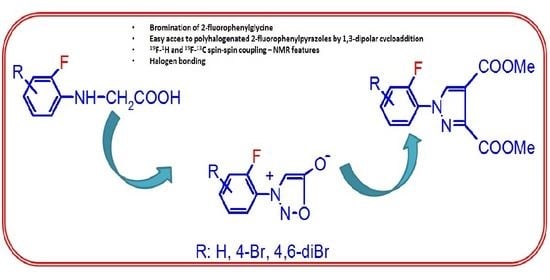
Synthesis of 1-(2-Fluorophenyl)pyrazoles by 1,3-Dipolar Cycloaddition of the Corresponding Sydnones
Abstract
:1. Introduction
2. Results and Discussion
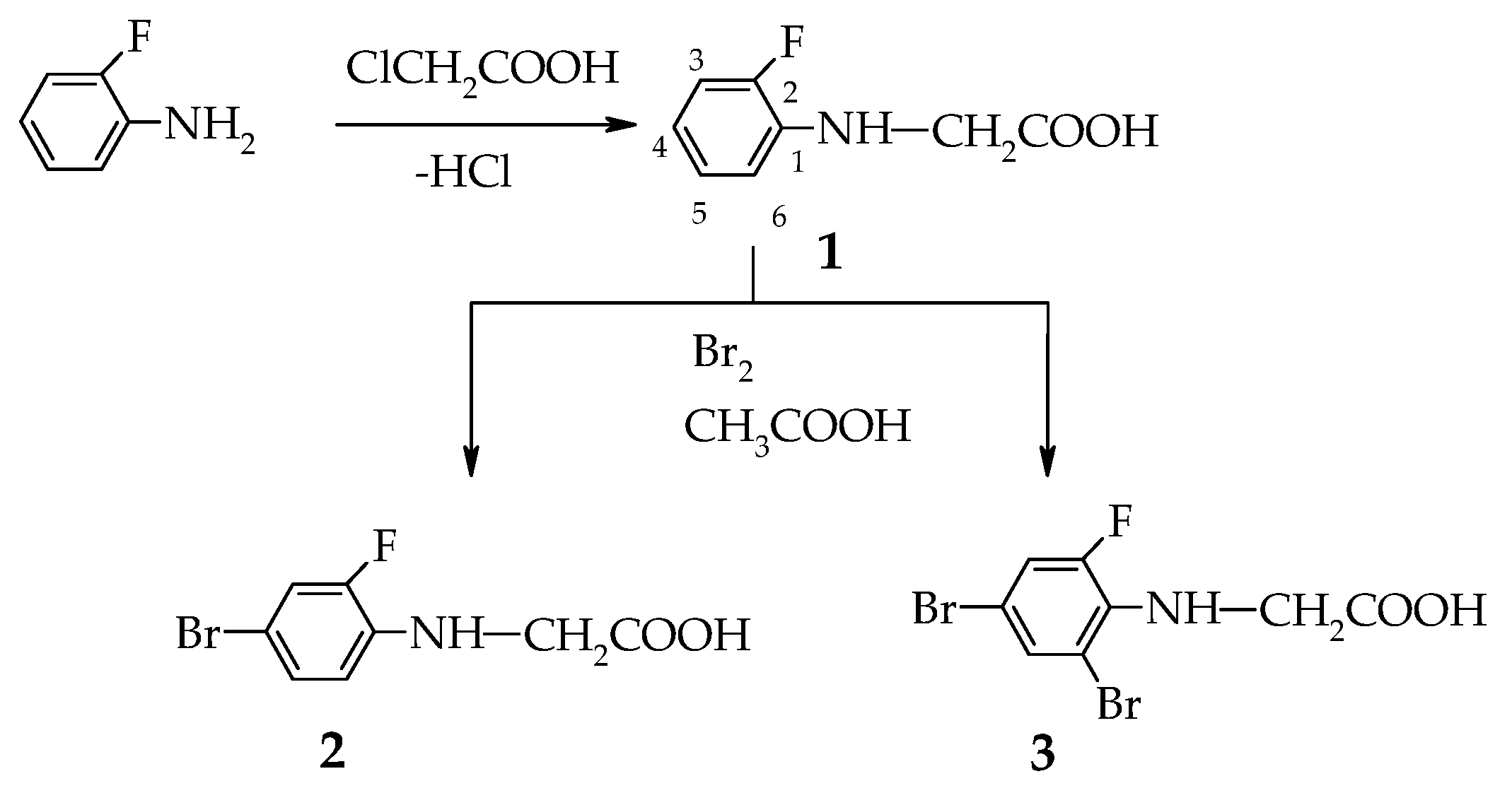
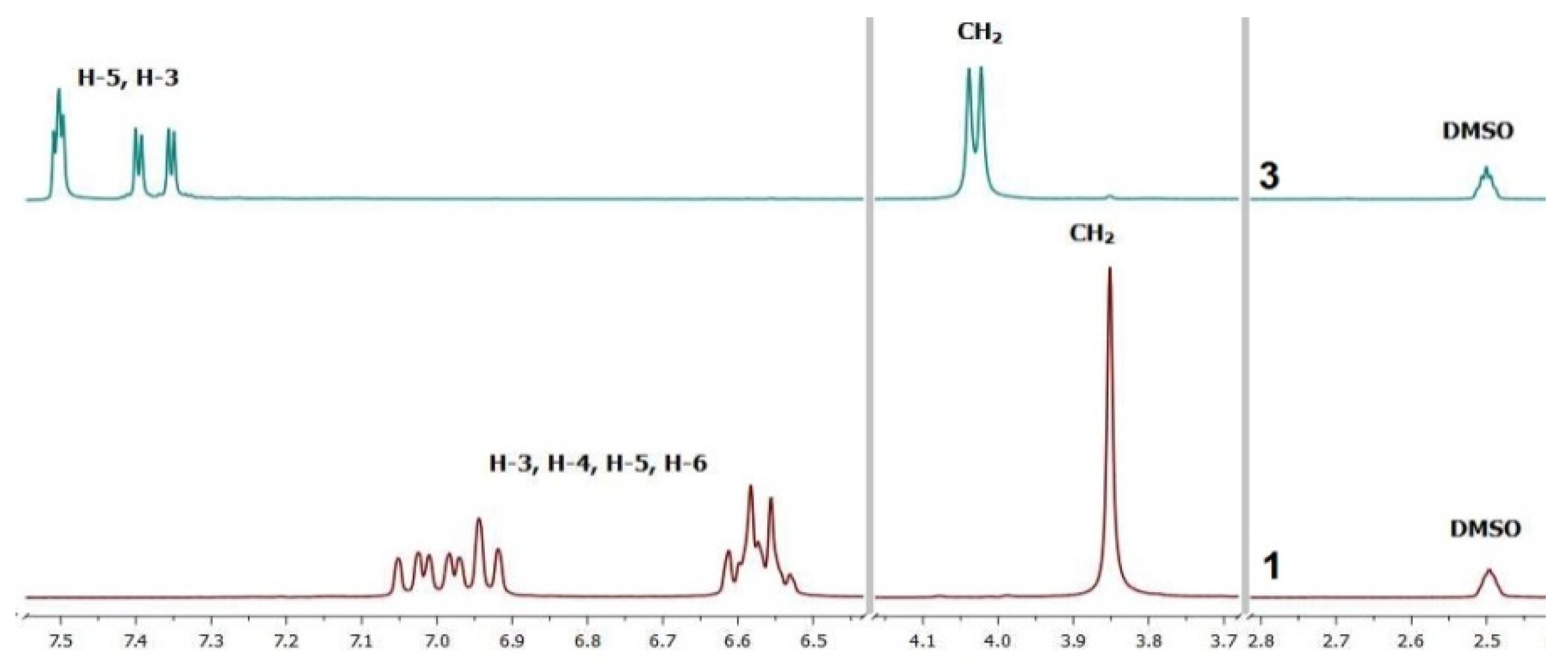
2.1. Synthesis and Spectral Analysis
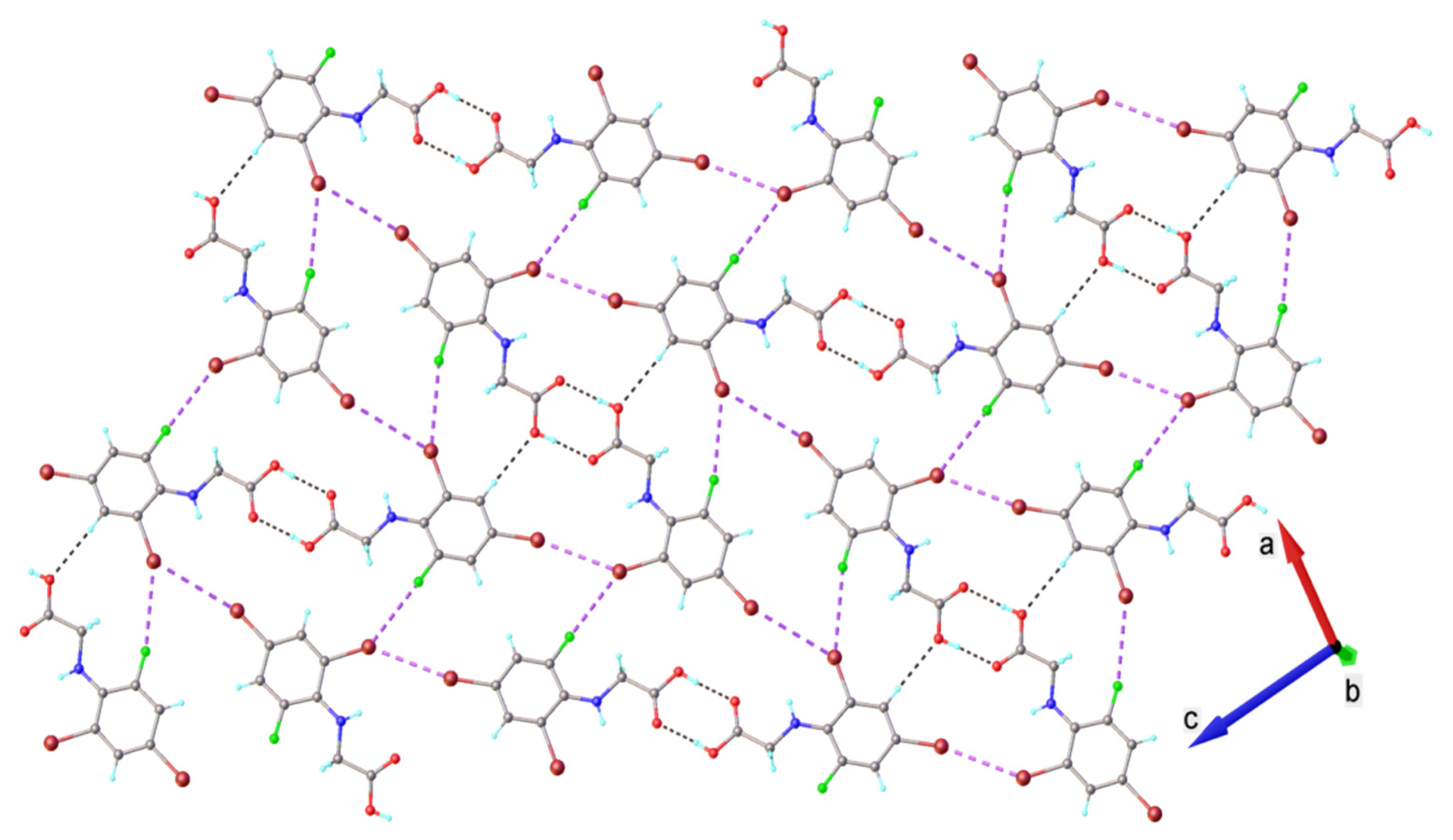
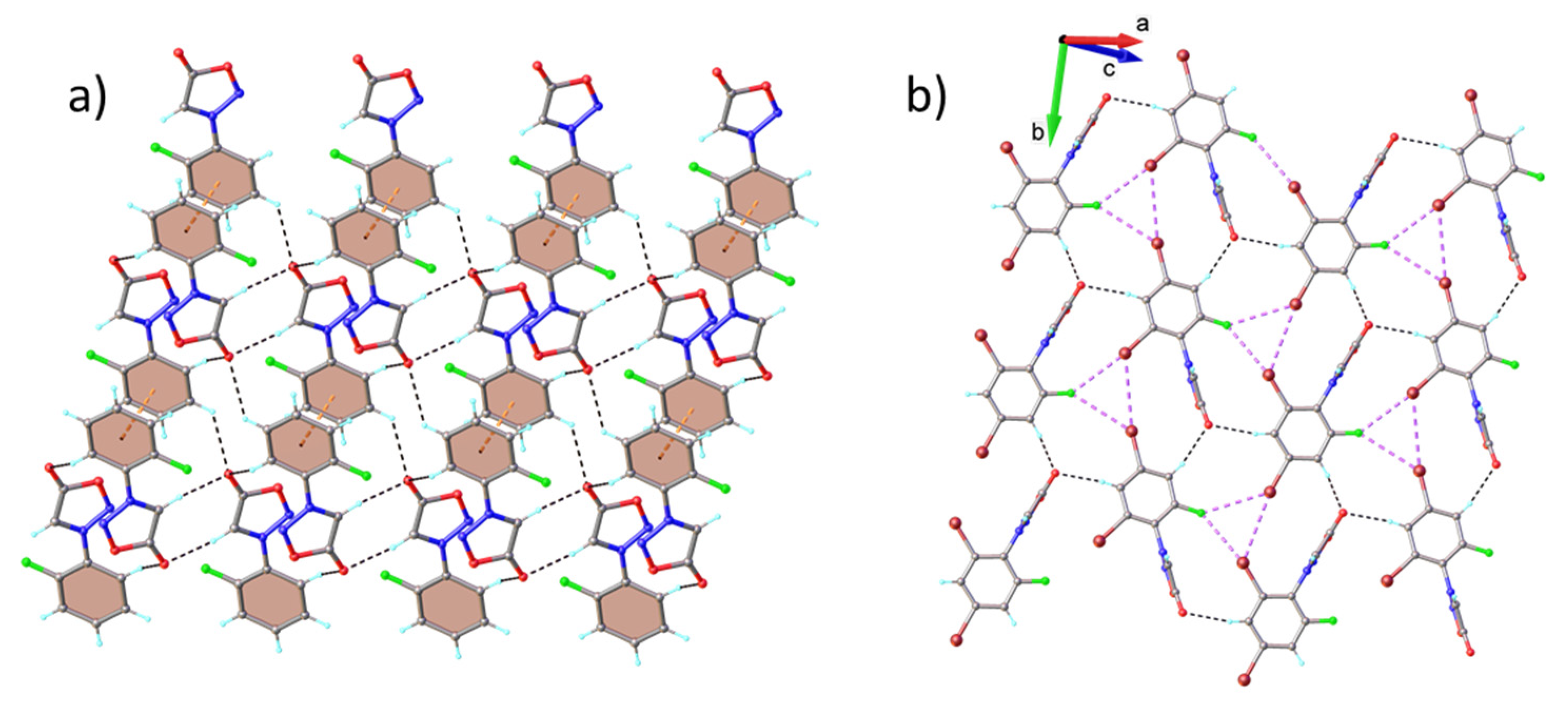
2.2. X-ray Diffraction Analysis
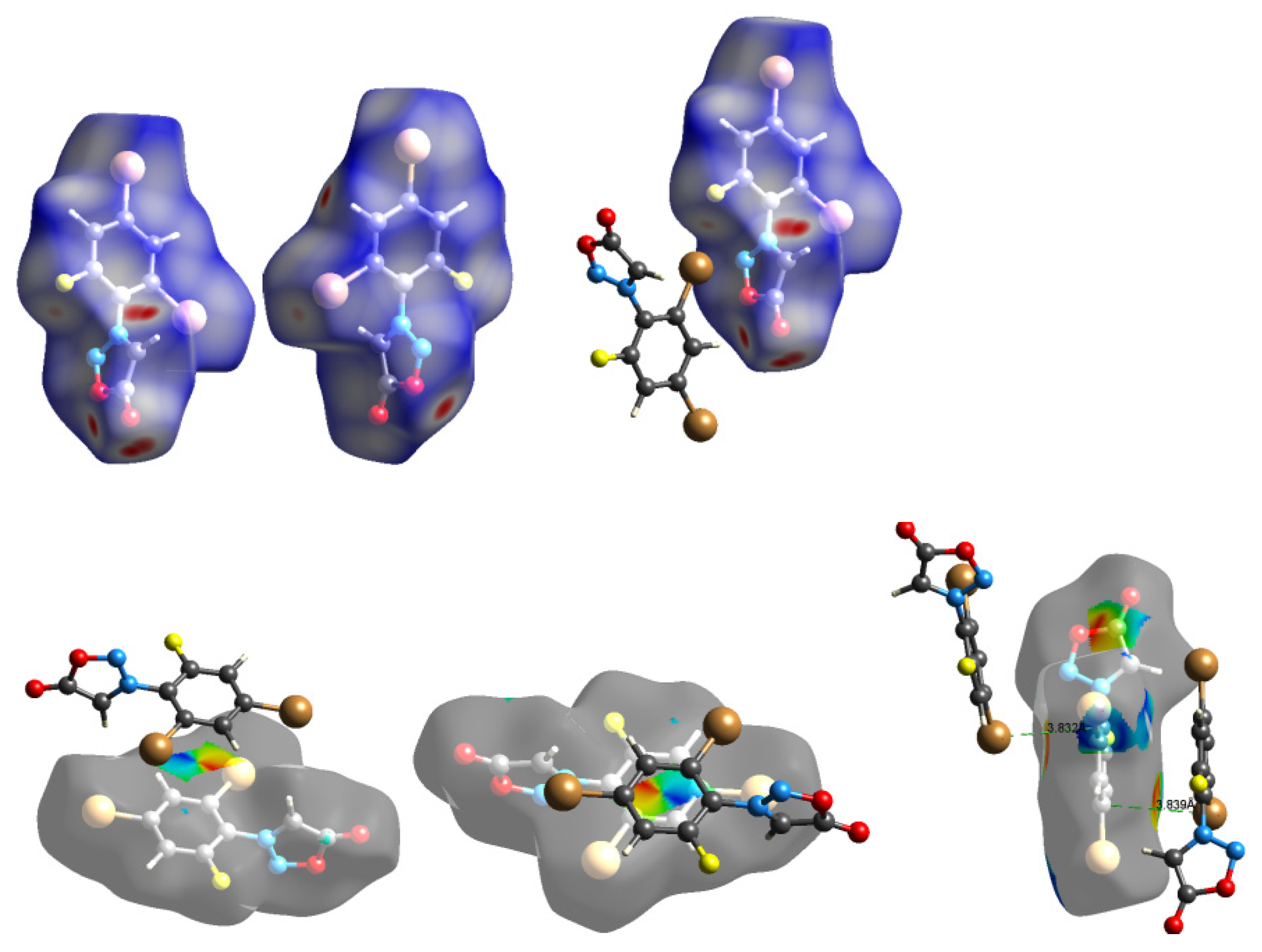
2.3. Hirshfeld Analysis
3. Materials and Methods
3.1. Procedures for Synthesis of Acids 1–3
3.2. Procedures for Synthesis of Sydnones 4a–c
3.3. Genereal Procedure for Synthesis of Pyrazoles 5a–c
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Breugst, M.; Reissig, H. The Huisgen Reaction: Milestones of the 1,3-Dipolar Cycloaddition. Angew. Chem. Int. Ed. 2020, 59, 12293–12307. [Google Scholar] [CrossRef] [Green Version]
- Trauner, D. Rolf Huisgen (1920–2020). Nat. Chem. Biol. 2020, 16, 711. [Google Scholar] [CrossRef]
- Albota, F.; Draghici, C.; Caira, M.R.; Dumitrascu, F. 1,3-Dipolar cycloaddition between acetylenic dipolarophiles and sydnone-N-ylides as bis(1,3-dipoles). Tetrahedron 2015, 71, 9095–9100. [Google Scholar] [CrossRef]
- Antoci, V.; Moldoveanu, C.; Danac, R.; Mangalagiu, V.; Zbancioc, G. Huisgen [3 + 2] Dipolar Cycloadditions of Phthalazinium Ylides to Activated Symmetric and Non-Symmetric Alkynes. Molecules 2020, 25, 4416. [Google Scholar] [CrossRef]
- Caira, M.R.; Georgescu, E.; Georgescu, F.; Popa, M.M.; Dumitrascu, F. 7-Methoxy-pyrrolo[1,2-a]quinolines via quinolinium N-ylides. Arkivoc 2009, 2009, 242–253. [Google Scholar] [CrossRef] [Green Version]
- Dumitrascu, F.; Georgescu, E.; Georgescu, F.; Popa, M.M.; Dumitrescu, D. Synthesis of Pyrrolo[2,1-a]isoquinolines by Multicomponent 1,3-Dipolar Cycloaddition. Molecules 2013, 18, 2635–2645. [Google Scholar] [CrossRef] [PubMed]
