Solar Driven Photocatalytic Activity of Porphyrin Sensitized TiO2: Experimental and Computational Studies
Abstract
:1. Introduction
2. Results and Discussion
2.1. Electronic Absorption Spectra of PP1 and PP2 and Their Metal Complexes
2.2. Photo-Physical Characterization of PP1, PP2 and Their Metal Complexes
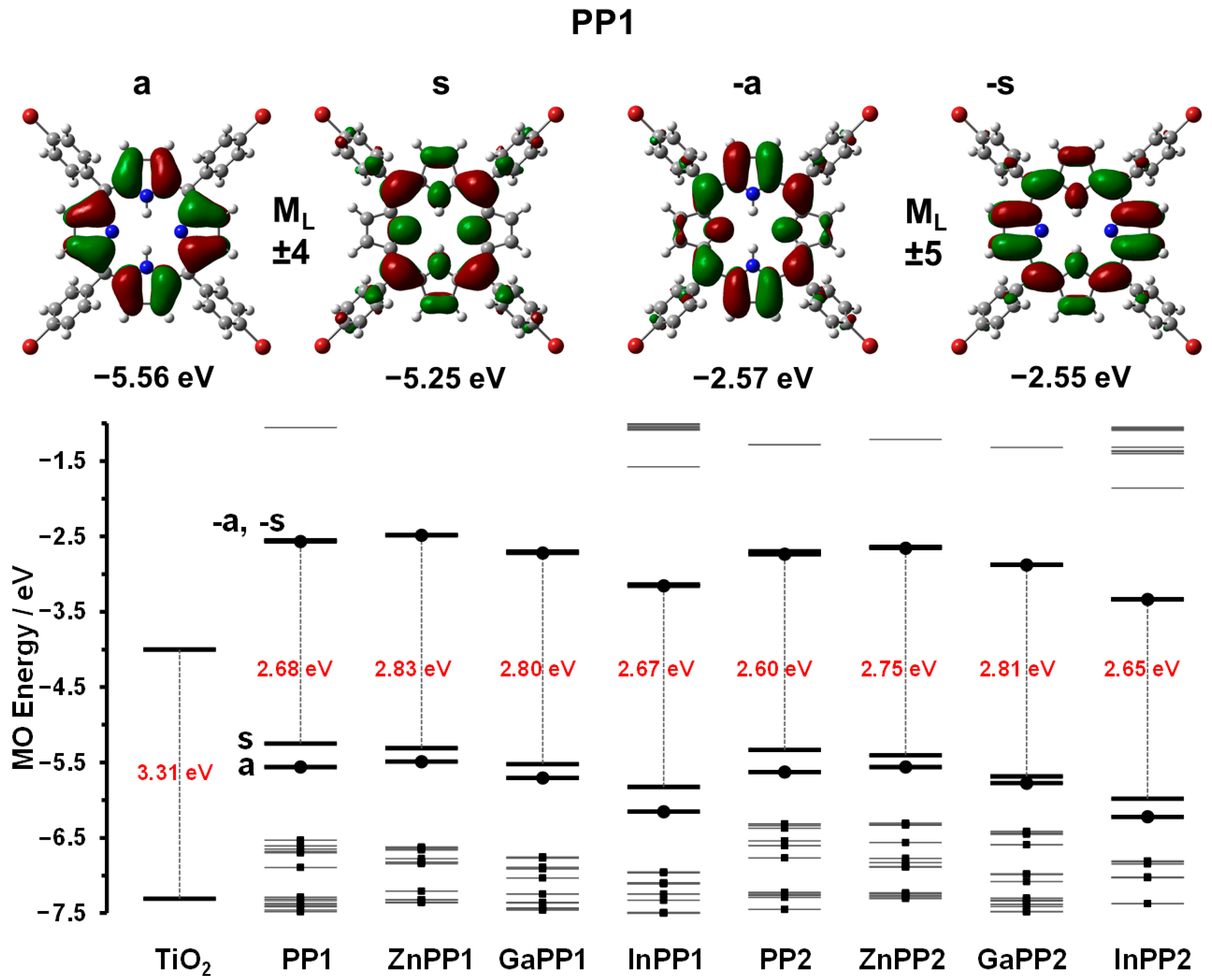
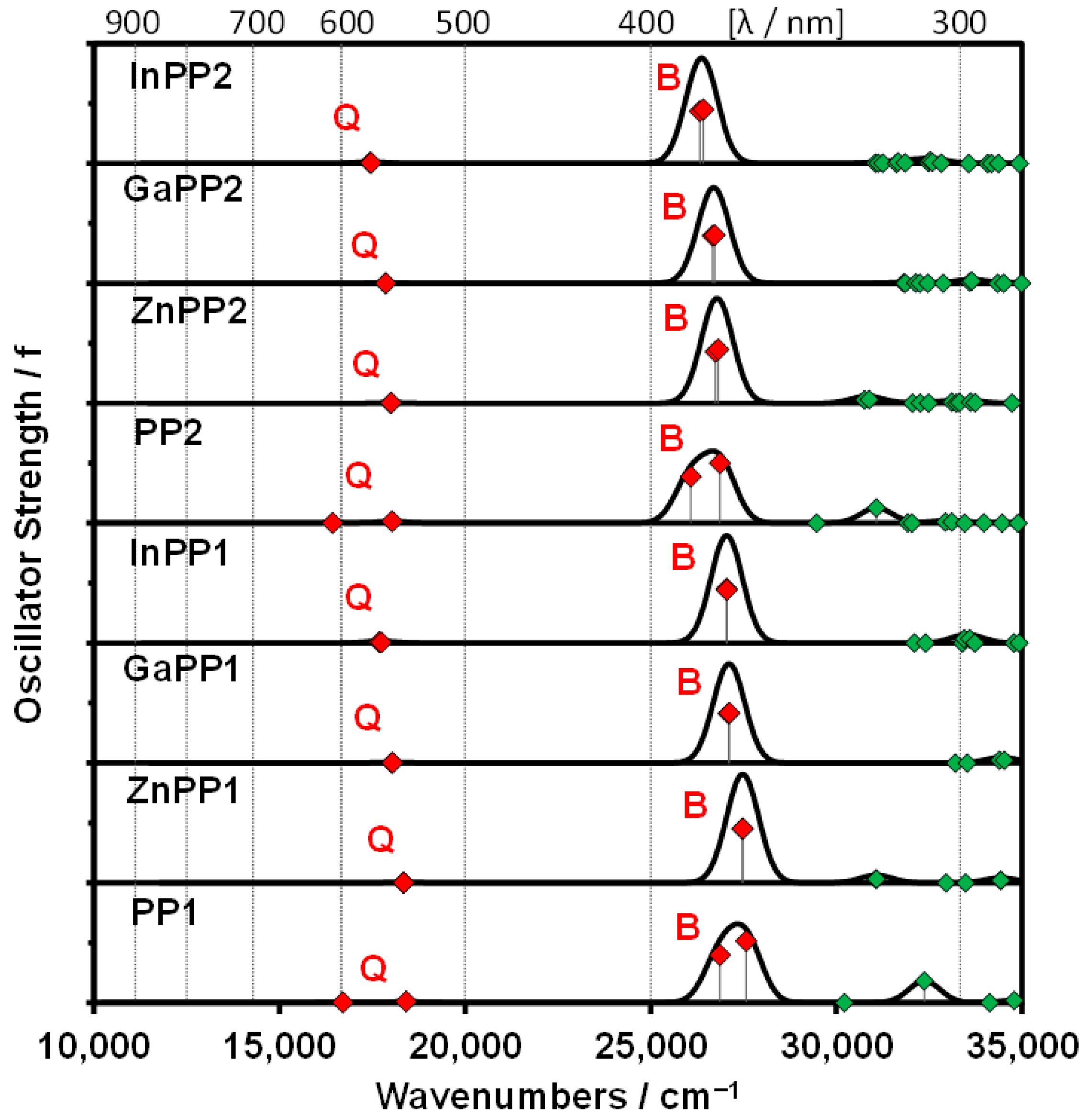
2.3. Density Function Theory Calculations
2.4. Suitability for Electron Injection
2.5. Characterization of the Porphyrin-TiO2 (P-TiO2) Photocatalysts by Diffuse Reflectance Spectroscopy
2.6. Photocatalytic Activity of the Photocatalysts
2.7. Mechanism of Photocatalysis
3. Materials and Methods
3.1. Materials
3.2. Instrumentation
3.3. Synthesis of Porphyrins
3.4. Photophysical Parameters
3.5. Theoretical Calculations
3.6. Supporting Porphyrins on TiO2 P25
3.7. Photocatalytic Hydrogen Generation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- British Petroleum Company. BP Statistical Review of World Energy; British Petroleum Company: London, UK, 1981. [Google Scholar]
- Perera, F. Pollution from Fossil-Fuel Combustion Is the Leading Environmental Threat to Global Pediatric Health and Equity: Solutions Exist. Int. J. Environ. Res. Public. Health 2017, 15, 16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dougherty, W.; Kartha, S.; Rajan, C.; Lazarus, M.; Bailie, A.; Runkle, B.; Fencl, A. Greenhouse Gas Reduction Benefits and Costs of a Large-Scale Transition to Hydrogen in the USA. Energy Policy 2009, 37, 56–67. [Google Scholar] [CrossRef]
- Hirscher, M. Handbook of Hydrogen Storage: New Materials for Future Energy Storage; John Wiley & Sons: Hoboken, NJ, USA, 2010; ISBN 978-3-527-62981-7. [Google Scholar]
- Teichmann, D.; Arlt, W.; Wasserscheid, P.; Freymann, R. A Future Energy Supply Based on Liquid Organic Hydrogen Carriers (LOHC). Energy Environ. Sci. 2011, 4, 2767. [Google Scholar] [CrossRef]
- Winter, C.-J.; Nitsch, J. Hydrogen as an Energy Carrier: Technologies, Systems, Economy; Springer Science & Business Media: Heidelberg, Germany, 2012; ISBN 978-3-642-61561-0. [Google Scholar]
- Dunn, S. Hydrogen Futures: Toward a Sustainable Energy System. Int. J. Hydrog. Energy 2002, 27, 235–264. [Google Scholar] [CrossRef]
- Balat, M. Potential Importance of Hydrogen as a Future Solution to Environmental and Transportation Problems. Int. J. Hydrog. Energy 2008, 33, 4013–4029. [Google Scholar] [CrossRef]
