Chemical, Thermal and Antioxidant Properties of Lignins Solubilized during Soda/AQ Pulping of Orange and Olive Tree Pruning Residues
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemical Composition of Lignins
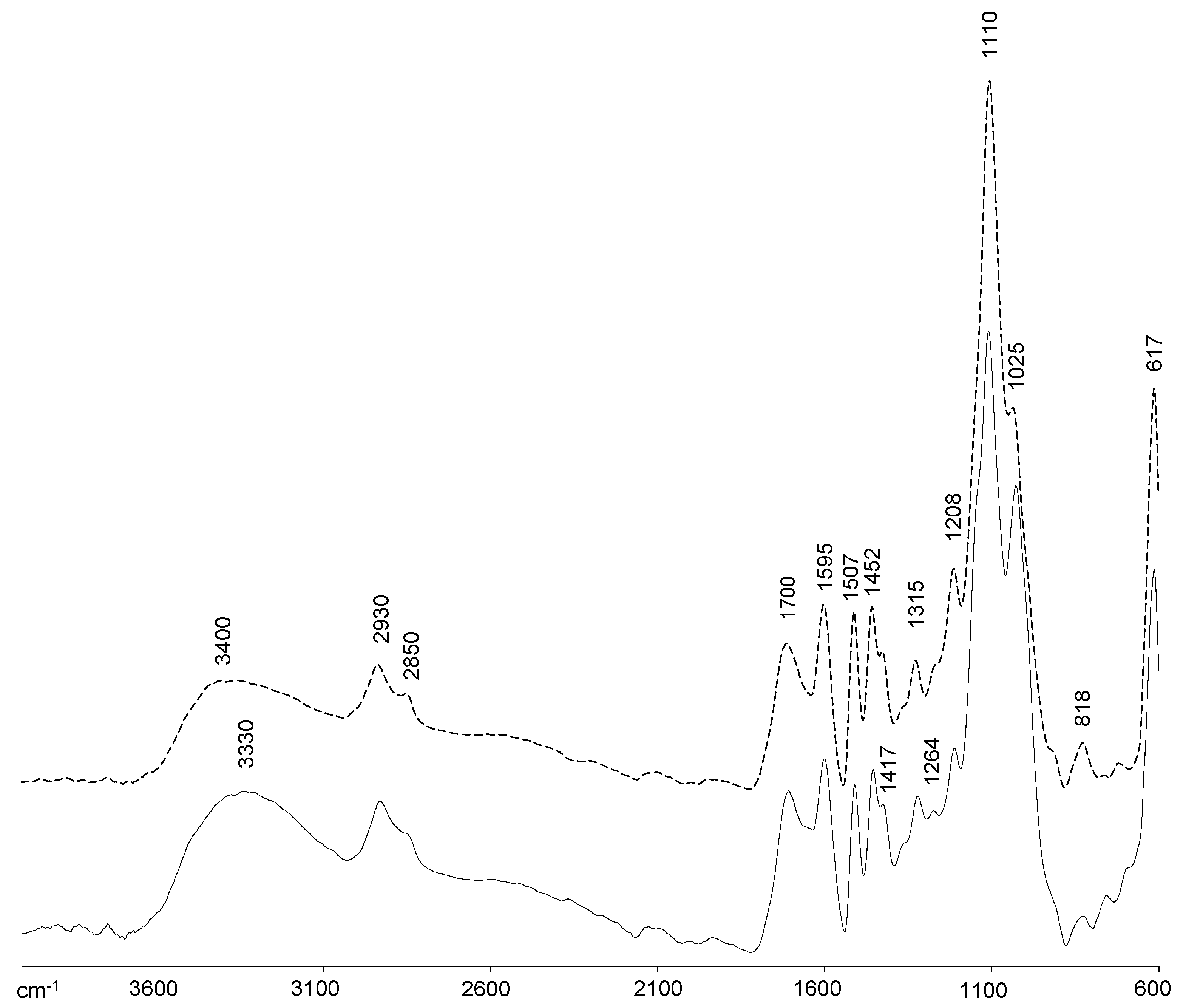
2.2. FTIR Spectra Analysis of Lignins
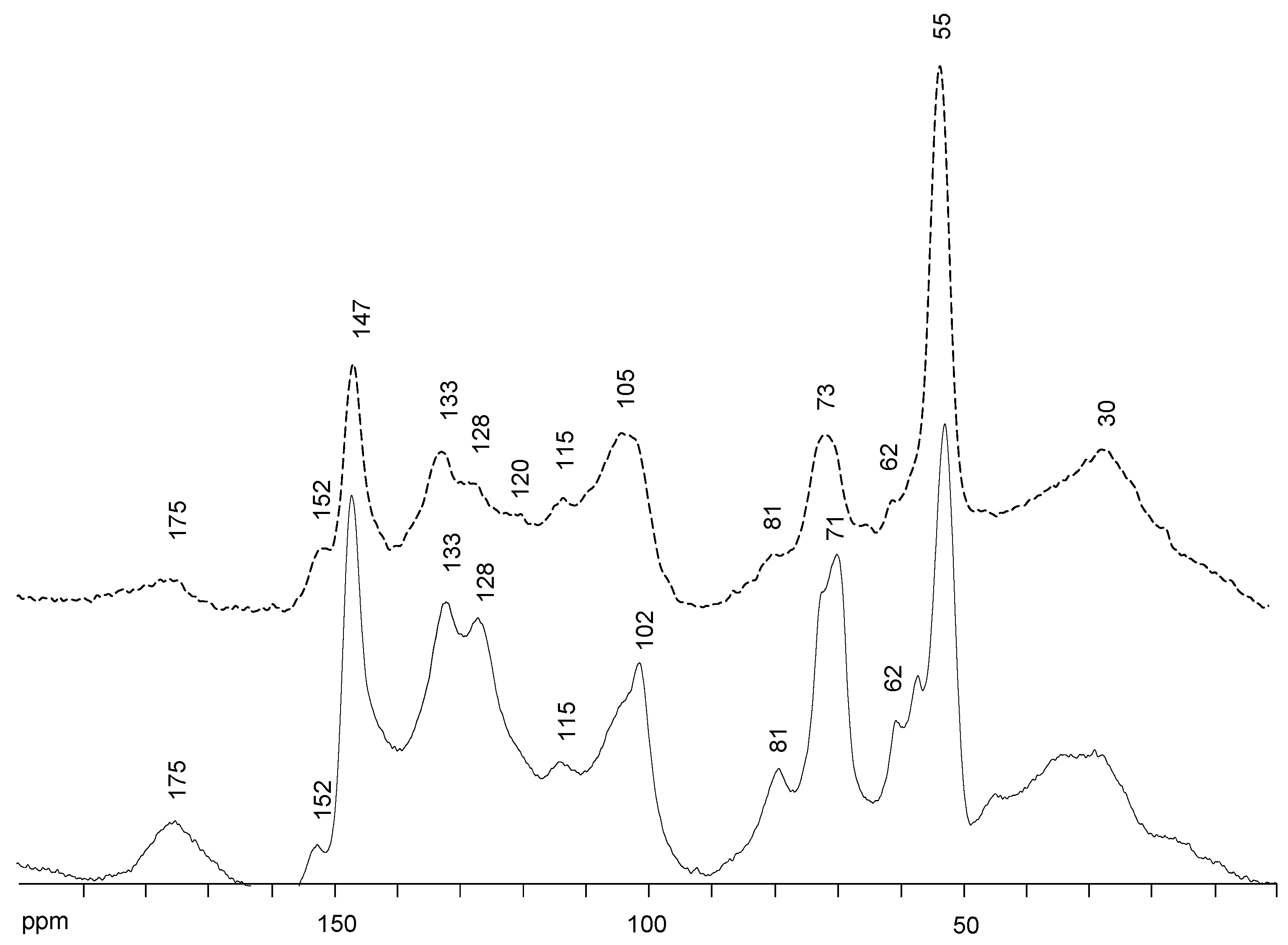
2.3. Solid State 13C NMR Spectra Analysis of Lignins
2.4. 2D NMR Spectra Analysis of Lignins
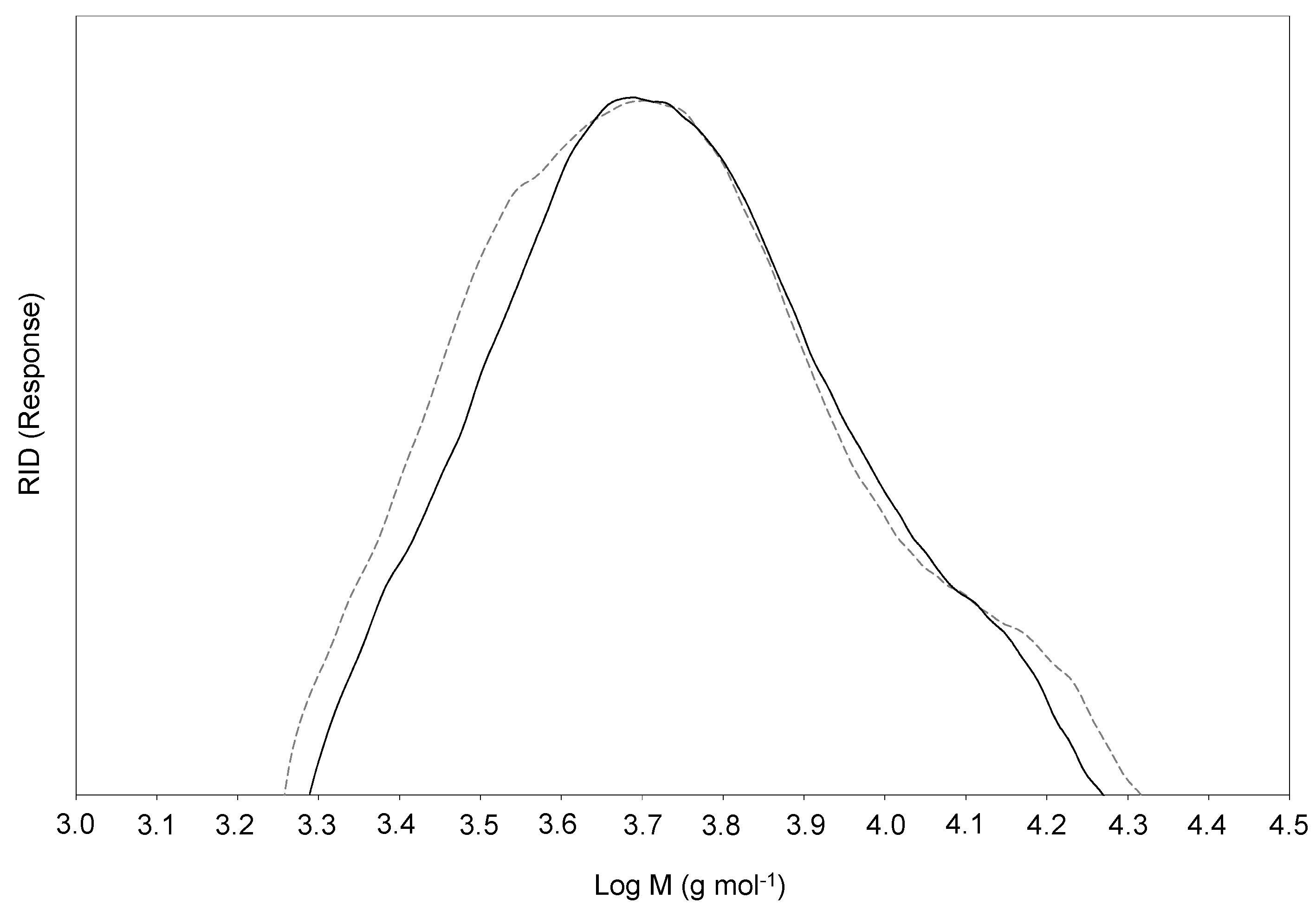
2.5. SEC Analysis
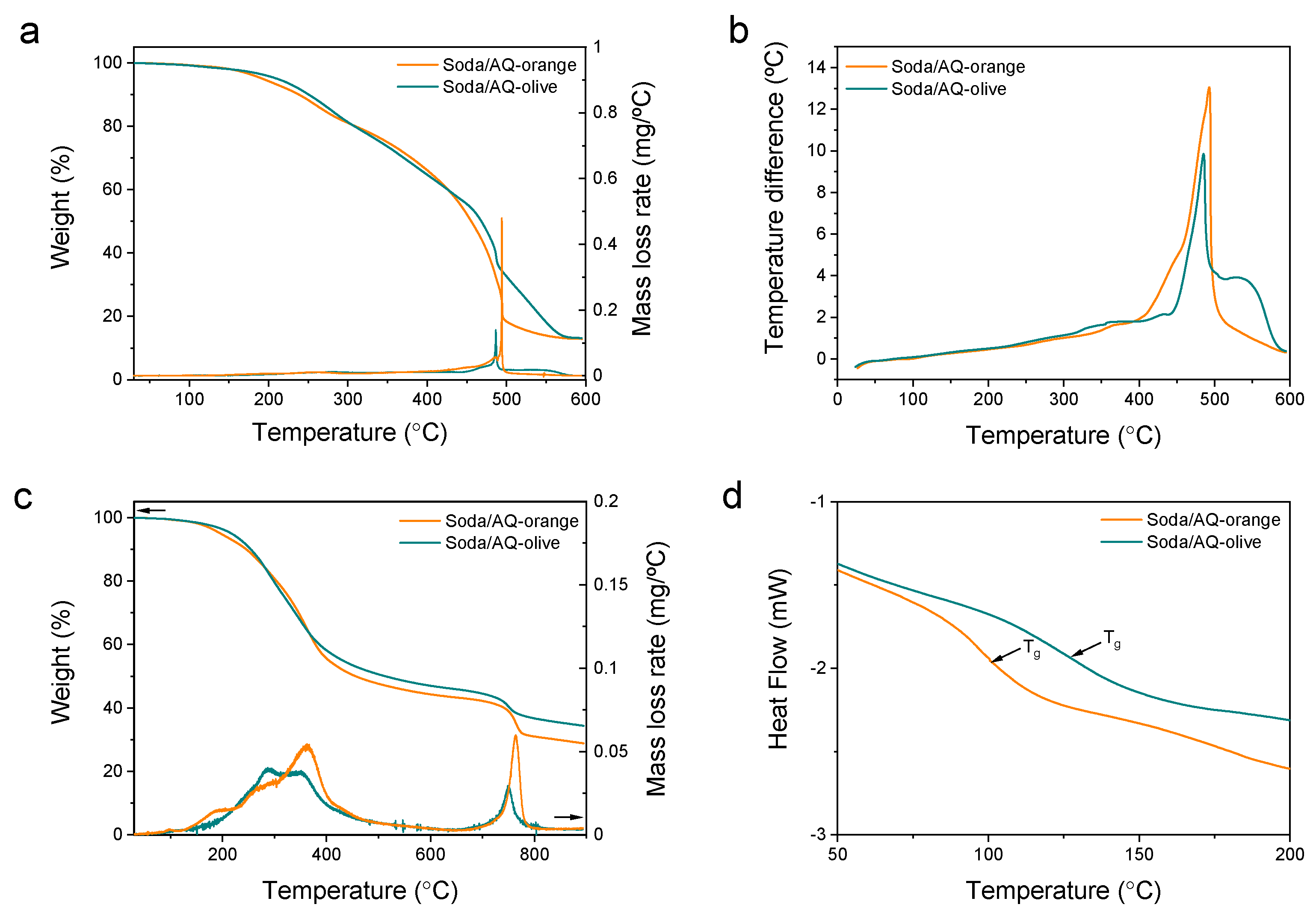
2.6. Thermal Analysis
2.7. Antioxidant Properties
3. Materials and Methods
3.1. Raw Materials and Chemicals
3.2. Pulps and Lignins Production
3.3. Lignins Characterization
3.4. Thermal Lignins Characterization
3.5. Antioxidant Activity of Lignins
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Fillat, Ú.; Martín-Sampedro, R.; González, Z.; Ferrer, A.; Ibarra, D.; Eugenio, M.E. Biobleaching of orange tree pruning cellulose pulp with xylanase and laccase mediator systems. Cellul. Chem. Technol. 2017, 51, 55–65. [Google Scholar]
- Susmozas, A.; Moreno, A.D.; Romero-García, J.M.; Manzanares, P.; Ballesteros, M. Designing an olive tree pruning biorefinery for the production of bioethanol, xylitol and antioxidants: A techno-economic assessment. Holzforschung 2019, 73, 15–23. [Google Scholar] [CrossRef]
- Oliva, J.M.; Negro, M.J.; Álvarez, C.; Manzanares, P.; Moreno, A.D. Fermentation strategies for the efficient use of olive tree pruning biomass from a flexible biorefinery approach. Fuel 2020, 277, 118171. [Google Scholar] [CrossRef]
