PEG-POSS Star Molecules Blended in Polyurethane with Flexible Hard Segments: Morphology and Dynamics
Abstract
:1. Introduction
2. Results
2.1. Scanning Electron Microscopy
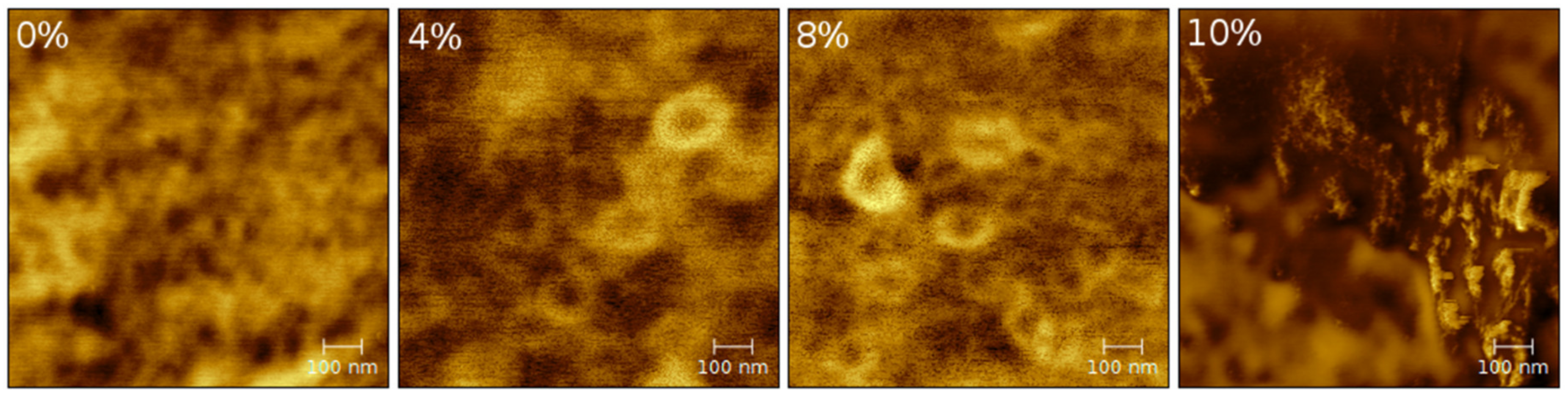
2.2. Atomic Force Microscopy
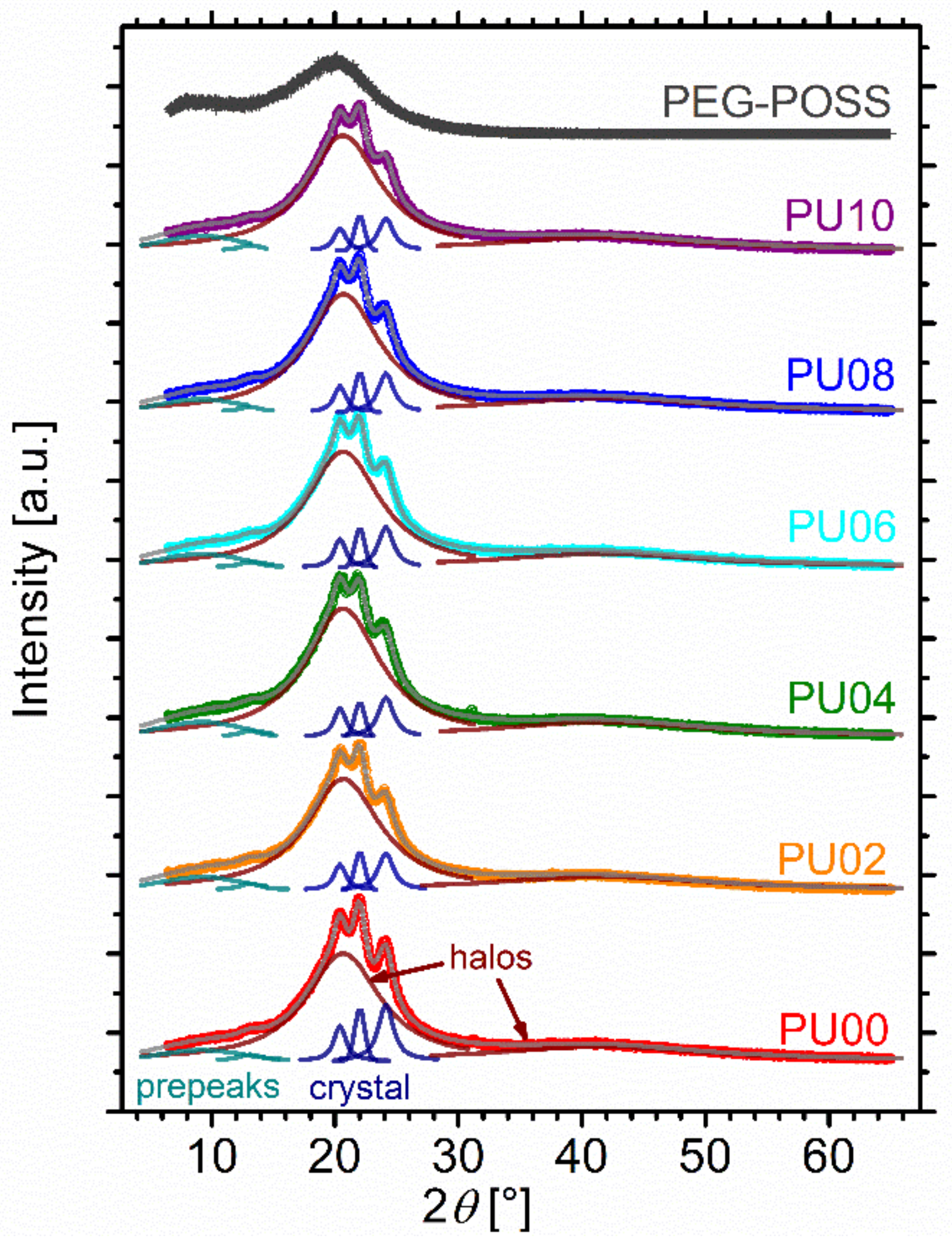
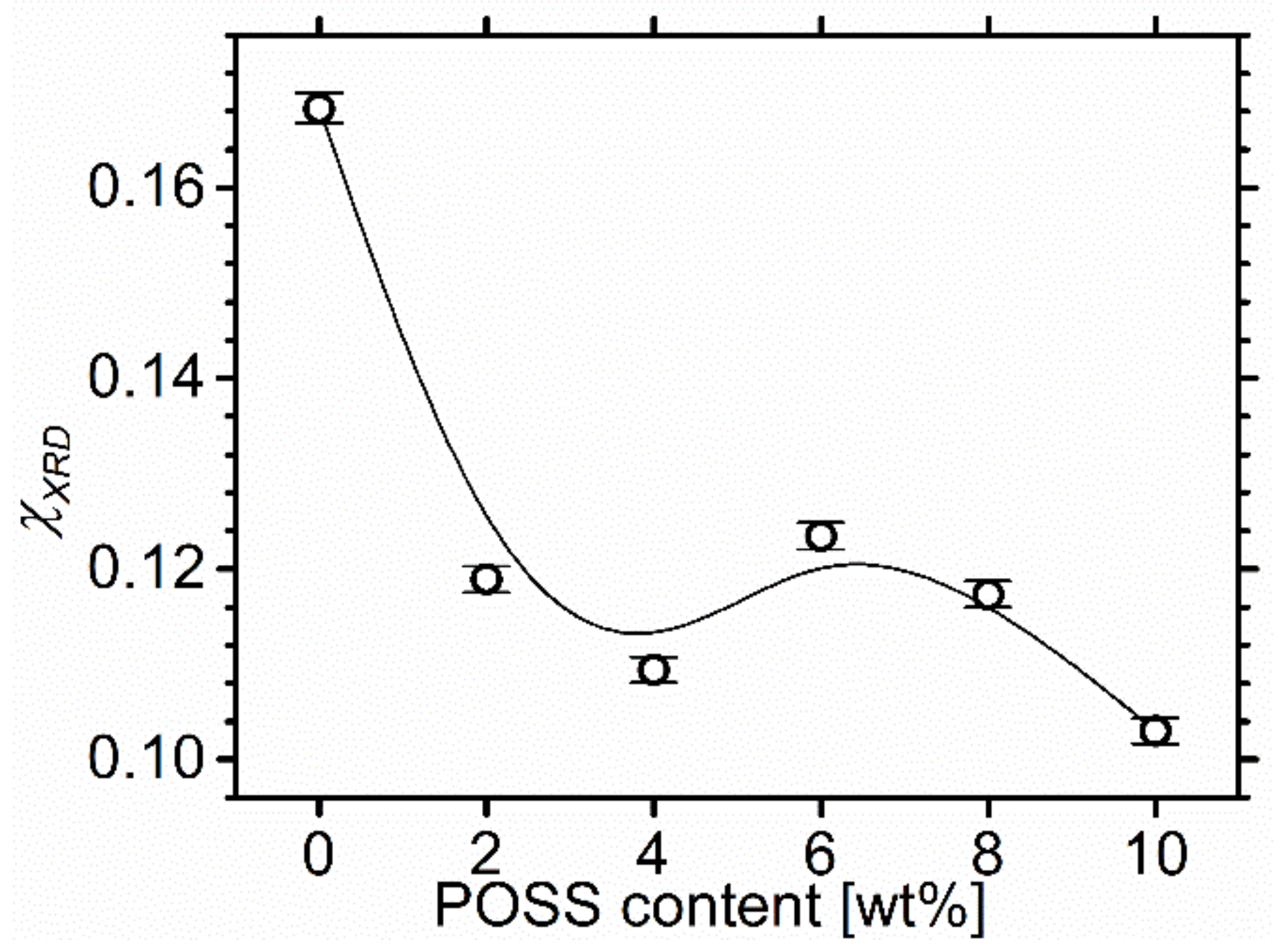
2.3. X-ray Diffraction
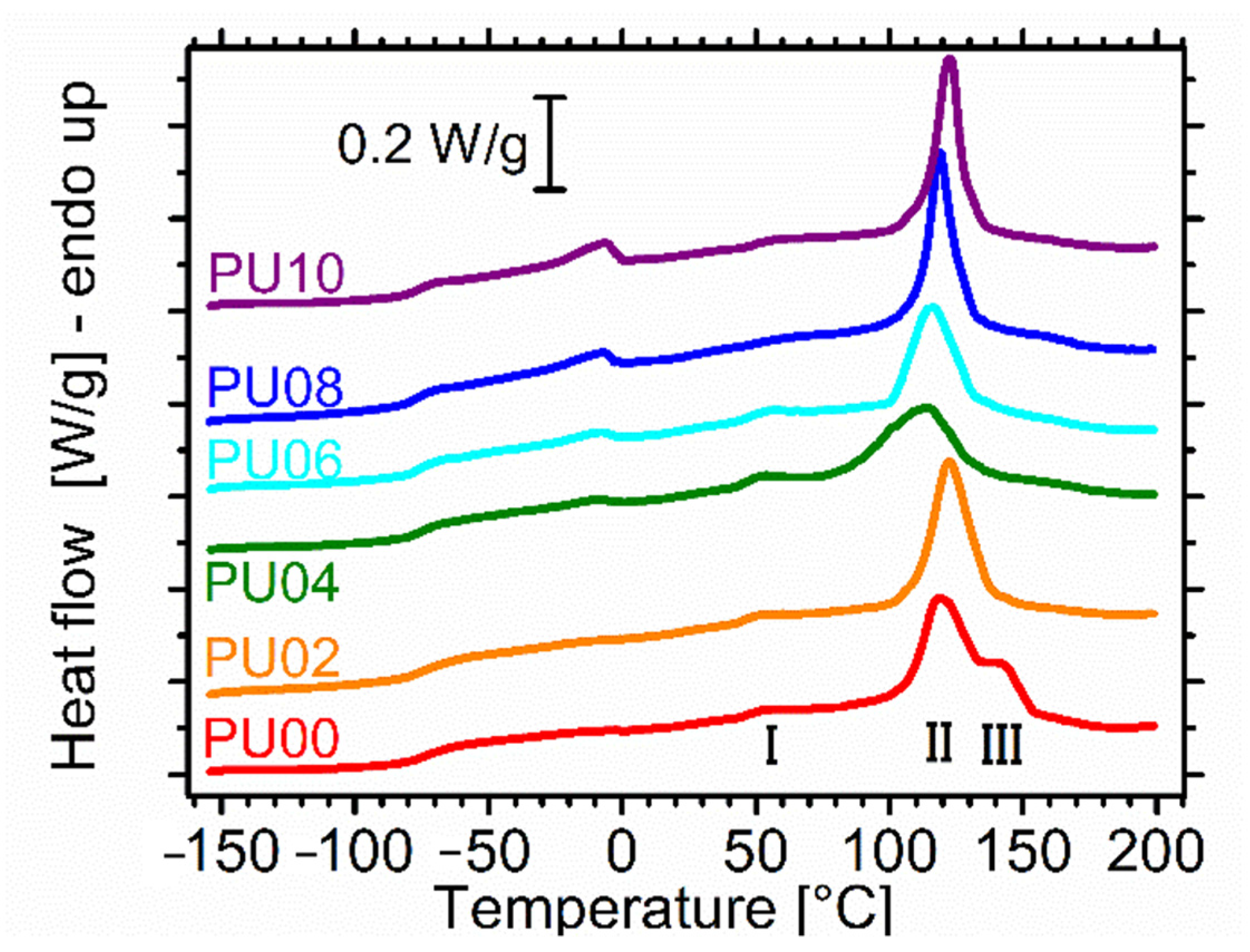
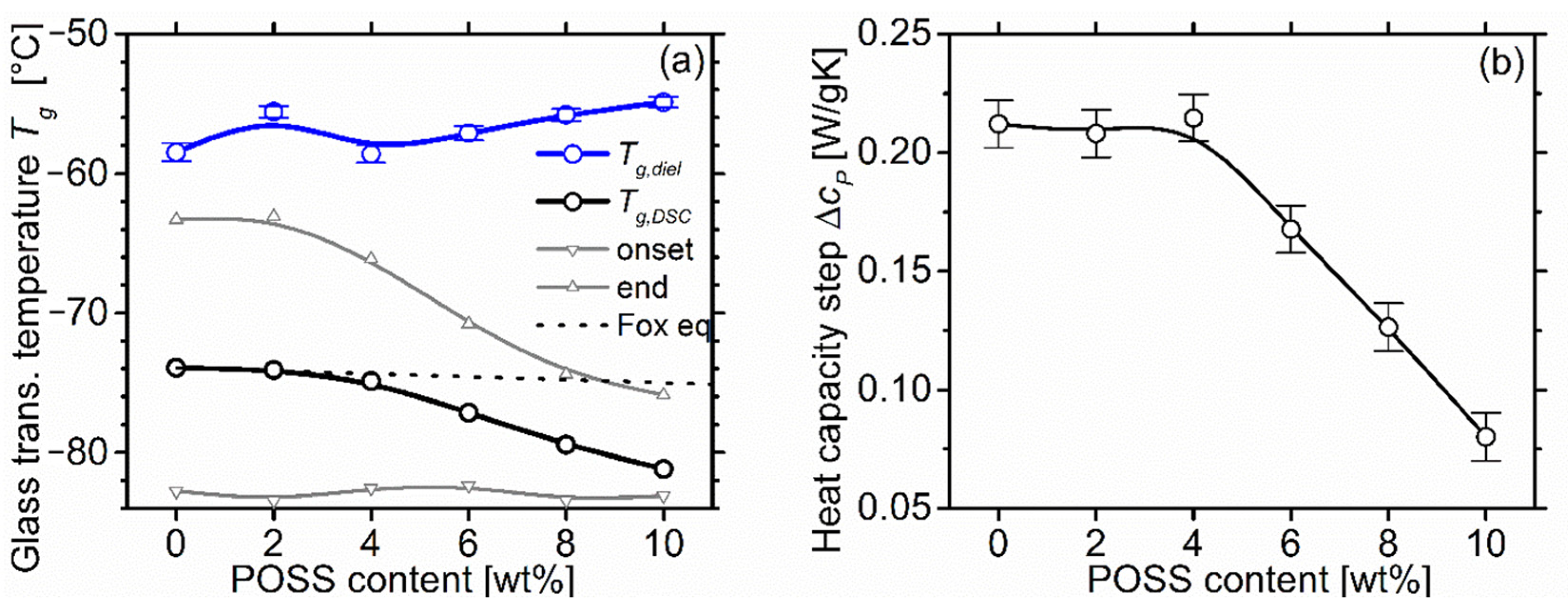
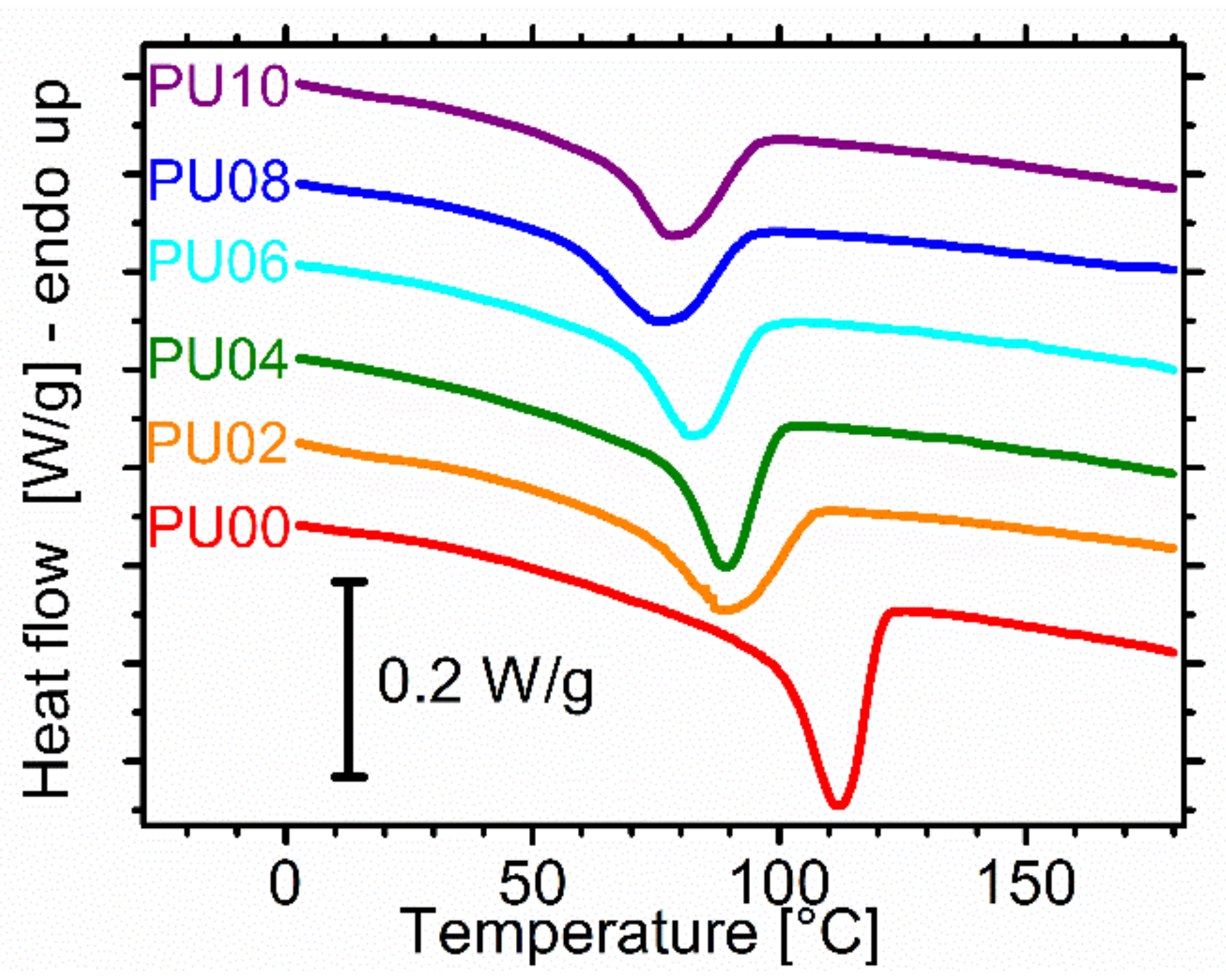
2.4. Differential Scanning Calorimetry
2.5. Dielectric Relaxation Spectroscopy
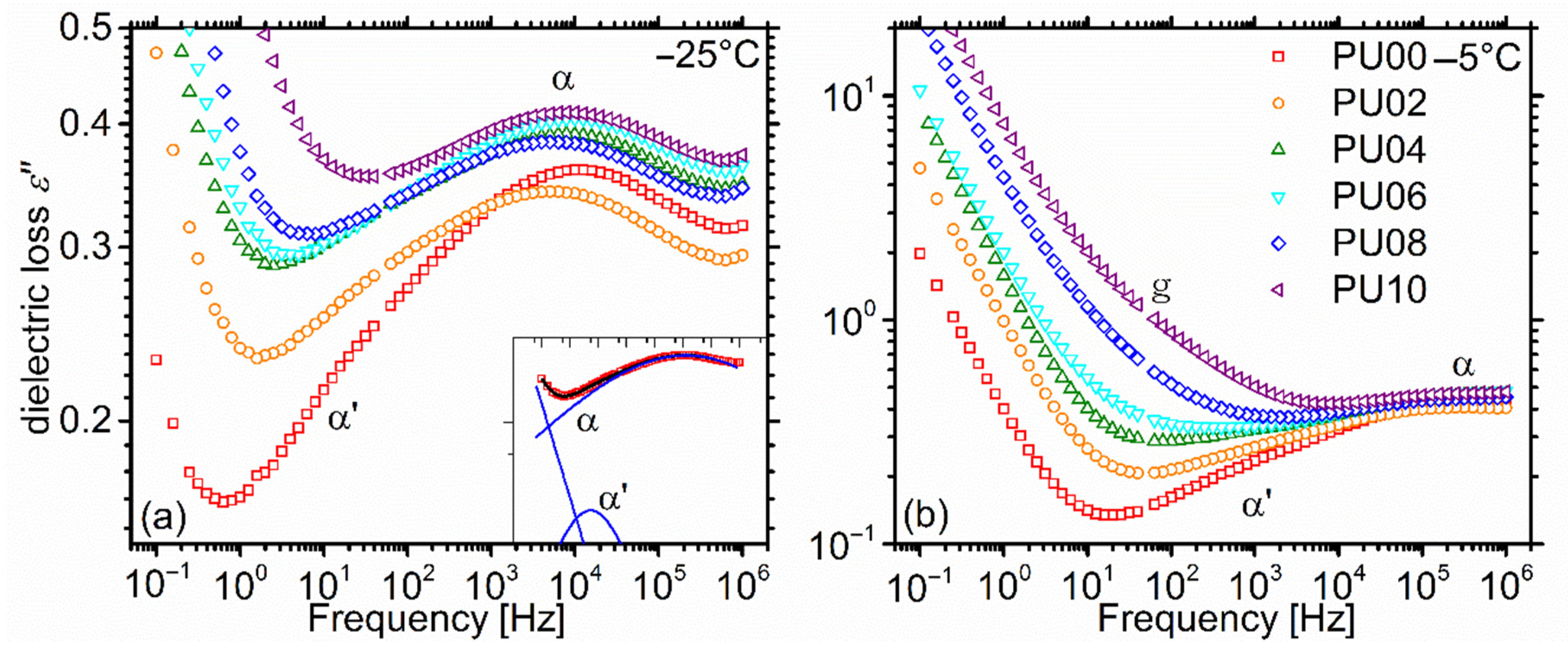
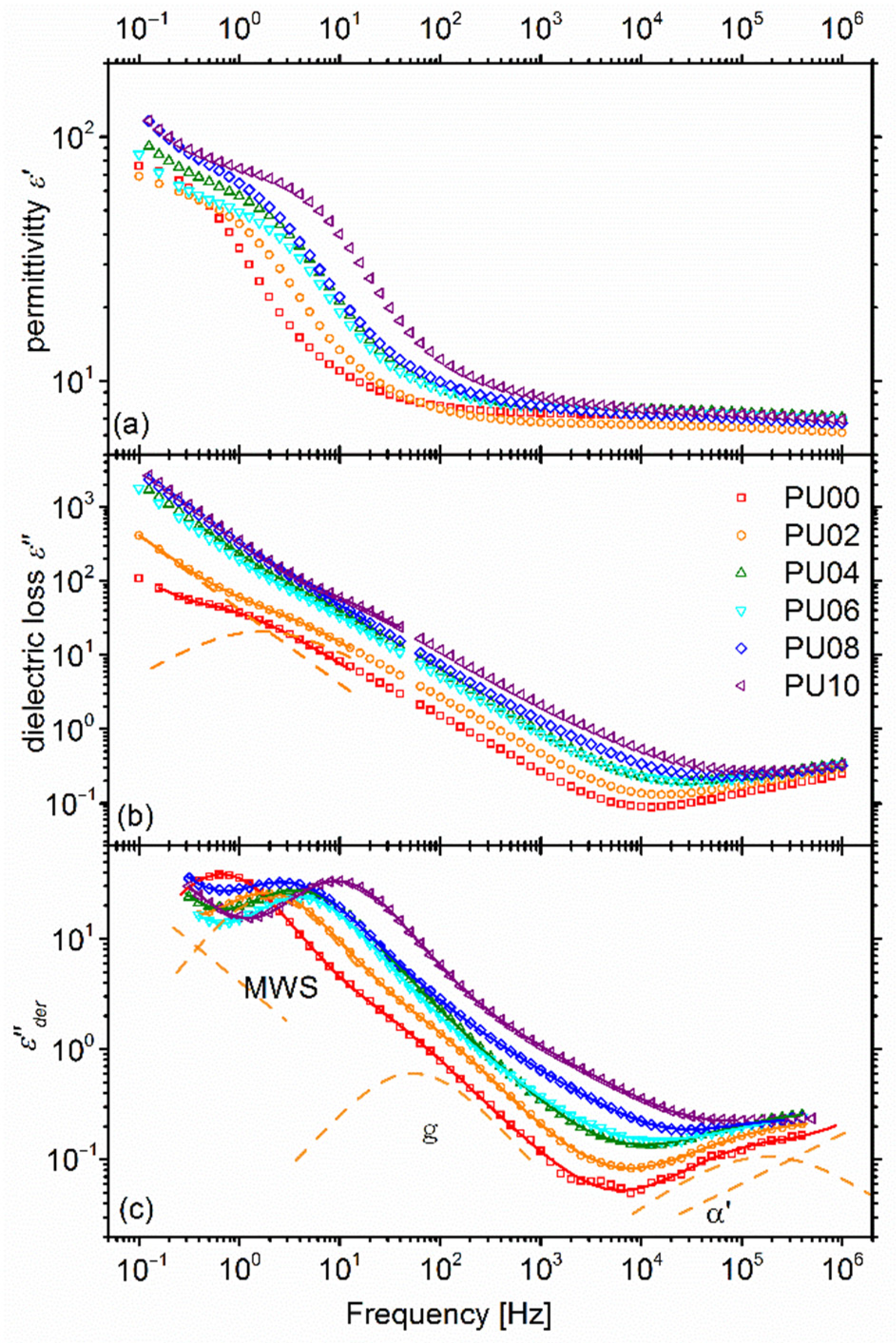
2.5.1. Overall Behaviour
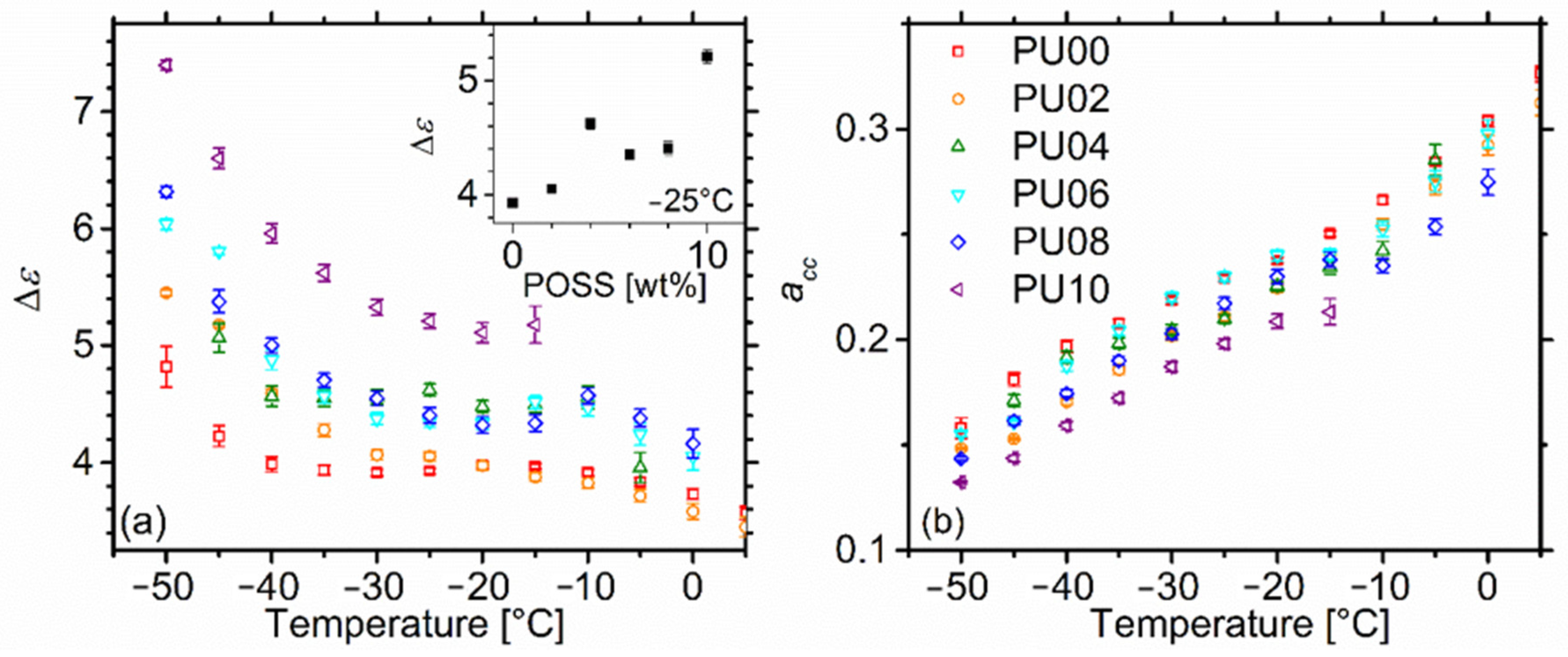
2.5.2. Dynamic Glass Transition—α Relaxation
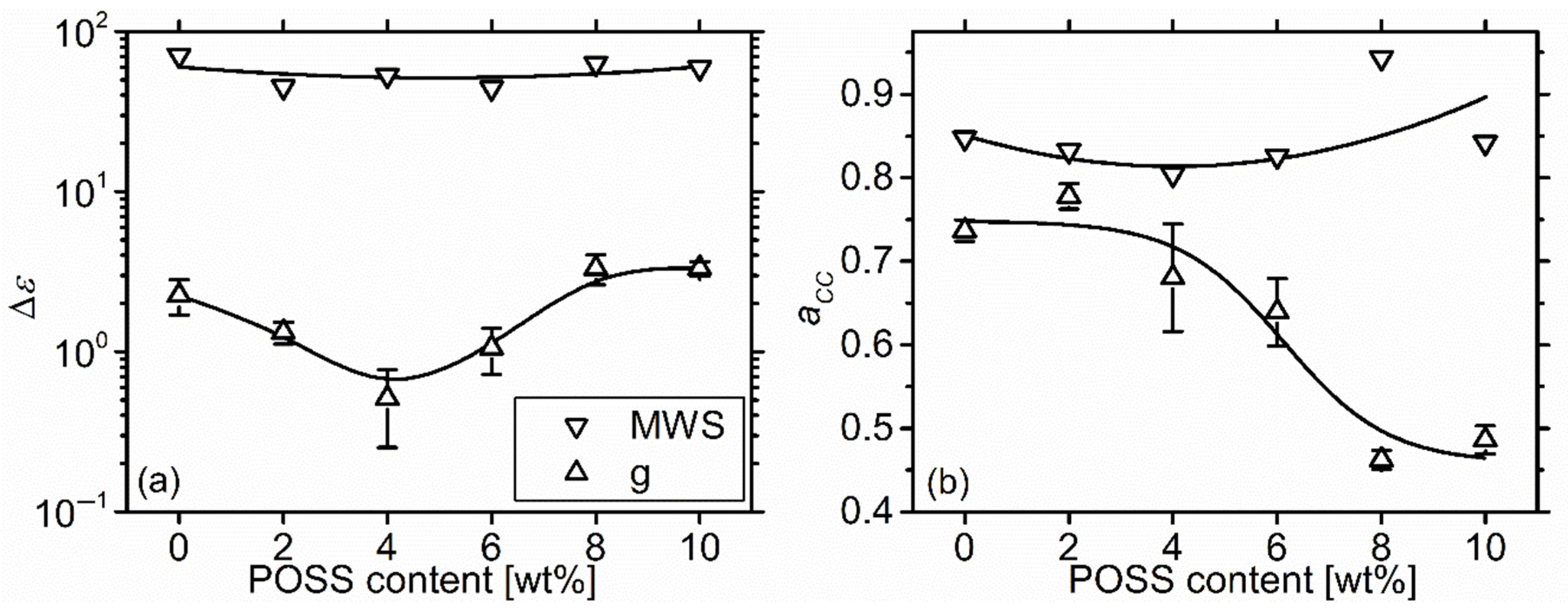
2.5.3. Higher Temperature Relaxations—Maxwell-Wagner-Sillars Process
2.5.4. Dc Conductivity
3. Discussion
4. Materials and Methods
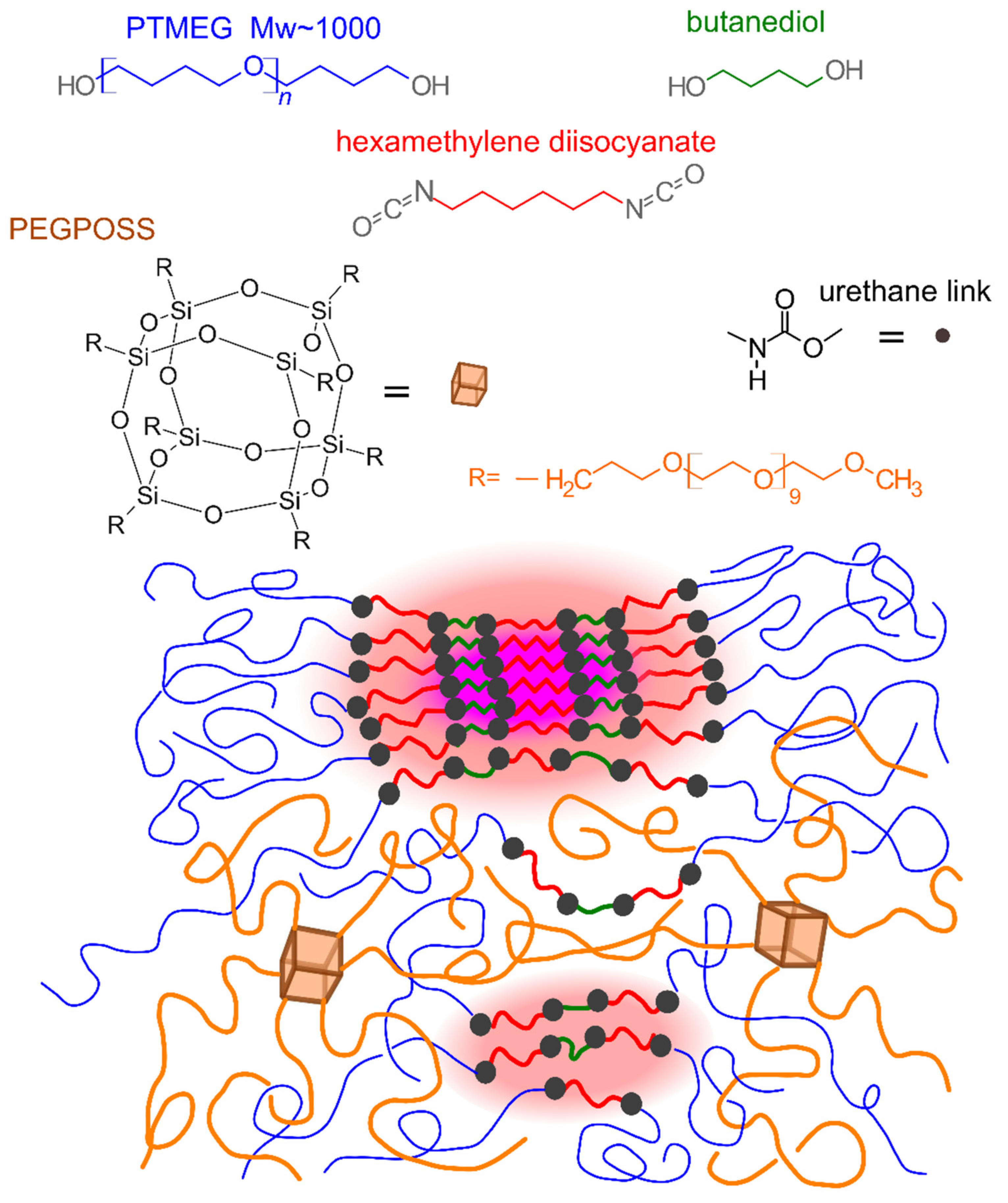
4.1. Materials
4.2. Synthesis
4.3. Scanning Electron Microscopy
4.4. Atomic Force Microscopy
4.5. X-ray Diffraction
4.6. Differential Scanning Calorimetry
4.7. Dielectric Relaxation Spectroscopy
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Raftopoulos, K.N.; Pielichowski, K. Segmental dynamics in hybrid polymer/POSS nanomaterials. Prog. Polym. Sci. 2016, 52, 136–187. [Google Scholar] [CrossRef]
- Hao, N.; Böhning, M.; Goering, H.; Schönhals, A.; Bo, M.; Scho, A. Nanocomposites of Polyhedral Oligomeric Phenethylsilsesquioxanes and Poly(bisphenol A carbonate) as Investigated by Dielectric Spectroscopy. Macromolecules 2007, 40, 2955–2964. [Google Scholar] [CrossRef]
- Xavier Perrin, F.; Viet Nguyen, T.B.; Margaillan, A. Linear and branched alkyl substituted octakis(dimethylsiloxy)octasilsesquioxanes: WAXS and thermal properties. Eur. Polym. J. 2011, 47, 1370–1382. [Google Scholar] [CrossRef]
- Tegou, E.; Bellas, V.; Gogolides, E.; Argitis, P. Polyhedral oligomeric silsesquioxane (POSS) acrylate copolymers for microfabrication: Properties and formulation of resist materials. Microelectron. Eng. 2004, 73–74, 238–243. [Google Scholar] [CrossRef]