- Cecconi, B.; Manfredi, N.; Montini, T.; Fornasiero, P.; Abbotto, A. Dye-Sensitized Solar Hydrogen Production: The Emerging Role of Metal-Free Organic Sensitizers: Dye-Sensitized Solar Hydrogen Production: The Emerging Role of Metal-Free Organic Sensitizers. Eur. J. Org. Chem. 2016, 2016, 5194–5215. [Google Scholar] [CrossRef]
- Lanterna, A.E.; Scaiano, J.C. Photoinduced Hydrogen Fuel Production and Water Decontamination Technologies. Orthogonal Strategies with a Parallel Future? ACS Energy Lett. 2017, 2, 1909–1910. [Google Scholar] [CrossRef] [Green Version]
- Hainer, A.S.; Hodgins, J.S.; Sandre, V.; Vallieres, M.; Lanterna, A.E.; Scaiano, J.C. Photocatalytic Hydrogen Generation Using Metal-Decorated TiO2: Sacrificial Donors vs True Water Splitting. ACS Energy Lett. 2018, 3, 542–545. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.; Park, Y.; Park, H. Solar Hydrogen Production Coupled with the Degradation of a Dye Pollutant Using TiO2 Modified with Platinum and Nafion. Int. J. Photoenergy 2014, 2014, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.S.; Venkateswarlu, P.; Rao, V.R.; Rao, G.N. Synthesis, Characterization and Optical Properties of Zinc Oxide Nanoparticles. Int. Nano Lett. 2013, 3. [Google Scholar] [CrossRef] [Green Version]
- Tang, G.; Abas, A.; Wang, S. Photocatalytic Degradation and Hydrogen Production of TiO 2 /Carbon Fiber Composite Using Bast as a Carbon Fiber Source. Int. J. Photoenergy 2018, 2018, 1–8. [Google Scholar] [CrossRef]
- Rahimi, R.; Zargari, S.; Yousefi, A.; Yaghoubi Berijani, M.; Ghaffarinejad, A.; Morsali, A. Visible Light Photocatalytic Disinfection of E. Coli with TiO2–Graphene Nanocomposite Sensitized with Tetrakis(4-Carboxyphenyl)Porphyrin. Appl. Surf. Sci. 2015, 355, 1098–1106. [Google Scholar] [CrossRef]
- Yadav, L.S.R.; Manjunath, K.; Kavitha, C.; Nagaraju, G. An Investigation of Hydrogen Generation and Antibacterial Activity of TiO2 Nanoparticles Synthesized by the Ionic Liquid Aided Ionothermal Method. J. Sci. Adv. Mater. Devices 2018, 3, 181–187. [Google Scholar] [CrossRef]
- Daghrir, R.; Drogui, P.; Robert, D. Modified TiO2 for Environmental Photocatalytic Applications: A Review. Ind. Eng. Chem. Res. 2013, 52, 3581–3599. [Google Scholar] [CrossRef]