- Reissig, H.-U.; Zimmer, R. Münchnones—New Facets after 50 Years. Angew. Chem. Int. Ed. 2014, 53, 9708–9710. [Google Scholar] [CrossRef] [PubMed]
- Kawase, M.; Sakagami, H.; Motohashi, N. The Chemistry of Bioactive Mesoionic Heterocycles. In Bioactive Heterocycles VII; Springer: Berlin/Heidelberg, Germany, 2007; pp. 135–152. [Google Scholar]
- Albota, F.; Stanescu, M.D. The state of the art in sydnones chemistry and applications. Rev. Roum. Chem. 2017, 62, 711–734. [Google Scholar]
- Cherepanov, I.A.; Moiseev, S.K. Recent developments in the chemistry of sydnones and sydnone imines. Adv. Heterocycl. Chem. 2020, 131, 49–164. [Google Scholar] [CrossRef]
- Nájera, C.; Sansano, J.M.; Yus, M. 1,3-Dipolar cycloadditions of azomethine imines. Org. Biomol. Chem. 2015, 13, 8596–8636. [Google Scholar] [CrossRef] [Green Version]
- Hein, C.D.; Liu, X.-M.; Wang, D. Click Chemistry, A Powerful Tool for Pharmaceutical Sciences. Pharm. Res. 2008, 25, 2216–2230. [Google Scholar] [CrossRef] [Green Version]
- Padwa, A.; Pearson, W.H. Synthetic Applications of 1,3-Dipolar Cycloaddition Chemistry Toward Heterocycles and Natural Products; John Wiley & Sons: Hoboken, NJ, USA, 2002. [Google Scholar]
- Browne, D.L.; Harrity, J.P. Recent developments in the chemistry of sydnones. Tetrahedron 2010, 66, 553–568. [Google Scholar] [CrossRef]
- Albota, F.; Caira, M.R.; Draghici, C.; Dumitrascu, F.; Dumitrescu, D.E. Sydnone C-4 heteroarylation with an indolizine ring via Chichibabin indolizine synthesis. Beilstein J. Org. Chem. 2016, 12, 2503–2510. [Google Scholar] [CrossRef] [Green Version]
- Dumitrascu, F.; Draghici, C.; Crangus, C.; Caproiu, M.T.; Mitan, C.I.; Dumitrescu, D.; Raileanu, D. Atropisomerism of New Sterically Hindered 1-Arylpyrazoles. Rev. Roum. Chim 2002, 47, 315–318. [Google Scholar]