- PG1190—PEG POSS Cage Mixture—Hybrid. Available online: https://hybridplastics.com/product/https-hybridplastics-com-wp-content-uploads-2019-06-pg11903-pdf/ (accessed on 29 November 2020).
- Wang, M.; Xing, R.; Wu, H.; Pan, F.; Zhang, J.; Ding, H.; Jiang, Z. Nanocomposite membranes based on alginate matrix and high loading of pegylated POSS for pervaporation dehydration. J. Membr. Sci. 2017, 538, 86–95. [Google Scholar] [CrossRef]
- Johnson, T.J.; Gupta, K.M.; Fabian, J.; Albright, T.H.; Kiser, P.F. Segmented polyurethane intravaginal rings for the sustained combined delivery of antiretroviral agents dapivirine and tenofovir. Eur. J. Pharm. Sci. 2010, 39, 203–212. [Google Scholar] [CrossRef]
- Gupta, D.; Madhukar, A.; Choudhary, V. Effect of functionality of polyhedral oligomeric silsesquioxane [POSS] on the properties of sulfonated poly(ether ether ketone) [SPEEK] based hybrid nanocomposite proton exchange membranes for fuel cell applications. Int. J. Hydrogen Energy 2013, 38, 12817–12829. [Google Scholar] [CrossRef]
- Rahman, M.M.; Filiz, V.; Shishatskiy, S.; Abetz, C.; Neumann, S.; Bolmer, S.; Khan, M.M.; Abetz, V. PEBAX® with PEG functionalized POSS as nanocomposite membranes for CO2 separation. J. Membr. Sci. 2013, 437, 286–297. [Google Scholar] [CrossRef] [Green Version]
- You, X.; Ma, T.; Su, Y.; Wu, H.; Wu, M.; Cai, H.; Sun, G.; Jiang, Z. Enhancing the permeation flux and antifouling performance of polyamide nanofiltration membrane by incorporation of PEG-POSS nanoparticles. J. Membr. Sci. 2017, 540, 454–463. [Google Scholar] [CrossRef]
- Rahman, M.M.; Filiz, V.; Shishatskiy, S.; Abetz, C.; Georgopanos, P.; Khan, M.M.; Neumann, S.; Abetz, V. Influence of Poly(ethylene glycol) Segment Length on CO2 Permeation and Stability of PolyActive Membranes and Their Nanocomposites with PEG POSS. ACS Appl. Mater. Interfaces 2015, 7, 12289–12298. [Google Scholar] [CrossRef]
- Jung, C.H.; Hwang, I.T.; Jung, C.H.; Choi, J.H. Preparation of flexible PLA/PEG-POSS nanocomposites by melt blending and radiation crosslinking. Radiat. Phys. Chem. 2014, 102, 23–28. [Google Scholar] [CrossRef]
- Lungova, M.; Krutyeva, M.; Pyckhout-Hintzen, W.; Wischnewski, A.; Monkenbusch, M.; Allgaier, J.; Ohl, M.; Sharp, M.; Richter, D. Nanoscale Motion of Soft Nanoparticles in Unentangled and Entangled Polymer Matrices. Phys. Rev. Lett. 2016, 117, 147803. [Google Scholar] [CrossRef] [Green Version]