- Elhage, A.A.; Scaiano, J.C.; Lanterna, A.E. Dressing up for the occasion: The many faces of decorated titanium dioxide in photocatalysis. In Photoactive Inorganic Nanoparticles; Elsevier: Amsterdam, The Netherlands, 2019; pp. 73–108. ISBN 978-0-12-814531-9. [Google Scholar]
- Horng-Huey, K.; Chen, H.-T.; Yen, F.-L.; Lu, W.-C.; Kuo, C.-W.; Wang, M.-C. Preparation of TiO2 Nanocrystallite Powders Coated with 9 Mol% ZnO for Cosmetic Applications in Sunscreens. Int. J. Mol. Sci. 2012, 13, 1658–1669. [Google Scholar] [CrossRef] [Green Version]
- Yaron, P. Application of TiO2 Photocatalysis for Air Treatment: Patents’ Overview. Appl. Catal. B Environ. 2010, 99, 448–460. [Google Scholar] [CrossRef]
- Zhang, J.; Pan, M.; Luo, C.; Chen, X.; Kong, J.; Zhou, T. A Novel Composite Paint (TiO2/Fluorinated Acrylic Nanocomposite) for Antifouling Application in Marine Environments. J. Environ. Chem. Eng. 2016, 4, 2545–2555. [Google Scholar] [CrossRef]
- Dong, H.; Zeng, G.; Tang, L.; Fan, C.; Zhang, C.; He, X.; He, Y. An Overview on Limitations of TiO2-Based Particles for Photocatalytic Degradation of Organic Pollutants and the Corresponding Countermeasures. Water Res. 2015, 79, 128–146. [Google Scholar] [CrossRef]
- Khan, M.; Cao, W. Preparation of Y-Doped TiO2 by Hydrothermal Method and Investigation of Its Visible Light Photocatalytic Activity by the Degradation of Methylene Blue. J. Mol. Catal. Chem. 2013, 376, 71–77. [Google Scholar] [CrossRef]
- Senarathna, U.L.N.H.; Fernando, S.S.N.; Gunasekara, T.D.C.P.; Weerasekera, M.M.; Hewageegana, H.G.S.P.; Arachchi, N.D.H.; Siriwardena, H.D.; Jayaweera, P.M. Enhanced Antibacterial Activity of TiO2 Nanoparticle Surface Modified with Garcinia Zeylanica Extract. Chem. Cent. J. 2017, 11. [Google Scholar] [CrossRef] [Green Version]
- Schultz, D.M.; Yoon, T.P. Solar Synthesis: Prospects in Visible Light Photocatalysis. Science 2014, 343, 1239176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schneider, J.; Bahnemann, D.W. Undesired Role of Sacrificial Reagents in Photocatalysis. J. Phys. Chem. Lett. 2013, 4, 3479–3483. [Google Scholar] [CrossRef]
- Leung, D.Y.C.; Fu, X.; Wang, C.; Ni, M.; Leung, M.K.H.; Wang, X.; Fu, X. Hydrogen Production over Titania-Based Photocatalysts. ChemSusChem 2010, 3, 681–694. [Google Scholar] [CrossRef] [PubMed]
- Teh, C.M.; Mohamed, A.R. Roles of Titanium Dioxide and Ion-Doped Titanium Dioxide on Photocatalytic Degradation of Organic Pollutants (Phenolic Compounds and Dyes) in Aqueous Solutions: A Review. J. Alloys Compd. 2011, 509, 1648–1660. [Google Scholar] [CrossRef]