- Espinosa, E.; Arrebola, R.I.; Bascón-Villegas, I.; Sánchez-Gutiérrez, M.; Domínguez-Robles, J.; Rodríguez, A. Industrial application of orange tree nanocellulose as papermaking reinforcement agent. Cellulose 2020, 27, 10781–10797. [Google Scholar] [CrossRef]
- Fillat, Ú.; Wicklein, B.; Martín-Sampedro, R.; Ibarra, D.; Ruiz-Hitzky, E.; Valencia, C.; Sarrión, A.; Castro, E.; Eugenio, M.E. Assessing cellulose nanofiber production from olive tree pruning residue. Carbohydr. Polym. 2018, 179, 252–261. [Google Scholar] [CrossRef] [PubMed]
- Martín-Sampedro, R.; Rodríguez, A.; Requejo, A.; Eugenio, M.E. Improvement of TCF bleaching of olive tree pruning residue pulp by addition of a laccase and/or xylanase pre-treatment. Bioresources 2012, 7, 1488–1503. [Google Scholar] [CrossRef] [Green Version]
- Cara, C.; Ruiz, E.; Carvalheiro, F.; Moura, P.; Ballesteros, I.; Castro, E.; Gírio, F. Production, purification and characterisation of oligosaccharides from olive tree pruning autohydrolysis. Ind. Crops Prod. 2012, 40, 225–231. [Google Scholar] [CrossRef] [Green Version]
- Mateo, S.; Puentes, J.G.; Moya, A.J.; Sánchez, S. Ethanol and xylitol production by fermentation of acid hydrolysate from olive pruning with Candida tropicalis NBRC 0618. Bioresour. Technol. 2015, 190, 1–6. [Google Scholar] [CrossRef]