- Miller, J.A.; Lin, S.B.; Hwang, K.K.S.; Wu, K.S.; Gibson, P.E.; Cooper, S.L. Properties of polyether-polyurethane block copolymers: Effects of hard segment length distribution. Macromolecules 1985, 18, 32–44. [Google Scholar] [CrossRef]
- Abouzahr, S.; Wilkes, G.L.; Ophir, Z. Structure-property behaviour of segmented polyether-MDI-butanediol based urethanes: Effect of composition ratio. Polymer 1982, 23, 1077–1086. [Google Scholar] [CrossRef]
- Koberstein, J.T.; Russell, T.P. Simultaneous SAXS-DSC Study of Multiple Endothermic Behavior in Polyether-Based Polyurethane Block Copolymers. Macromolecules 1986, 19, 714–720. [Google Scholar]
- Koberstein, J.T.; Leung, L.M. Compression-Molded Polyurethane Block Copolymers. 2. Evaluation of Microphase Compositions. Macromolecules 1992, 25, 6205–6213. [Google Scholar] [CrossRef]
- Leung, L.M.; Koberstein, J.T. Small-angle scattering analysis of hard-microdomain structure and microphase mixing in polyurethane elastomers. J. Polym. Sci. Polym. Phys. Ed. 1985, 23, 1883–1913. [Google Scholar]
- Koberstein, J.T.; Stein, R.S. Small-angle X-ray scattering studies of microdomain structure in segmented polyurethane elastomers. J. Polym. Sci. Polym. Phys. Ed. 1983, 21, 1439–1472. [Google Scholar] [CrossRef]
- Leung, L.M.; Koberstein, J.T. DSC Annealing Study of Microphase Separation and Multiple Endothermic Behavior in Polyether-Based Polyurethane Block Copolymers. Macromolecules 1986, 19, 706–713. [Google Scholar] [CrossRef]
- Raftopoulos, K.N.; Janowski, B.; Apekis, L.; Pielichowski, K.; Pissis, P. Molecular mobility and crystallinity in polytetramethylene ether glycol in the bulk and as soft component in polyurethanes. Eur. Polym. J. 2011, 47, 2120–2133. [Google Scholar] [CrossRef]
- Szefer, E.; Stafin, K.; Leszczyńska, A.; Zając, P.; Hebda, E.; Raftopoulos, K.N.; Pielichowski, K. Morphology, dynamics, and order development in a thermoplastic polyurethane with melt blended POSS. J. Polym. Sci. Part B Polym. Phys. 2019, 57, 1133–1142. [Google Scholar] [CrossRef]