- Dumitrascu, F.; Draghici, C.; Dumitrescu, D.; Tarko, L.; Raileanu, D. Direct Iodination of Sydnones and Their Cycloadditions to Form 5-Iodopyrazoles. Eur. J. Org. Chem. 1997, 1997, 2613–2616. [Google Scholar] [CrossRef]
- Brown, D.C.; Turnbull, K. Improved Method for the Iodination of Sydnones. Synth. Commun. 2013, 43, 3233–3237. [Google Scholar] [CrossRef]
- Aleem, A.S.; Turnbull, K. Halogenation of 3-(3,5-Dimethoxyphenyl)sydnone. Org. Prep. Proced. Int. 2015, 47, 87–93. [Google Scholar] [CrossRef]
- Nashashibi, I.F.; Tumey, J.M.; Owens, B.L.; Turnbull, K. Chlorination of 3-Arylsydnones with Iodine Monochloride. Org. Prep. Proced. Int. 2017, 49, 59–63. [Google Scholar] [CrossRef]
- Decuypere, E.; Specklin, S.; Gabillet, S.; Audisio, D.; Liu, H.; Plougastel, L.; Kolodych, S.; Taran, F. Copper(I)-Catalyzed Cycloaddition of 4-Bromosydnones and Alkynes for the Regioselective Synthesis of 1,4,5-Trisubstituted Pyrazoles. Org. Lett. 2014, 17, 362–365. [Google Scholar] [CrossRef]
- Liu, H.; Audisio, D.; Plougastel, L.; Decuypere, E.; Buisson, D.-A.; Koniev, O.; Kolodych, S.; Wagner, A.; Elhabiri, M.; Krzyczmonik, A.; et al. Ultrafast Click Chemistry with Fluorosydnones. Angew. Chem. Int. Ed. 2016, 55, 12073–12077. [Google Scholar] [CrossRef]
- Decuypère, E.; Plougastel, L.; Audisio, D.; Taran, F. Sydnone–alkyne cycloaddition: Applications in synthesis and bioconjugation. Chem. Commun. 2017, 53, 11515–11527. [Google Scholar] [CrossRef]
- Plougastel, L.; Lamaa, D.; Yen-Pon, E.; Audisio, D.; Taran, F. Fluorogenic probes based on polycyclic sydnone scaffolds. Tetrahedron 2020, 76, 131250. [Google Scholar] [CrossRef]
- Abdualkader, A.M.; Taher, M.; Yusoff, N.I.N. Mesoionic sydnone: A review in their chemical and biological properties. Int. J. Pharm. Pharm. Sci. 2017, 9, 1–9. [Google Scholar] [CrossRef]