- Jafari, T.; Moharreri, E.; Amin, A.S.; Miao, R.; Song, W.; Suib, S.L. Photocatalytic Water Splitting-The Untamed Dream: A Review of Recent Advances. Mol. Basel Switz. 2016, 21, 900. [Google Scholar] [CrossRef]
- Altobello, S.; Bignozzi, C.A.; Caramori, S.; Larramona, G.; Quici, S.; Marzanni, G.; Lakhmiri, R. Sensitization of TiO2 with Ruthenium Complexes Containing Boronic Acid Functions. J. Photochem. Photobiol. Chem. 2004, 166, 91–98. [Google Scholar] [CrossRef]
- Karthikeyan, S.; Lee, J.Y. Zinc-Porphyrin Based Dyes for Dye-Sensitized Solar Cells. J. Phys. Chem. A 2013, 117, 10973–10979. [Google Scholar] [CrossRef]
- Kumar, P.R.; Britto, N.J.; Kathiravan, A.; Neels, A.; Jaccob, M.; Mothi, E.M. Synthesis and Electronic Properties of A 3 B-Thienyl Porphyrins: Experimental and Computational Investigations. New J. Chem. 2019, 43, 1569–1580. [Google Scholar] [CrossRef]
- Gouterman, M. Spectra of Porphyrins. J. Mol. Spectrosc. 1961, 6, 138–163. [Google Scholar] [CrossRef]
- Michl, J. Magnetic Circular Dichroism of Aromatic Molecules. Tetrahedron 1984, 40, 3845–3934. [Google Scholar] [CrossRef]
- Mack, J. Expanded, Contracted, and Isomeric Porphyrins: Theoretical Aspects. Chem. Rev. 2017, 117, 3444–3478. [Google Scholar] [CrossRef]
- Greco, J.A.; Rossi, A.; Birge, R.R.; Brückner, C. A Spectroscopic and Theoretical Investigation of a Free-Base Meso- Trithienylcorrole. Photochem. Photobiol. 2014, 90, 402–414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ojadi, E.C.A.; Linschitz, H.; Gouterman, M.; Walter, R.I.; Lindsey, J.S.; Wagner, R.W.; Droupadi, P.R.; Wang, W. Sequential Protonation of Meso-[p-(Dimethylamino)Phenyl]Porphyrins: Charge-Transfer Excited States Producing Hyperporphyrins. J. Phys. Chem. 1993, 97, 13192–13197. [Google Scholar] [CrossRef]
- Fonda, H.N.; Gilbert, J.V.; Cormier, R.A.; Sprague, J.R.; Kamioka, K.; Connolly, J.S. Spectroscopic, Photophysical, and Redox Properties of Some Meso-Substituted Free-Base Porphyrins. J. Phys. Chem. 1993, 97, 7024–7033. [Google Scholar] [CrossRef]
- Hayashi, S.; Inokuma, Y.; Osuka, A. Meso-Tris(Oligo-2,5-Thienylene)-Substituted Subporphyrins. Org. Lett. 2010, 12, 4148–4151. [Google Scholar] [CrossRef]