- Nanda, A.K.; Wicks, D.A.; Madbouly, S.A.; Otaigbe, J.U. Nanostructured Polyurethane/POSS Hybrid Aqueous Dispersions Prepared by Homogeneous Solution Polymerization. Macromolecules 2006, 39, 7037–7043. [Google Scholar] [CrossRef]
- Madbouly, S.A.; Otaigbe, J.U.; Nanda, A.K.; Wicks, D.A. Rheological behavior of POSS/polyurethane-urea nanocomposite films prepared by homogeneous solution polymerization in aqueous dispersions. Macromolecules 2007, 40, 4982–4991. [Google Scholar]
- Raftopoulos, K.N.; Janowski, B.; Apekis, L.; Pissis, P.; Pielichowski, K. Direct and indirect effects of POSS on the molecular mobility of Polyurethanes with varying segment Mw. Polymer 2013, 54, 2745–2754. [Google Scholar] [CrossRef]
- Raftopoulos, K.N.; Pandis, C.; Apekis, L.; Pissis, P.; Janowski, B.; Pielichowski, K.; Jaczewska, J. Polyurethane-POSS hybrids: Molecular dynamics studies. Polymer 2010, 51, 709–718. [Google Scholar] [CrossRef]
- Janowski, B.; Pielichowski, K. Thermo (oxidative) stability of novel polyurethane/POSS nanohybrid elastomers. Thermochim. Acta 2008, 478, 51–53. [Google Scholar] [CrossRef]
- Raftopoulos, K.N.; Jancia, M.; Aravopoulou, D.; Hebda, E.; Pielichowski, K.; Pissis, P. POSS along the Hard Segments of Polyurethane. Phase Separation and Molecular Dynamics. Macromolecules 2013, 46, 7378–7386. [Google Scholar] [CrossRef]
- Raftopoulos, K.N.; Koutsoumpis, S.; Jancia, M.; Lewicki, J.P.J.P.; Kyriakos, K.; Mason, H.E.; Harley, S.J.S.J.; Hebda, E.; Papadakis, C.M.C.M.; Pielichowski, K.; et al. Reduced Phase Separation and Slowing of Dynamics in Polyurethanes with Three-Dimensional POSS-Based Cross-Linking Moieties. Macromolecules 2015, 48, 1429–1441. [Google Scholar] [CrossRef]
- Monticelli, O.; Fina, A.; Cavallo, D.; Gioffredi, E.; Delprato, G. On a novel method to synthesize POSS-based hybrids: An example of the preparation of TPU based system. Express Polym. Lett. 2013, 7, 966–973. [Google Scholar] [CrossRef] [Green Version]
- Fina, A.; Monticelli, O.; Camino, G. POSS-based hybrids by melt/reactive blending. J. Mater. Chem. 2010, 20, 9297. [Google Scholar] [CrossRef]