- Ansari, A.; Ali, A.; Asif, M.; Shamsuzzaman, S. Review: Biologically active pyrazole derivatives. New J. Chem. 2016, 41, 16–41. [Google Scholar] [CrossRef]
- Bonacorso, H.G.; Pittaluga, E.P.; Porte, L.M.; Junges, A.F.; Libero, F.M.; Zanatta, N.; Martins, M.A. New 4-fluoroalkyl substituted N-phenylpyrazoles: Synthesis promoted by DAST and multinuclear NMR analysis. J. Fluor. Chem. 2015, 176, 44–50. [Google Scholar] [CrossRef]
- Wilcken, R.; Zimmermann, M.O.; Lange, A.; Joerger, A.; Boeckler, F.M. Principles and Applications of Halogen Bonding in Medicinal Chemistry and Chemical Biology. J. Med. Chem. 2013, 56, 1363–1388. [Google Scholar] [CrossRef]
- Hernandes, M.Z.; Cavalcanti, S.M.T.; Moreira, D.R.; Junior, W.F.D.A.; Leite, A.L. Halogen Atoms in the Modern Medicinal Chemistry: Hints for the Drug Design. Curr. Drug Targets 2010, 11, 303–314. [Google Scholar] [CrossRef] [PubMed]
- Ojima, I. Use of Fluorine in the Medicinal Chemistry and Chemical Biology of Bioactive Compounds-A Case Study on Fluorinated Taxane Anticancer Agents. ChemBioChem 2004, 5, 628–635. [Google Scholar] [CrossRef] [PubMed]
- Filler, R.; Saha, R. Fluorine in medicinal chemistry: A century of progress and a 60-year retrospective of selected highlights. Futur. Med. Chem. 2009, 1, 777–791. [Google Scholar] [CrossRef] [PubMed]
- Hunter, L. The C–F bond as a conformational tool in organic and biological chemistry. Beilstein J. Org. Chem. 2010, 6, 38. [Google Scholar] [CrossRef] [Green Version]
- Gillis, E.P.; Eastman, K.J.; Hill, M.D.; Donnelly, D.J.; Meanwell, N.A. Applications of Fluorine in Medicinal Chemistry. J. Med. Chem. 2015, 58, 8315–8359. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, J.; Gu, Z.; Wang, S.; Zhu, W.; Aceña, J.L.; Soloshonok, V.A.; Izawa, K.; Liu, H. Next Generation of Fluorine-Containing Pharmaceuticals, Compounds Currently in Phase II–III Clinical Trials of Major Pharmaceutical Companies: New Structural Trends and Therapeutic Areas. Chem. Rev. 2016, 116, 422–518. [Google Scholar] [CrossRef] [PubMed]
