Impact of Crystal Habit on the Dissolution Rate and In Vivo Pharmacokinetics of Sorafenib Tosylate
Abstract
:1. Introduction
2. Results and Discussion
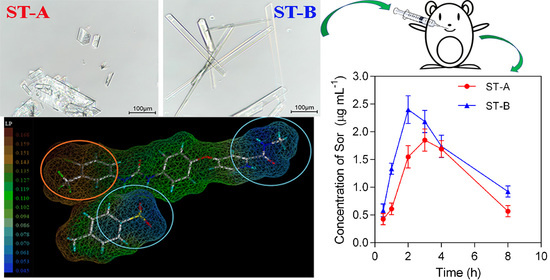
2.1. Morphology of Crystals
2.2. Differential Scanning Calorimetry (DSC)
2.3. Powder X-ray Diffraction (PXRD)
2.4. Face Indexation
2.5. Molecular Modeling
2.6. X-ray Photoelectron Spectroscopy (XPS)
2.7. Dissolution Rate of Sor-Tos Crystal Habit in Water and Gastric Juice pH 1.2 Acid Solution
2.8. In Vivo Pharmacokinetics
3. Materials and Methods
3.1. Materials and Chemicals
3.2. Crystallization Experiment
3.3. Characterization of Crystallized Solid Forms
3.3.1. Optical Microscopy
3.3.2. Differential Scanning Calorimetry (DSC)
3.3.3. Powder X-ray Diffraction (PXRD)
3.3.4. X-ray Photoelectron Spectroscopy (XPS)
3.3.5. Molecular Modeling
3.3.6. Face Indexation
3.4. Dissolution Rate Measurement
3.5. In Vivo Pharmacokinetics
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Tran, P.; Pyo, Y.-C.; Kim, D.-H.; Lee, S.-E.; Kim, J.-K.; Park, J.-S. Overview of the Manufacturing Methods of Solid Dispersion Technology for Improving the Solubility of Poorly Water-Soluble Drugs and Application to Anticancer Drugs. Pharmaceutics 2019, 11, 132. [Google Scholar] [CrossRef] [Green Version]
- Sathisaran, I.; Dalvi, S.V. Engineering Cocrystals of Poorly Water-Soluble Drugs to Enhance Dissolution in Aqueous Medium. Pharmaceutics 2018, 10, 108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poovi, G.; Damodharan, N. Lipid nanoparticles: A challenging approach for oral delivery of BCS Class-II drugs. Futur. J. Pharm. Sci. 2018, 4, 191–205. [Google Scholar] [CrossRef]
- Pauli, G.; Tang, W.-L.; Li, S.-D. Development and Characterization of the Solvent-Assisted Active Loading Technology (SALT) for Liposomal Loading of Poorly Water-Soluble Compounds. Pharmaceutics 2019, 11, 465. [Google Scholar] [CrossRef] [Green Version]
- Chen, F.; Fang, Y.; Zhao, R.; Le, J.; Zhang, B.; Huang, R.; Chen, Z.; Shao, J. Evolution in medicinal chemistry of sorafenib derivatives for hepatocellular carcinoma. Eur. J. Med. Chem. 2019, 179, 916–935. [Google Scholar] [CrossRef]
- Iyer, R.; Fetterly, G.; Lugade, A.; Thanavala, Y. Sorafenib: A clinical and pharmacologic review. Expert Opin. Pharmacother. 2010, 11, 1943–1955. [Google Scholar] [CrossRef] [PubMed]
- Porta, C.; Paglino, C.; Imarisio, I.; Ferraris, E. Sorafenib tosylate in advanced kidney cancer: Past, present and future. Anti-Cancer Drugs 2009, 20, 409–415. [Google Scholar] [CrossRef]
- Gadaleta-Caldarola, G.; Infusino, S.; Divella, R.; Mazzocca, A.; Rose, F.D.; Filippelli, G.; Brandi, M.; Ferraro, E.; Abbate, I. Sorafenib: 10 years after the first pivotal trial. Future Oncol. 2015, 11, 1863–1880. [Google Scholar] [CrossRef]
- Wang, X.-Q.; Fan, J.-M.; Liu, Y.-O.; Zhao, B.; Jia, Z.-R.; Zhang, Q. Bioavailability and pharmacokinetics of sorafenib suspension, nanoparticles and nanomatrix for oral administration to rat. Int. J. Pharm. 2011, 419, 339–346. [Google Scholar] [CrossRef]
- Jiang, S.; Qin, Y.; Wu, S.; Xu, S.; Li, K.; Yang, P.; Zhao, K.; Lin, L.; Gong, J. Solubility Correlation and Thermodynamic Analysis of Sorafenib Free Base and Sorafenib Tosylate in Monosolvents and Binary Solvent Mixtures. J. Chem. Eng. Data 2016, 62, 259–267. [Google Scholar] [CrossRef]