- Koutsoumpis, S.; Raftopoulos, K.N.; Jancia, M.; Pagacz, J.; Hebda, E.; Papadakis, C.M.; Pielichowski, K.; Pissis, P. POSS moieties with PEG vertex groups as diluent in Polyurethane elastomers: Morphology and phase separation. Macromolecules 2016, 49, 6507–6517. [Google Scholar] [CrossRef]
- Majka, T.M.; Raftopoulos, K.N.; Pielichowski, K. The influence of POSS nanoparticles on selected thermal properties of polyurethane-based hybrids. J. Therm. Anal. Calorim. 2017. [Google Scholar] [CrossRef] [Green Version]
- Fox, T. Influence of diluent and of copolymer composition on the glass temperature of a polymer system. Bull. Am. Phys. Soc. 1956, 1, 123–132. [Google Scholar]
- Koutsoumpis, S.; Ozimek, J.; Raftopoulos, K.N.; Hebda, E.; Klonos, P.; Papadakis, C.M.; Pielichowski, K.; Pissis, P. Polyurethanes with POSS pendent on flexible hard segments: Morphology and glass transition. Polymer 2018, 147, 225–236. [Google Scholar] [CrossRef]
- Fragiadakis, D.; Pissis, P.; Bokobza, L. Glass transition and molecular dynamics in poly (dimethylsiloxane)/silica nanocomposites. Polymer 2005, 46, 6001–6008. [Google Scholar] [CrossRef]
- Sargsyan, A.; Tonoyan, A.; Davtyan, S.; Schick, C. The amount of immobilized polymer in PMMA SiO2 nanocomposites determined from calorimetric data. Eur. Polym. J. 2007, 43, 3113–3127. [Google Scholar] [CrossRef]
- Koberstein, J.T.; Galambos, A.F. Multiple melting in segmented polyurethane block copolymers. Macromolecules 1992, 25, 5618–5624. [Google Scholar] [CrossRef]
- Yang, J.H.; Chun, B.C.; Chung, Y.-C.; Cho, J.H. Comparison of thermal/mechanical properties and shape memory effect of polyurethane block-copolymers with planar or bent shape of hard segment. Polymer 2003, 44, 3251–3258. [Google Scholar] [CrossRef]
- Hernandez, R.; Weksler, J.; Padsalgikar, A.; Runt, J. Microstructural Organization of Three-Phase Polydimethylsiloxane-Based Segmented Polyurethanes. Macromolecules 2007, 40, 5441–5449. [Google Scholar]
- Pangon, A.; Dillon, G.P.; Runt, J. Influence of mixed soft segments on microphase separation of polyurea elastomers. Polymer 2014, 55, 1837–1844. [Google Scholar] [CrossRef]
- Janowski, B.; Pielichowski, K.; Kwiatkowski, R. Uklady nanohybrydowe poliuretan (PUR)/funkcjonalizo-wany silseskwioksan (PHIPOSS). Cz. II. Rentgenowskie badania strukturalne metodamiWAXD i SAXS. Polimery 2014, 59, 44–56. [Google Scholar] [CrossRef]