- Volle, J.-N.; Schlosser, M. Fluorine-Sacrificial Cyclizations as an Access to 5-Fluoropyrazoles. Eur. J. Org. Chem. 2000, 2000, 823–828. [Google Scholar] [CrossRef]
- Borkin, D.A.; Puscau, M.; Carlson, A.; Solan, A.; Wheeler, K.A.; Török, B.; Dembinski, R. Synthesis of diversely 1,3,5-trisubstituted pyrazoles via 5-exo-dig cyclization. Org. Biomol. Chem. 2012, 10, 4505. [Google Scholar] [CrossRef]
- Vankayalapaty, H.M.; Horrigan, S. Compositions and Methods for Inhibition of tbl-1 Binding to Disease-Associated Molecules. WO 2013/071232, 16 May 2013. [Google Scholar]
- Baranoff, E.; Bolink, H.J.; Constable, E.C.; Delgado, M.; Haussinger, D.; Housecroft, C.E.; Nazeeruddin, M.K.; Neuburger, M.; Orti, E.; Schneider, G.E.; et al. Tuning the photophysical properties of cationic iridium(III) complexes containing cyclometallated 1-(2,4-difluorophenyl)-1H-pyrazole through functionalized 2,2′-bipyridine ligands: Blue but not blue enough. Dalton Trans. 2013, 42, 1073–1087. [Google Scholar] [CrossRef]
- Dumitrescu, D.; Shova, S.; Man, I.C.; Caira, M.R.; Popa, M.M.; Dumitrascu, F. 5-Iodo-1-Arylpyrazoles as Potential Benchmarks for Investigating the Tuning of the Halogen Bonding. Crystals 2020, 10, 1149. [Google Scholar] [CrossRef]
- Popa, M.M.; Man, I.C.; Draghici, C.; Shova, S.; Caira, M.R.; Dumitrascu, F.; Dumitrescu, D. Halogen bonding in 5-iodo-1-arylpyrazoles investigated in the solid state and predicted by solution13C-NMR spectroscopy. CrystEngComm 2019, 21, 7085–7093. [Google Scholar] [CrossRef]
- Caira, M.; Dumitrascu, F.; Georgescu, E.; Georgescu, F.; Popa, M.M.; Draghici, B.; Dumitrescu, D.G. A Novel Approach for the Synthesis of N-Arylpyrroles. Synlett 2009, 2009, 3336–3340. [Google Scholar] [CrossRef]