- Ralph, J.; Lundquist, K.; Brunow, G.; Lu, F.; Kim, H.; Schatz, P.F.; Marita, J.M.; Hadfield, R.D.; Ralph, S.A.; Christensen, J.H. Lignins: Natural polymers from oxidative coupling of 4-hydroxyphenyl-propanoids. Phytochem. Rev. 2003, 3, 29–60. [Google Scholar] [CrossRef]
- Susmozas, A.; Martín-Sampedro, R.; Eugenio, M.E.; Iglesias, R.; Manzanares, P.; Moreno, A.D. Process strategies for the transition of 1G to advanced bioethanol production. Processes 2020, 8, 1310. [Google Scholar] [CrossRef]
- Ragauskas, A.J.; Beckham, G.T.; Biddy, M.J.; Chandra, R.; Chen, F.; Davis, M.F.; Davison, B.H.; Dixon, R.A.; Gilna, P.; Keller, M.; et al. Lignin valorization: Improving lignin processing in the biorefinery. Science 2014, 344, 1–10. [Google Scholar] [CrossRef]
- Brännvall, E. Overview of pulp and paper processes. In Pulp and Paper Chemistry Technology; Ek, M., Gellerstedt, G., Henriksson, G., Eds.; De Gruyter: Berlin, Germany, 2009; pp. 1–12. [Google Scholar]
- Gosselink, R.J.A. Lignin as a Renewable Aromatic Resource for the Chemical Industry. Ph.D. Thesis, Wageningen University, Wageningen, NL, USA, 2011. [Google Scholar]
- Eugenio, M.E.; Ibarra, D.; Martín-Sampedro, R.; Espinosa, E.; Bascón, I.; Rodríguez, A. Alternative raw materials for pulp and paper production in the concept of a lignocellulosic biorefinery. In Cellulose; Rodríguez, A., Eugenio, M.E., Eds.; IntechOpen: London, UK, 2019; pp. 1–26. [Google Scholar] [CrossRef] [Green Version]