- Hojabri, L.; Kong, X.; Narine, S.S. Fatty acid-derived diisocyanate and biobased polyurethane produced from vegetable oil: Synthesis, polymerization, and characterization. Biomacromolecules 2009, 10, 884–891. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.K.; Shieh, T.S.; Chui, J.Y. Studies on the first DSC endotherm of polyurethane hard segment based on 4,4′-diphenylmethane diisocyanate and 1,4-butanediol. Macromolecules 1998, 31, 1312–1320. [Google Scholar] [CrossRef]
- Chen, T.K.; Chui, J.Y.; Shieh, T.S. Glass Transition Behaviors of a Polyurethane Hard Segment based on 4, 4-Diisocyanatodiphenylmethane and 1, 4-Butanediol and the Calculation of Microdomain Composition. Macromolecules 1997, 30, 5068–5074. [Google Scholar] [CrossRef]
- Pissis, P.; Apekis, L.; Christodoulides, C.; Niaounakis, M.; Kyritsis, A.; Nedbal, J. Water effects in polyurethane block copolymers. J. Polym. Sci. Part B Polym. Phys. 1996, 34, 1529–1539. [Google Scholar] [CrossRef]
- Georgoussis, G.; Kyritsis, A.; Bershtein, V.A.; Fainleib, A.M.; Pissis, P. Dielectric studies of chain dynamics in homogeneous semi-interpenetrating polymer networks. J. Polym. Sci. Part B Polym. Phys. 2000, 38, 3070–3087. [Google Scholar] [CrossRef]
- Savelyev, Y.V.; Akhranovich, E.R.; Grekov, A.P.; Privalko, E.G.; Korskanov, V.V.; Shtompel, V.I.; Privalko, V.P.; Pissis, P.; Kanapitsas, A. Influence of chain extenders and chain end groups on properties of segmented polyurethanes. II. Dielectric study. Polymer 1998, 39, 3425–3429. [Google Scholar] [CrossRef]
- Hamon, B.V. Maxwell-Wagner Loss and Absorption Currents in Dielectrics. Aust. J. Phys. 1953, 6, 304. [Google Scholar] [CrossRef]
- Fragiadakis, D.; Runt, J. Molecular Dynamics of Segmented Polyurethane Copolymers: In fl uence of Soft Segment Composition. Macromolecules 2013, 46, 4184–4190. [Google Scholar] [CrossRef]
- Cole, K.S.; Cole, R.H. Dispersion and absorption in dielectrics I. Alternating current characteristics. J. Chem. Phys. 1941, 9, 341. [Google Scholar]