- Savjani, K.T.; Gajjar, A.K.; Savjani, J.K. ChemInform Abstract: Drug Solubility: Importance and Enhancement Techniques. ISRN Pharm. 2013, 44, 195727–195737. [Google Scholar] [CrossRef]
- Yang, G.; Kubota, N.; Sha, Z.; Louhi-Kultanen, A.M.; Wang, J. Crystal Shape Control by Manipulating Supersaturation in Batch Cooling Crystallization. Cryst. Growth Des. 2006, 6, 2799–2803. [Google Scholar] [CrossRef]
- Serrano, D.R.; O’Connell, P.; Paluch, K.J.; Walsh, D.; Healy, A.M. Cocrystal habit engineering to improve drug dissolution and alter derived powder properties. J. Pharm. Pharmacol. 2016, 68, 665–677. [Google Scholar] [CrossRef] [Green Version]
- Miletic, T.; Kyriakos, K.; Graovac, A.; Ibric, S. Spray-dried voriconazole–cyclodextrin complexes: Solubility, dissolution rate and chemical stability. Carbohydr. Polym. 2013, 98, 122–131. [Google Scholar] [CrossRef] [PubMed]
- Rasenack, N.; Müller, B.W. Micron-Size Drug Particles: Common and Novel Micronization Techniques. Pharm. Dev. Technol. 2004, 9, 1–13. [Google Scholar] [CrossRef]
- Sun, C.; Grant, D.J.W. Influence of crystal shape on the tableting performance of L-lysine monohydrochloride dihydrate. J. Pharm. Sci. 2001, 90, 569–579. [Google Scholar] [CrossRef]
- Banga, S.; Chawla, G.; Varandani, D.; Mehta, B.R.; Bansal, A.K. Modification of the crystal habit of celecoxib for improved processability. J. Pharm. Pharmacol. 2010, 59, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Tari, T.; Szabó-Révész, P.; Aigner, Z. Comparative Study of Different Crystallization Methods in the Case of Cilostazol Crystal Habit Optimization. Crystals 2019, 9, 295. [Google Scholar] [CrossRef] [Green Version]
- Laad, P.; Shete, G.; Modi, S.R.; Bansal, A.K. Differential surface properties of commercial crystalline telmisartan samples. Eur. J. Pharm. Sci. 2013, 49, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Modi, S.R.; Dantuluri, A.K.R.; Puri, V.; Pawar, Y.B.; Nandekar, P.; Sangamwar, A.T.; Perumalla, S.R.; Sun, C.C.; Bansal, A.K. Impact of Crystal Habit on Biopharmaceutical Performance of Celecoxib. Cryst. Growth Des. 2013, 13, 2824–2832. [Google Scholar] [CrossRef]
- Ren, Y.; Shen, J.; Yu, K.; Phan, C.U.; Chen, G.; Liu, J.; Hu, X.; Feng, J. Impact of Crystal Habit on Solubility of Ticagrelor. Crystals 2019, 9, 556. [Google Scholar] [CrossRef] [Green Version]
- Nokhodchi, A.; Bolourtchian, N.; Dinarvand, R. Dissolution and mechanical behaviors of recrystallized carbamazepine from alcohol solution in the presence of additives. J. Cryst. Growth 2005, 274, 573–584. [Google Scholar] [CrossRef]
- Kumar, D.; Thipparaboina, R.; Sreedhar, B.; Shastri, N.R. The role of surface chemistry in crystal morphology and its associated properties. CrystEngComm 2015, 17, 6646–6650. [Google Scholar] [CrossRef]
- Virupaxappa, B.S.; Shivaprasad, K.H.; Kulkani, R.M. Latha, Research Journal of Pharmaceutical, Biological and Chemical Sciences. Hydroll. Process. 2012, 26, 3393–3404. [Google Scholar]
- Katritzky, A.R.; Chen, K.; Wang, Y.; Karelson, M.; Lucic, B.; Trinajstic, N.; Suzuki, T.; Schuurmann, G. Prediction of liquid viscosity for organic compounds by a quantitative structure±property relationship. J. Phys. Org. Chem. 2000, 13, 80–86. [Google Scholar] [CrossRef]