- Huang, X.; Nakanishi, K.; Berova, N. Porphyrins and Metalloporphyrins: Versatile Circular Dichroic Reporter Groups for Structural Studies. Chirality 2000, 12, 237–255. [Google Scholar] [CrossRef]
- Saenz, C.; Ethirajan, M.; Iacobucci, G.; Pandey, A.; Missert, J.R.; Dobhal, M.P.; Pandey, R.K. Indium as a Central Metal Enhances the Photosensitizing Efficacy of Benzoporphyrin Derivatives. J. Porphyr. Phthalocyanines 2011, 15, 1310–1316. [Google Scholar] [CrossRef] [PubMed]
- Kim, D. Multiporphyrin Arrays: Fundamentals and Applications; CRC Press: Boca Raton, FL, USA, 2012; ISBN 978-981-4364-28-7. [Google Scholar]
- Kumar, P.P.; Premaladha, G.; Maiya, B.G. Porphyrin-Anthraquinone Dyads: Synthesis, Spectroscopy and Photochemistry. J. Chem. Sci. 2005, 117, 193–201. [Google Scholar] [CrossRef] [Green Version]
- Sen, P.; Hirel, C.; Andraud, C.; Aronica, C.; Bretonniere, Y.; Mohammed, A.; Ågren, H.; Minaev, B.; Minaeva, V.; Baryshnikov, G.; et al. Fluorescence and FTIR Spectra Analysis of Trans-A(2)B(2)-Substituted Di- and Tetra-Phenyl Porphyrins. Materials 2010, 3, 4446–4475. [Google Scholar] [CrossRef]
- Figueiredo, T.L.C.; Johnstone, R.A.W.; Sørensen, A.M.P.S.; Burget, D.; Jacques, P. Determination of Fluorescence Yields, Singlet Lifetimes and Singlet Oxygen Yields of Water-Insoluble Porphyrins and Metalloporphyrins in Organic Solvents and in Aqueous Media. Photochem. Photobiol. 1999, 69, 517–528. [Google Scholar] [CrossRef]
- Gupta, I.; Ravikanth, M. Fluorescence Properties of Meso-Tetrafurylporphyrins. J. Chem. Sci. 2005, 117, 161–166. [Google Scholar] [CrossRef] [Green Version]
- Nyarko, E.; Hanada, N.; Habib, A.; Tabata, M. Fluorescence and Phosphorescence Spectra of Au(III), Pt(II) and Pd(II) Porphyrins with DNA at Room Temperature. Inorganica Chim. Acta 2004, 357, 739–745. [Google Scholar] [CrossRef]
- Zakavi, S.; Hoseini, S. The Absorption and Fluorescence Emission Spectra of Meso-Tetra(Aryl)Porphyrin Dications with Weak and Strong Carboxylic Acids: A Comparative Study. RSC Adv. 2015, 5, 106774–106786. [Google Scholar] [CrossRef]
- Managa, M.; Britton, J.; Amuhaya, E.K.; Nyokong, T. Photophysical Properties of GaCl 5,10,15,20-Tetra(1-Pyrenyl)Porphyrinato Incorporated into Pluronic F127 Micelle. J. Lumin. 2017, 185, 34–41. [Google Scholar] [CrossRef]