- Caira, M.; Dumitrascu, F.; Draghici, B.; Caproiu, M.; Dumitrescu, D.G. A Novel Approach for the Synthesis of Highly Fluorescent Pyrrolo[1,2-b]pyridazines. Synlett 2008, 2008, 813–816. [Google Scholar] [CrossRef]
- Georgescu, E.; Georgescu, F.; Popa, M.M.; Draghici, C.; Tarko, L.; Dumitrascu, F. Efficient One-Pot, Three-Component Synthesis of a Library of Pyrrolo[1,2-c]pyrimidine Derivatives. ACS Comb. Sci. 2012, 14, 101–107. [Google Scholar] [CrossRef]
- Earl, J.C.; Mackney, A.W. The action of acetic anhydride on N-nitrosophenylglycine and some of its derivatives. J. Chem. Soc. 1935, 899–900. [Google Scholar] [CrossRef]
- Bellas, M.; Suschitzky, H. Syntheses of heterocyclic compounds. Part XII. Halogen-substituted 3-arylsydnones. J. Chem. Soc. C 1966, 189–192. [Google Scholar] [CrossRef]
- Wolff, S.K.; Grimwood, D.J.; McKinnon, J.J.; Turner, M.J.; Jayatilaka, D.; Spackman, M.A. CrystalExplorer; Version 3.1; University of Western Australia: Perth, WA, Australia, 2012. [Google Scholar]
- Oxford Diffraction. CrysAlisPro Software System; Version 1.171.41.64; Rigaku Corporation: Oxford, UK, 2015. [Google Scholar]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A Found. Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. 2015, 71, 3–8. [Google Scholar]
- McKinnon, J.J.; Jayatilaka, D.; Spackman, M. Towards quantitative analysis of intermolecular interactions with Hirshfeld surfaces. Chem. Commun. 2007, 3814–3816. [Google Scholar] [CrossRef] [PubMed]
- Spackman, M.A.; Jayatilaka, D. Hirshfeld surface analysis. CrystEngComm 2009, 11, 19–32. [Google Scholar] [CrossRef]




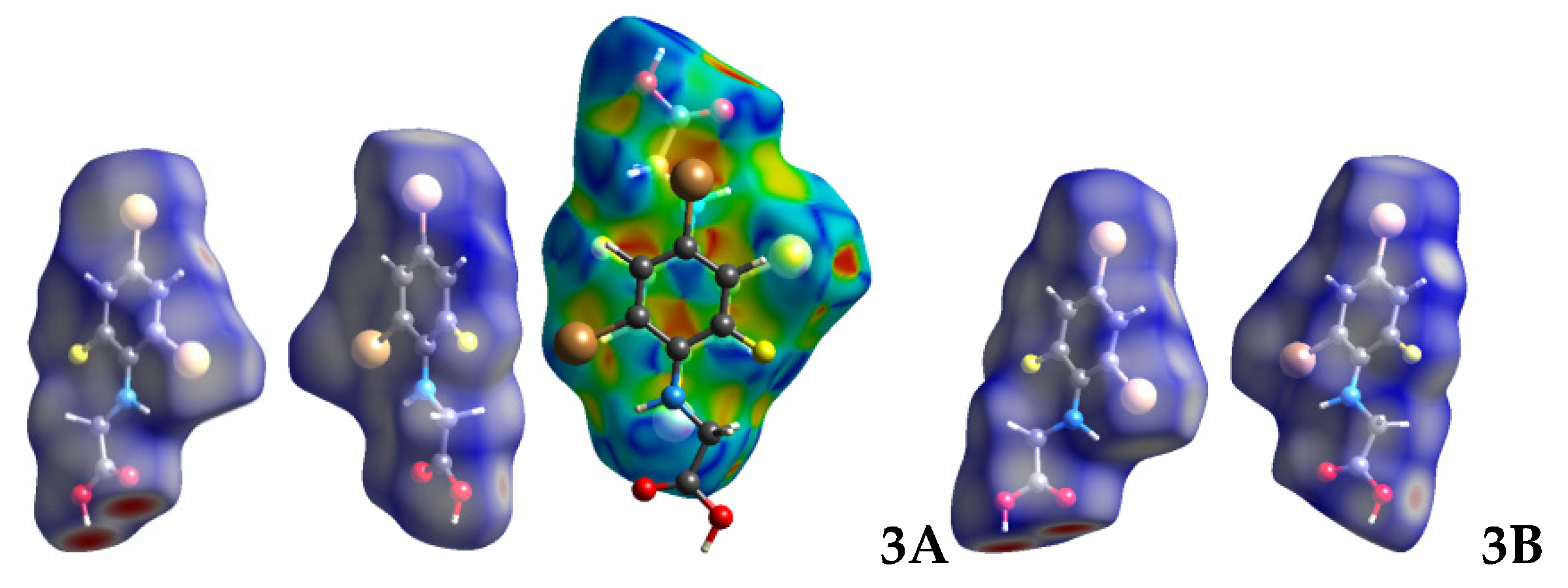
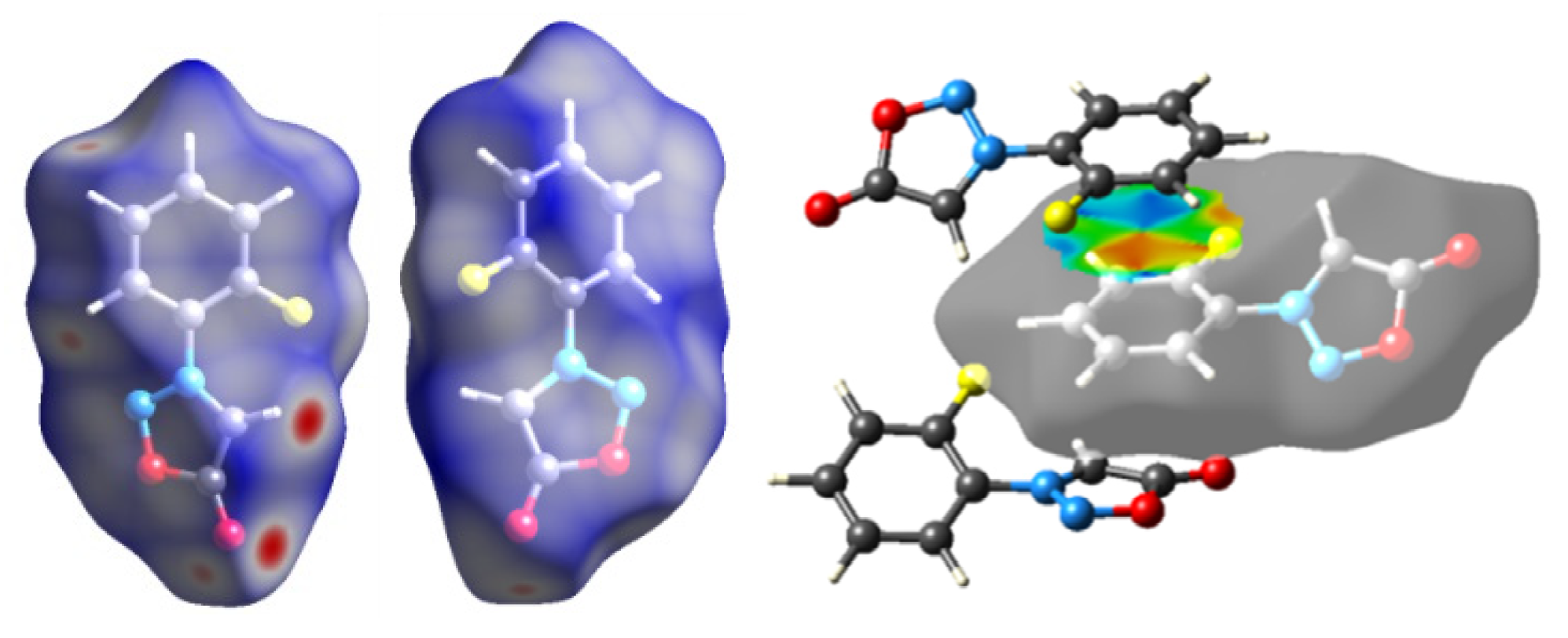
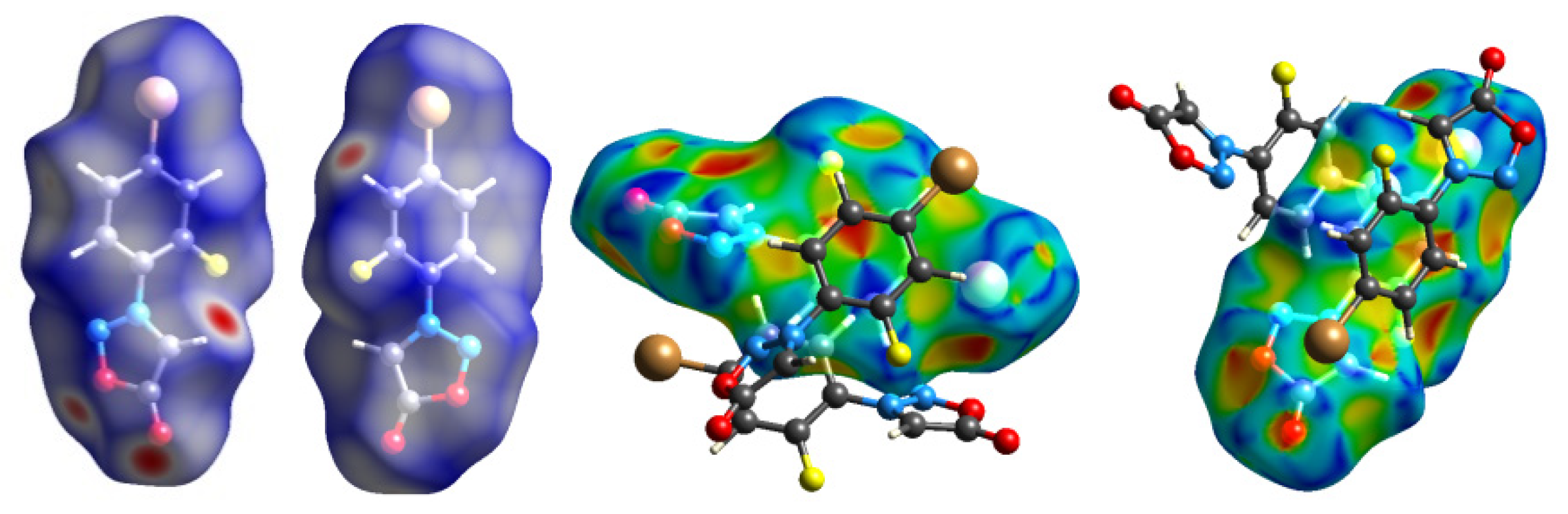
| No. | C-3 | C-4 | C-5 | C-1′ | C-2′ | C-3′ | C-4′ | C-5′ | C-6′ |