- Del Río, J.C.; Rencoret, J.; Marques, G.; Gutierrez, A.; Ibarra, D.; Santos, J.I.; Jimenez-Barbero, J.; Zhang, L.; Martínez, A.T. Highly acylated (acetylated and/or p-coumaroylated) native lignins from diverse herbaceous plants. J. Agric. Food. Chem. 2008, 56, 9525–9534. [Google Scholar] [CrossRef]
- Mansfield, S.D.; Kim, H.; Lu, F.; Ralph, J. Whole plant cell wall characterization using solution-state 2D NMR. Nat. Protocol. 2012, 7, 1579–1589. [Google Scholar] [CrossRef] [PubMed]
- Rencoret, J.; Marques, G.; Ana Gutiérrez, A.; Ibarra, D.; Li, J.; Gellerstedt, G.; Santos, J.I.; Jiménez-Barbero, J.; Martínez, A.T.; del Río, J.C. Structural characterization of milled wood lignins from different eucalypt species. Holzforschung 2008, 62, 514–526. [Google Scholar] [CrossRef]
- Alekhina, M.; Ershova, O.; Ebert, A.; Heikkinen, S.; Sixta, H. Softwood kraft lignin for value-added applications: Fractionation and structural characterization. Ind. Crops Prod. 2015, 66, 220–228. [Google Scholar] [CrossRef]
- Martín-Sampedro, R.; Santos, J.I.; Fillat, Ú.; Eugenio, M.E.; Wicklein, B.; Jiménez-López, L.; Ibarra, D. Chemical and thermal analysis of lignin streams from Robinia pseudoacacia L. generated during organosolv and acid hydrolysis pre-treatments and subsequent enzymatic hydrolysis. Int. J. Biol. Macromol. 2019, 140, 311–322. [Google Scholar] [CrossRef]
- Martín-Sampedro, R.; Santos, J.I.; Fillat, Ú.; Wicklein, B.; Eugenio, M.E.; Ibarra, D. Characterization of lignins from Populus alba L. generated as by-products in different transformation processes: Kraft pulping, organosolv and acid hydrolysis. Int. J. Biol. Macromol. 2019, 126, 18–29. [Google Scholar] [CrossRef] [PubMed]