- Paredes-Gil, K.; Mendizabal, F.; Páez-Hernández, D.; Arratia-Pérez, R. Electronic Structure and Optical Properties Calculation of Zn-Porphyrin with N-Annulated Perylene Adsorbed on TiO2 Model for Dye-Sensitized Solar Cell Applications: A DFT/TD-DFT Study. Comput. Mater. Sci. 2017, 126, 514–527. [Google Scholar] [CrossRef]
- Santhanamoorthi, N.; Lo, C.-M.; Jiang, J.-C. Molecular Design of Porphyrins for Dye-Sensitized Solar Cells: A DFT/TDDFT Study. J. Phys. Chem. Lett. 2013, 4, 524–530. [Google Scholar] [CrossRef] [PubMed]
- Auwärter, W.; Écija, D.; Klappenberger, F.; Barth, J.V. Porphyrins at Interfaces. Nat. Chem. 2015, 7, 105–120. [Google Scholar] [CrossRef]
- Antolini, E. Photo-Assisted Methanol Oxidation on Pt-TiO2 Catalysts for Direct Methanol Fuel Cells: A Short Review. Appl. Catal. B Environ. 2018, 237, 491–503. [Google Scholar] [CrossRef]
- Kim, W.; Tachikawa, T.; Majima, T.; Li, C.; Kim, H.-J.; Choi, W. Tin-Porphyrin Sensitized TiO2 for the Production of H2 under Visible Light. Energy Environ. Sci. 2010, 3, 1789. [Google Scholar] [CrossRef] [Green Version]
- Phillips, K.R.; Jensen, S.C.; Baron, M.; Li, S.-C.; Friend, C.M. Sequential Photo-Oxidation of Methanol to Methyl Formate on TiO2 (110). J. Am. Chem. Soc. 2013, 135, 574–577. [Google Scholar] [CrossRef]
- Adler, A.D.; Longo, F.R.; Finarelli, J.D.; Goldmacher, J.; Assour, J.; Korsakoff, L. A Simplified Synthesis for Meso-Tetraphenylporphine. J. Org. Chem. 1967, 32, 476. [Google Scholar] [CrossRef]
- Lindsey, J.S.; Schreiman, I.C.; Hsu, H.C.; Kearney, P.C.; Marguerettaz, A.M. Rothemund and Adler-Longo Reactions Revisited: Synthesis of Tetraphenylporphyrins under Equilibrium Conditions. J. Org. Chem. 1987, 52, 827–836. [Google Scholar] [CrossRef]
- Hong, T.-N.; Sheu, Y.-H.; Jang, K.-W.; Chen, J.-H.; Wang, S.-S.; Wang, J.-C.; Wang, S.-L. A New Synthesis of Acetato Porphyrinato Indium(III) from Indium(III) Oxide and X-Ray Crystal Structures of In(Tpyp)(OAc) and In(Tmpp)(OAc). Polyhedron 1996, 15, 2647–2654. [Google Scholar] [CrossRef]
- Managa, M.; Ngoy, B.P.; Mafukidze, D.; Britton, J.; Nyokong, T. Photophysical Studies of Meso-Tetrakis(4-Nitrophenyl) and Meso-Tetrakis(4-Sulfophenyl) Gallium Porphyrins Loaded into Pluronic F127 Polymeric Micelles. J. Photochem. Photobiol. Chem. 2017, 348, 179–187. [Google Scholar] [CrossRef]
- Hu, Y.; Geissinger, P.; Woehl, J.C. Potential of Protoporphyrin IX and Metal Derivatives for Single Molecule Fluorescence Studies. J. Lumin. 2011, 131, 477–481. [Google Scholar] [CrossRef]