|---|---|---|---|---|---|---|---|---|---|
| Chemical Shift (ppm), 19F-13C Coupling Constant J (Hz) | |||||||||
| 1 [45] | - | - | - | 136.3 J = 11.6 | 151.0 J = 237.7 | 114.4 J = 18.0 | 116.1 J = 6.9 | 124.7 J = 3.2 | 112.1 J = 3.8 |
| 2 | - | - | - | 136.0 J = 11.5 | 150.6 J = 242.0 | 117.4 J = 21.8 | 105.4 J = 9.2 | 127.4 J = 3.7 | 113.5 J = 4.6 |
| 3 | - | - | - | 134.0 J = 10.6 | 150.7 J = 245.5 | 119.1 J = 24.9 | 106.7 J = 10.9 | 130.2 J = 3.0 | 111.1 J = 6.7 |
| 4a [45] | - | 97.1 J ~ 0.7 | - | 123.0 J = 8.9 | 154.4 J = 257.4 | 117.9 J = 20.0 | 134.0 J = 8.3 | 125.8 J = 3.8 | 125.0 J ~ 0.9 |
| 4b | - | 97.0 J ~ 0.7 | - | 121.4 J = 9.0 | 153.9 J = 262.0 | 121.6 J = 22.0 | 127.3 J = 9.1 | 129.1 J = 3.8 | 125.7 Small J |
| 4c | - | 99.4 No J | - | 121.9 J = 14.9 | 156.0 J = 261.0 | 120.5 J = 22.3 | 127.5 J = 10.0 | 132.2 J = 3.6 | 121.2 Small J |
| 5a | 144.7 | 116.3 | 135.7 J = 10.0 | 129.7 J = 9.4 | 153.8 J = 251.0 | 116.9 J = 20.0 | 129.8 J = 8.0 | 125.2 J = 3.6 | 125.1 Small J |
| 5b | 144.8 | 116.5 | 136.5 J = 10.0 | 126.2 J = 9.4 | 154.3 J = 257.2 | 120.6 J = 22.0 | 122.0 J = 8.8 | 128.7 J = 3.4 | 126.0 J ~ 0.7 |
| 5c | 145.1 | 116.4 | 137.1 No J | 126.7 J = 14.8 | 157.9 J = 262.2 | 119.7 J = 22.0 | 125.0 J = 10.1 | 131.7 J = 3.6 | 123.2 Small J |
| Parameter |  3 | 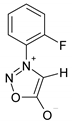 4a |  4b | 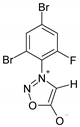 4c |
|---|---|---|---|---|
| Empirical formula | C8H6Br2FNO2 | C8H5FN2O2 | C8H4BrFN2O2 | C8H3Br2FN2O2 |
| Fw | 326.96 | 180.14 | 259.04 | 337.94 |
| space group | P-1 | P21/c | I2/a | P21/n |
| a [Å] | 8.8727(6) | 6.7072(5) | 13.8035(9) | 10.3170(8) |
| b [Å] | 10.4079(7) | 12.6000(11) | 8.6487(4) | 9.1652(5) |
| c [Å] | 12.7466(11) | 9.3015(6) | 14.9402(7) | 10.5526(8) |
| α [°] | 107.218(7) | 90 | 90 | 90 |