- Santos, J.I.; Martín-Sampedro, R.; Fillat, Ú.; Oliva, J.M.; Negro, M.J.; Ballesteros, M.; Eugenio, M.E.; Ibarra, D. Evaluating lignin-rich fermentation residues from biochemical ethanol production of wheat straw and olive tree-pruning by FTIR and 2D-NMR. Int. J. Polym. Technol. 2015, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Domínguez-Robles, J.; Sánchez, R.; Espinosa, E.; Savy, D.; Mazzei, P.; Piccolo, A.; Rodríguez, A. Isolation and characterization of gramineae and fabaceae soda lignins. Int. J. Mol. Sci. 2017, 18, 327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prinsen, P.; Rencoret, J.; Gutiérrez, A.; Liitiä, T.; Tamminen, T.; Colodette, J.L.; Berbis, M.A.; Jiménez-Barbero, J.; Martínez, A.; del Río, J.C. Modification of the lignin structure during alkaline delignification of eucalyptus wood by Kraft, Soda-AQ, and Soda-O2 cooking. Ind. Eng. Chem. Res. 2013, 52, 15702–15712. [Google Scholar] [CrossRef] [Green Version]
- Zhao, C.; Huang, J.; Yang, L.; Yue, F.; Lu, F. Revealing structural differences between alkaline and kraft lignins by HSQC NMR. Ind. Eng. Chem. Res. 2019, 58, 5707–5714. [Google Scholar] [CrossRef]
- Lawoko, M.; Henriksson, G.; Gellerstedt, G. Characterization of lignin-carbohydrate complexes (LCCs) of spruce wood (Picea abies L.) isolated with two methods. Holzforschung 2006, 60, 156–161. [Google Scholar] [CrossRef]