- Maiti, N.C.; Ravikanth, M. Effects of Non-Planarity and β-Substitution on the Singlet-Excited-State Properties of Basket-Handle Porphyrins. J. Chem Soc Faraday Trans. 1996, 92, 1095–1100. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision E. 01; Gaussian Inc.: Wallingford, CT, USA, 2015. [Google Scholar]
- Lundqvist, M.J.; Nilsing, M.; Persson, P.; Lunell, S. DFT Study of Bare and Dye-Sensitized TiO2 Clusters and Nanocrystals. Int. J. Quantum Chem. 2006, 106, 3214–3234. [Google Scholar] [CrossRef]
- Duan, M.; Li, J.; Mele, G.; Wang, C.; Lü, X.; Vasapollo, G.; Zhang, F. Photocatalytic Activity of Novel Tin Porphyrin/TiO2 Based Composites. J. Phys. Chem. C 2010, 114, 7857–7862. [Google Scholar] [CrossRef]


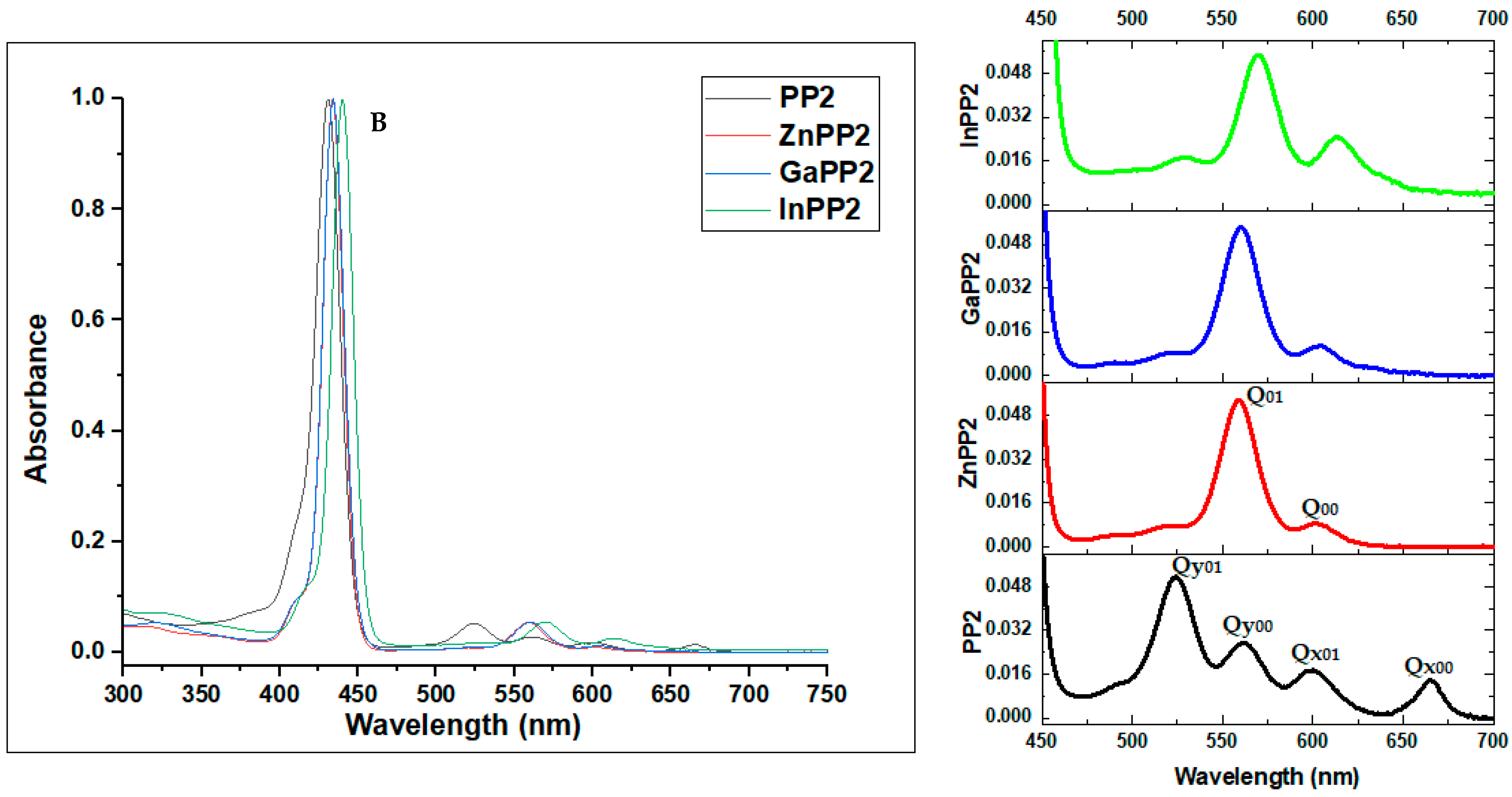


| Soret Band (nm) (ε × 105) (L mol−1 cm−1) | Q Bands (nm) (ε × 103) (L mol−1 cm−1) | ||||
| Qy01 | Qy00 | Qx01 | Qx00 | ||
| H2TPP | 421 | 515 | 549 | 591 | 649 |
| PP1 | 421 (2.1) | 517 (9.4) | 550 (4.5) | 593 (2.7) | 650 (2.1) |
| PP2 | 431 (2.6) | 524 (13.5) | 561 (7.0) | 600 (4.5) | 665 (3.7) |
| Soret Band (nm) (ε × 105) (L mol−1 cm−1) | Q Bands (nm) (ε × 103) (L mol−1 cm−1) | ||||
| Q01 | Q00 | ||||
| ZnPP1 | 425 (9.6) | 550 (52.8) | 589 (10.6) | ||
| InPP1 | 430 (5.4) | 563 (31.0) | 602 (13.2) | ||
| GaPP1 | 424 (6.9) | 553 (29.0) | 592 (6.2) | ||
| ZnPP2 | 433 (8.0) | 559 (51.9) | 602 (8.5) | ||
| InPP2 | 439 (3.0) | 570 (15.7) | 613 (6.6) | ||
| GaPP2 | 434 (3.9) | 561 (22.7) | 604 (4.7) | ||
| λem (nm) | Stokes Shift cm−1 | ΦF | (108 s−1) | (108 s−1) | |||
|---|---|---|---|---|---|---|---|
| PP1 | 654 | 717 (sh) | 8460 | 0.019 | 1.55 ± 0.02 | 0.123 | 6.33 |
| ZnPP1 | 600 (sh) | 649 | 8120 | 0.010 | 0.38 ± 0.01 | 0.255 | 26.1 |
| GaPP1 | 597 (sh) | 650 | 8200 | 0.013 | 0.48 ± 0.01 | 0.269 | 20.6 |
| InPP1 | 610 (sh) | 660 | 8100 | 0.006 | 0.24 ± 0.02 | 0.233 | 41.4 |
| PP2 | 670 | 730 (sh) | 8280 | 0.017 | 1.37 ± 0.02 | 0.124 | 7.18 |
| ZnPP2 | 621 (sh) | 659 | 7920 | 0.012 | 0.54 ± 0.01 | 0.219 | 18.3 |
| GaPP2 | 624 (sh) | 663 | 7960 | 0.013 | 0.53 ± 0.01 | 0.240 | 18.6 |
| InPP2 | 633 | 673 (sh) | 6980 | 0.010 | 0.29 ± 0.02 | 0.331 | 34.2 |
| Band a | # b | Calc. c | Exp. d | Wave Function e | ||||
|---|---|---|---|---|---|---|---|---|
| PP1 | Q | 1 | 16.7 | 599 | 0.01 | 15.4 | 650 | 59% s → −s; 40% a → −a; … |
| Q | 2 | 18.4 | 543 | 0.04 | 17.1 | 586 | 59% s → −a; 40% a → −s; … | |