- Vassiliadou, O.; Chrysostomou, V.; Pispas, S.; Klonos, P.A.; Kyritsis, A. Molecular Dynamics and Crystallization in Polymers based on Ethylene Glycol Methacrylates (EGMA) with Melt Memory Characteristics: From Linear Oligomers to Comb-like Polymers. Soft Matter 2021. [Google Scholar] [CrossRef] [PubMed]
- Angell, C.A. Relaxation in liquids, polymers and plastic crystals-strong/fragile patterns and problems. J. Non. Cryst. Solids 1991, 131, 13–31. [Google Scholar] [CrossRef]
- Kremer, F.; Schönhals, A. Broadband Dielectric Spectroscopy; Kremer, F., Schönhals, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2003; ISBN 9783540434078. Chapter 4; p. 99. [Google Scholar]
- Bershtein, V.A.; Egorova, L.M.; Yakushev, P.N.; Pissis, P.; Sysel, P.; Brozova, L. Molecular dynamics in nanostructured polyimide-silica hybrid materials and their thermal stability. J. Polym. Sci. Part B Polym. Phys. 2002, 40, 1056–1069. [Google Scholar] [CrossRef]
- Wübbenhorst, M.; van Turnhout, J. Analysis of complex dielectric spectra. I. One-dimensional derivative techniques and three-dimensional modelling. J. Non. Cryst. Solids 2002, 305, 40–49. [Google Scholar] [CrossRef]
- Balko, J.; Fernández-d’Arlas, B.; Pöselt, E.; Dabbous, R.; Müller, A.J.; Thurn-Albrecht, T. Clarifying the Origin of Multiple Melting of Segmented Thermoplastic Polyurethanes by Fast Scanning Calorimetry. Macromolecules 2017, 50, 7672–7680. [Google Scholar] [CrossRef]
- Tocha, E.; Janik, H.; Debowski, M.; Vancso, G.J. Morphology of polyurethanes revisited by complementary AFM and TEM. J. Macromol. Sci. Part B 2002, 41, 1291–1304. [Google Scholar]
- Aneja, A.; Wilkes, G.L. A systematic series of model “PTMO” based segmented polyurethanes reinvestigated using atomic force microscopy. Polymer 2003, 44, 7221–7228. [Google Scholar] [CrossRef]
- Fernandez-D’Arlas, B.; Eceiza, A. Structure-property relationship in high urethane density polyurethanes. J. Polym. Sci. Part B Polym. Phys. 2016, 54, 739–746. [Google Scholar] [CrossRef]
- Castagna, A.M.; Fragiadakis, D.; Lee, H.; Choi, T.; Runt, J. The Role of Hard Segment Content on the Molecular Dynamics of Poly(tetramethylene oxide)-Based Polyurethane Copolymers. Macromolecules 2011, 44, 7831–7836. [Google Scholar] [CrossRef]