- Fodil Cherif, M.; Trache, D.; Brosse, N.; Benaliouche, F.; Ahmed Fouzi Tarchoun, A.F. Comparison of the physicochemical properties and thermal stability of organosolv and kraft lignins from hardwood and softwood biomass for their potential valorization. Waste Biomass Valor. 2020, 11, 6541–6553. [Google Scholar] [CrossRef]
- Ibarra, D.; del Río, J.C.; Gutiérrez, A.; Rodríguez, I.M.; Romero, J.; Martínez, M.J.; Martínez, A.T. Isolation of high-purity residual lignins from eucalypt paper pulps by cellulase and proteinase treatments followed by solvent extraction. Enzyme Microb. Technol. 2004, 35, 173–181. [Google Scholar] [CrossRef] [Green Version]
- Tejado, A.; Peña, C.; Labidi, J.; Echeverria, J.M.; Mondragón, I. Physico-chemical characterization of lignins from different sources for use in phenol–formaldehyde resin synthesis. Bioresour. Technol. 2007, 98, 1655–1663. [Google Scholar] [CrossRef]
- Eugenio, M.E.; Martín-Sampedro, R.; Santos, J.I.; Wicklein, B.; Martín, J.A.; Ibarra, D. Properties versus application requirements of solubilized lignins from an elm clone during different pre-treatments. Int. J. Biol. Macromol. 2021, 181, 99–111. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-López, L.; Martín-Sampedro, R.; Eugenio, M.E.; Santos, J.I.; Sixto, H.; Cañellas, I.; Ibarra, D. Co-production of soluble sugars and lignin from short rotation white poplar and black locust crops. Wood Sci. Technol. 2020, 54, 1617–1643. [Google Scholar] [CrossRef]