| B | 3 | 26.9 | 372 | 1.44 | 23.7 | 421 | 57% a → −a; 32% s → −s; … | |
| B | 4 | 27.6 | 363 | 1.85 | 58% a → −s; 39% s → −a; … | |||
| ZnPP1 | Q | 1, 2 | 18.3 | 545 | 0.01 | 17.0 | 587 | 53% s → −a/−s; 46% a → −a/−s; … |
| B | 3, 4 | 27.5 | 364 | 1.63 | 23.5 | 425 | 46% a → −a/−s; 44% s → −a/−s; … | |
| GaPP1 | Q | 1, 2 | 18.0 | 554 | 0.01 | 17.0 | 589 | 50% s → −a/−s; 41% a → −a/−s; … |
| B | 3, 4 | 27.1 | 368 | 1.48 | 23.6 | 424 | 55% a → −a/−s; 42% s → −a/−s; … | |
| InPP1 | Q | 1, 2 | 17.7 | 565 | 0.03 | 16.6 | 601 | 60% s → −a/−s; 39% a → −a/−s; … |
| B | 3, 4 | 27.0 | 370 | 1.63 | 23.3 | 430 | 58% a → −a/−s; 37% s → −a/−s; … | |
| PP2 | Q | 1 | 16.4 | 609 | 0.01 | 15.0 | 665 | 57% s → −s; 42% a → −a; … |
| Q | 2 | 18.0 | 555 | 0.06 | 16.6 | 602 | 59% s → −a; 40% a → −s; … | |
| B | 3 | 26.1 | 383 | 1.40 | 23.2 | 431 | 54% a → −a; 34% s → −s; … | |
| B | 4 | 26.9 | 372 | 1.80 | 58% a → −s; 38% s → −a; … | |||
| ZnPP2 | Q | 1, 2 | 18.0 | 556 | 0.01 | 16.6 | 602 | 50% s → −a/−s; 48% a → −a/−s; … |
| B | 3, 4 | 26.7 | 374 | 1.54 | 23.1 | 433 | 49% a → −a/−s; 44% s → −a/−s; … | |
| GaPP2 | Q | 1, 2 | 17.9 | 560 | 0.00 | 16.6 | 603 | 50% s → −a/−s; 49% a → −a/−s; … |
| B | 3, 4 | 26.7 | 375 | 1.43 | 23.0 | 434 | 49% a → −a/−s; 48% s → −a/−s; … | |
| InPP2 | Q | 1, 2 | 17.4 | 573 | 0.02 | 16.2 | 616 | 55% s → −a/−s; 43% a → −a/−s; … |
| B | 3, 4 | 26.3 | 380 | 1.57 | 22.8 | 439 | 53% a → −a/−s; 40% s → −a/−s; … | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Otieno, S.; Lanterna, A.E.; Mack, J.; Derese, S.; Amuhaya, E.K.; Nyokong, T.; Scaiano, J.C. Solar Driven Photocatalytic Activity of Porphyrin Sensitized TiO2: Experimental and Computational Studies. Molecules 2021, 26, 3131. https://doi.org/10.3390/molecules26113131
Otieno S, Lanterna AE, Mack J, Derese S, Amuhaya EK, Nyokong T, Scaiano JC. Solar Driven Photocatalytic Activity of Porphyrin Sensitized TiO2: Experimental and Computational Studies. Molecules. 2021; 26(11):3131. https://doi.org/10.3390/molecules26113131
Chicago/Turabian StyleOtieno, Sebastian, Anabel E. Lanterna, John Mack, Solomon Derese, Edith K. Amuhaya, Tebello Nyokong, and Juan C. Scaiano. 2021. "Solar Driven Photocatalytic Activity of Porphyrin Sensitized TiO2: Experimental and Computational Studies" Molecules 26, no. 11: 3131. https://doi.org/10.3390/molecules26113131