- Yang, P.; Qin, C.; Du, S.; Jia, L.; Qin, Y.; Gong, J.; Wu, S. Crystal Structure, Stability and Desolvation of the Solvates of Sorafenib Tosylate. Crystals 2019, 9, 367. [Google Scholar] [CrossRef] [Green Version]
- Ravikumar, K.; Sridhar, B.; Rao, A.K.S.B.; Reddy, M.P. Sorafenib and its tosylate salt: A multikinase inhibitor for treating cancer. Acta Crystallogr. Sect. C Cryst. Struct. Commun. 2011, 67, o29–o32. [Google Scholar] [CrossRef] [PubMed]
- Kiang, Y.-H.; Yang, C.-Y.; Staples, R.J.; Jona, J. Crystal structure, crystal morphology, and surface properties of an investigational drug. Int. J. Pharm. 2009, 368, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Esparza, G.U.; Wu, S.; Segura-Ibarra, V.; Cara, F.E.; Evans, K.W.; Milosevic, M.; Ziemys, A.; Kojic, M.; Meric-Bernstam, F.; Ferrari, M.; et al. Polymer Nanoparticles Encased in a Cyclodextrin Complex Shell for Potential Site- and Sequence-Specific Drug Release. Adv. Funct. Mater. 2014, 24, 4753–4761. [Google Scholar] [CrossRef]
- Sybyl-X 2.1, Certara L.P. Louis, MO, USA. 2011. Available online: https://www.certara.com/pressrelease/certara-enhances-sybyl-x-drug-design-and-discovery-software-suite/ (accessed on 20 April 2021).
- Ghose, R.; Nijhof, V.; Brouwer, J.; Matsubara, Y.; Kaida, Y.; Takahashi, T. Shallow to very shallow, high-resolution reflection seismic using a portable vibrator system. Geophysics 1998, 63, 1295–1309. [Google Scholar] [CrossRef]
- Xia, D.; Cui, F.; Piao, H.; Cun, D.; Piao, H.; Jiang, Y.; Ouyang, M.; Quan, P. Effect of Crystal Size on the In Vitro Dissolution and Oral Absorption of Nitrendipine in Rats. Pharm. Res. 2010, 27, 1965–1976. [Google Scholar] [CrossRef] [PubMed]
- APEX3, SADABS and SAINT Bruker AXS Inc.: Madison, WI, USA. 2016. Available online: https://www.bruker.com/en/products-and-solutions/diffractometers-and-scattering-systems/single-crystal-x-ray-diffractometers/sc-xrd-software/apex.html (accessed on 20 April 2021).







| Crystal Habit | Elemental Composition (%) | (O + N + S)/(C + F + Cl) (%) | |||||
|---|---|---|---|---|---|---|---|
| C | F | Cl | S | O | N | ||
| ST-A | 42.17 | 16.66 | 7.82 | 3.38 | 20.41 | 9.56 | 50.0 |
| ST-B | 41.18 | 17.42 | 7.33 | 3.75 | 20.13 | 10.20 | 51.7 |
| Value | AUC (μg h mL−1) | Cmax (μg mL−1) | Tmax (h) |
|---|---|---|---|
| ST-A | 9.33 | 1.85 | 3 |
| ST-B | 11.93 | 2.41 | 2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Phan, C.U.; Shen, J.; Yu, K.; Mao, J.; Tang, G. Impact of Crystal Habit on the Dissolution Rate and In Vivo Pharmacokinetics of Sorafenib Tosylate. Molecules 2021, 26, 3469. https://doi.org/10.3390/molecules26113469
Phan CU, Shen J, Yu K, Mao J, Tang G. Impact of Crystal Habit on the Dissolution Rate and In Vivo Pharmacokinetics of Sorafenib Tosylate. Molecules. 2021; 26(11):3469. https://doi.org/10.3390/molecules26113469
Chicago/Turabian StylePhan, Chi Uyen, Jie Shen, Kaxi Yu, Jianming Mao, and Guping Tang. 2021. "Impact of Crystal Habit on the Dissolution Rate and In Vivo Pharmacokinetics of Sorafenib Tosylate" Molecules 26, no. 11: 3469. https://doi.org/10.3390/molecules26113469