- Del Río, J.C.; Gutiérrez, A.; Romero, J.; Martínez, M.J.; Martínez, A.T. Identification of residual lignin markers in eucalypt kraft pulps by Py-GC/MS. J. Anal. Appl. Pyr. 2001, 58, 425–439. [Google Scholar] [CrossRef] [Green Version]
- Sannigrahi, P.; Ragauskas, A.J. Characterization of fermentation residues from the production of bio-ethanol from lignocellulosic feedstocks. J. Biobased Mat. Bioeneg. 2011, 5, 514–519. [Google Scholar] [CrossRef]
- Santos, J.I.; Fillat, Ú.; Martín-Sampedro, R.; Eugenio, M.E.; Negro, M.J.; Ballesteros, I.; Rodríguez, A.; Ibarra, D. Evaluation of lignins from side-streams generated in an olive tree pruning-based biorefinery: Bioethanol production and alkaline pulping. Int. J. Biol. Macromol. 2017, 105, 238–251. [Google Scholar] [CrossRef]
- Giummarella, N.; Pylypchuk, V.; Sevastyanova, O.; Lawoko, M. New structures in Eucalyptus kraft lignin with complex mechanistic implications. ACS Sustain. Chem. Eng. 2020, 8, 10983–10994. [Google Scholar] [CrossRef]
- Lancefield, C.S.; Wienk, H.L.J.; Boelens, R.; Weckhuysen, B.M.; Bruijnincx, P.C.A. Identification of a diagnostic structural motif reveals a new reaction intermediate and condensation pathway in kraft lignin formation. Chem. Sci. 2018, 9, 6348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ralph, S.A.; Ralph, J.; Landucci, L. NMR Database of Lignin and Cell Wall Model Compounds. US Forest Products Laboratory, Madison, WI. 2006. Available online: Services/docs.htm?docids10491 (accessed on 21 March 2021).