| β [°] | 91.685(6) | 102.085(7) | 95.109(5) | 95.928(6) |
| γ [°] | 114.901(7) | 90 | 90 | 90 |
| V [Å3] | 1003.57(14) | 768.66(10) | 1776.51(17) | 992.49(12) |
| Z | 4 | 4 | 8 | 4 |
| rcalcd [g cm−3] | 2.164 | 1.557 | 1.937 | 2.262 |
| Crystal size [mm] | 0.30 × 0.20 × 0.20 | 0.30 × 0.10 × 0.10 | 0.30 × 0.20 × 0.20 | 0.30 × 0.20 × 0.20 |
| T [K] | 293 | 293 | 293 | 293 |
| μ [mm−1] | 8.064 | 0.131 | 4.616 | 8.161 |
| 2Θ range [°] | 4.588 to 58.638 | 5.524 to 50.038 | 5.448 to 50.05 | 5.258 to 52.722 |
| Reflections collected | 11,043 | 5284 | 3760 | 9203 |
| Independent reflections | 4731[Rint = 0.0491] | 1346[Rint = 0.0405] | 1559[Rint = 0.0552] | 2027[Rint = 0.0543] |
| Data/restraints/parameters | 4731/0/255 | 1346/0/118 | 1559/0/127 | 2027/0/136 |
| R1a | 0.0580 | 0.0451 | 0.0334 | 0.0455 |
| wR2b | 0.1037 | 0.1150 | 0.0392 | 0.0670 |
| GOF c | 0.992 | 1.098 | 1.021 | 1.076 |
| Largest diff. peak/hole [e Å−3] | 0.52/−0.48 | 0.17/−0.26 | 0.32/−0.49 | 0.49/−0.43 |
| CCDC No. | 2080828 | 2080829 | 2080830 | 2080831 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dumitrescu, D.; Shova, S.; Draghici, C.; Popa, M.M.; Dumitrascu, F. Synthesis of 1-(2-Fluorophenyl)pyrazoles by 1,3-Dipolar Cycloaddition of the Corresponding Sydnones. Molecules 2021, 26, 3693. https://doi.org/10.3390/molecules26123693
Dumitrescu D, Shova S, Draghici C, Popa MM, Dumitrascu F. Synthesis of 1-(2-Fluorophenyl)pyrazoles by 1,3-Dipolar Cycloaddition of the Corresponding Sydnones. Molecules. 2021; 26(12):3693. https://doi.org/10.3390/molecules26123693
Chicago/Turabian StyleDumitrescu, Denisa, Sergiu Shova, Constantin Draghici, Marcel Mirel Popa, and Florea Dumitrascu. 2021. "Synthesis of 1-(2-Fluorophenyl)pyrazoles by 1,3-Dipolar Cycloaddition of the Corresponding Sydnones" Molecules 26, no. 12: 3693. https://doi.org/10.3390/molecules26123693