CH3), 7.37 (dd, 1H, J = 7.9, 4.8 Hz, H-5′), 8.21 (dd, J = 8.2, 5.7 Hz, 1H, H-5″), 8.44 (dd, 1H, J = 4.8, 1.5 Hz, H-6′), 8.49 (s, 1H, H-2′), 8.58 (s, 1H, H-5), 8.78 (dd, 1H, J = 7.9, 1.5 Hz, H-4′), 8.95 (d, 1H, J = 5.7 Hz, H-6″), 9.23 (d, 1H, J = 8.2 Hz, H-4″), 9.59 (d, 1H, J = 1.7 Hz, H-2”); 13C (50 MHz, DMSO-d6): 31.6 (q), 107.6 (s), 116.9 (d), 117.3 (s), 117.5 (d), 127.5 (d), 130.2 (d), 131.6 (d), 132.9 (s), 139.1 (d), 140.4 (d), 142.3 (d), 143.2 (d), 146.8 (s), 147.6 (s), and 163.2 (s). Anal. Calcd for: C16H13BrN4S: C, 51.48; H, 3.51; N, 15.01%. Found: C, 51.76; H, 3.72; N, 14.92%.