- Klonos, P.; Pissis, P. Effects of interfacial interactions and of crystallization on rigid amorphous fraction and molecular dynamics in polylactide/silica nanocomposites: A methodological approach. Polymer 2017, 112, 228–243. [Google Scholar] [CrossRef]
- Klonos, P.; Kripotou, S.; Kyritsis, A.; Papageorgiou, G.Z.; Bikiaris, D.; Gournis, D.; Pissis, P. Glass transition and segmental dynamics in poly(l-lactic acid)/graphene oxide nanocomposites. Thermochim. Acta 2015, 617, 44–53. [Google Scholar] [CrossRef]
- Raftopoulos, K.N.; Łukaszewska, I.; Klonos, P.A.; Hebda, E.; Bukowczan, A.; Kyritsis, A.; Pielichowski, K. Molecular and charge mobility of a poloxamer in the bulk and as soft component in polyurethanes. Polymer 2019, 182, 121821. [Google Scholar] [CrossRef]





| POSS Content [wt%] | α Relaxation | dc Conductivity | ||||
|---|---|---|---|---|---|---|
| log(f∞/Hz) | D | T0 [K] | log(σ∞/[S/cm]) | D | T0 [K] | |
| 0 | 10.6 ± 0.5 | 5.2 ± 0.9 | 184 ± 4 | - | - | - |
| 2 | 11.1 ± 0.5 | 5.9 ± 0.9 | 184 ± 3 | −7.7 ± 0.1 | 3.7 ± 0.4 | 213 ± 4 |
| 4 | 11.5 ± 0.6 | 6.7 ± 1.2 | 178 ± 5 | −6.8 ± 0.3 | 5.6 ± 0.7 | 192 ± 5 |
| 6 | 10.7 ± 0.5 | 5.0 ± 0.7 | 186 ± 3 | −4.6 ± 1.0 | 19 ± 9 | 134 ± 24 |
| 8 | 10.3 ± 0.5 | 4.7 ± 0.6 | 188 ± 3 | −5.1 ± 0.3 | 14 ± 2 | 142 ± 9 |
| 10 | 10.5 ± 0.5 | 4.7 ± 0.7 | 189 ± 3 | −5.7 ± 0.2 | 9.6 ± 1.3 | 161 ± 7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raftopoulos, K.N.; Hebda, E.; Grzybowska, A.; Klonos, P.A.; Kyritsis, A.; Pielichowski, K. PEG-POSS Star Molecules Blended in Polyurethane with Flexible Hard Segments: Morphology and Dynamics. Molecules 2021, 26, 99. https://doi.org/10.3390/molecules26010099
Raftopoulos KN, Hebda E, Grzybowska A, Klonos PA, Kyritsis A, Pielichowski K. PEG-POSS Star Molecules Blended in Polyurethane with Flexible Hard Segments: Morphology and Dynamics. Molecules. 2021; 26(1):99. https://doi.org/10.3390/molecules26010099
Chicago/Turabian StyleRaftopoulos, Konstantinos N., Edyta Hebda, Anna Grzybowska, Panagiotis A. Klonos, Apostolos Kyritsis, and Krzysztof Pielichowski. 2021. "PEG-POSS Star Molecules Blended in Polyurethane with Flexible Hard Segments: Morphology and Dynamics" Molecules 26, no. 1: 99. https://doi.org/10.3390/molecules26010099