- Rencoret, J.; Gutiérrez, A.; Castro, E.; del Río, J.C. Structural characteristics of lignin in pruning residues of olive tree (Olea europea L.). Holzforschung 2019, 73, 25–34. [Google Scholar] [CrossRef]
- Balakshin, M.Y.; Capanema, E.A.; Chen, D.; Gracz, H.S. Elucidation of the structures of residual and dissolved pine kraft lignins using an HMQC NMR technique. J. Agric. Food. Chem. 2003, 51, 6116–6127. [Google Scholar] [CrossRef]
- Fernández-Costas, C.; Gouveia, S.; Sanromán, M.A.; Moldes, M. Structural characterization of Kraf lignins from different spent cooking liquors by 1D and 2D Nuclear Magnetic Resonance Spectroscopy. Biomass Bioenerg. 2014, 63, 156–166. [Google Scholar] [CrossRef]
- Ibarra, D.; Chávez, M.I.; Rencoret, J.; del Río, J.C.; Gutiérrez, A.; Romero, J.; Camarero, S.; Martínez, M.J.; Jiménez-Barbero, J.; Martínez, A.T. Lignin modification during Eucalyptus globulus kraft pulping followed by totally chlorine-free bleaching: A two-dimensional nuclear magnetic resonance, Fourier transform infrared, and pyrolysis-gas chromatography/mass spectrometry study. J. Agric. Food Chem. 2007, 55, 3477–3490. [Google Scholar] [CrossRef] [Green Version]
- Del Río, J.C.; Rencoret, J.; Prinsen, P.; Martínez, A.T.; Ralph, J.; Gutiérrez, A. Structural characterization of wheat straw lignin as revealed by analytical pyrolysis, 2D-NMR, and Reductive Cleavage Methods. J. Agric. Food Chem. 2012, 60, 5922–5935. [Google Scholar] [CrossRef] [Green Version]
- Del Río, J.C.; Prinsen, P.; Rencoret, J.; Nieto, L.; Jiménez-Barbero, J.; Ralph, J.; Martínez, A.T.; Gutiérrez, A. Structural Characterization of the Lignin in the Cortex and Pith of Elephant Grass (Pennisetum purpureum) Stems. J. Agric. Food Chem. 2012, 60, 3619–3634. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ralph, J. Hydroxycinnamates in lignification. Phytochem. Rev. 2010, 9, 65–83. [Google Scholar] [CrossRef]
- Marques, A.V.; Rencoret, J.; Gutiérrez, A.; del Río, J.C.; Pereira, H. Ferulates and lignins structural composition in cork. Holzforschung 2015, 70, 275–289. [Google Scholar] [CrossRef]
- Barneto, A.G.; Carmona, J.A.; Alfonso, J.E.M.; Alcaide, L.J. Use of autocatalytic kinetics to obtain composition of lignocellulosic materials. Bioresour. Technol. 2009, 100, 3963–3973. [Google Scholar] [CrossRef] [PubMed]
- Faravelli, T.; Frassoldati, A.; Migliavacca, G.; Ranzi, E. Detailed kinetic modeling of the thermal degradation of lignins. Biomass Bioenerg. 2010, 34, 290–301. [Google Scholar] [CrossRef]
- Sanghamitra, S.; Shrada, P.; Argyropoulos, A. Thermal properties of lignin in copolymers, blends, and composites; a review. Green Chem. 2015, 17, 4862–4887. [Google Scholar] [CrossRef]
- Espinoza-Acosta, J.L.; Torres-Chávez, P.I.; Ramírez-Wong, B.; López-Saiz, C.M.; Montaño-Leyva, B. Antioxidant, antimicrobial, and antimutagenic properties of technical lignins and their applications. Bioresources 2016, 11, 5452–5481. [Google Scholar] [CrossRef]
- Arshanitsa, A.; Ponomarenko, J.; Dizhbite, T.; Andersone, A.; Gosselink, R.J.A.; van der Putten, J.; Lauberts, M.; Telysheva, G. Fractionation of technical lignins as a tool for improvement of their antioxidant properties. J. Anal. Appl. Pyrolysis 2013, 103, 78–85. [Google Scholar] [CrossRef] [Green Version]
- Barclay, L.R.C.; Xi, F.; Norris, J.Q. Antioxidant properties of phenolic lignin model compounds. J. Wood Chem. Technol. 1997, 17, 73–90. [Google Scholar] [CrossRef]
- Lourençon, T.V.; Lima, G.G.; Ribeiro, C.S.P.; Hansel, F.A.; Maciel, G.M.; da Silva, K.; Winnischofer, S.M.B.; de Muniz, G.I.B.; Magalhaes, W.L.E. Antioxidant, antibacterial and antitumoural activities of kraft lignin from hardwood fractionated by acid precipitation. Int. J. Biol. Macromol. 2021, 166, 1535–1542. [Google Scholar] [CrossRef]
- Ratanasumarn, N.; Chitprasert, P. Cosmetic potential of lignin extracts from alkaline-treated sugarcane bagasse: Optimization of extraction conditions using response surface methodology. Int. J. Biol. Macromol. 2020, 153, 138–145. [Google Scholar] [CrossRef]
- Qazi, S.S.; Li, D.; Briens, C.; Berruti, F.; Abou-Zaid, M.M. Antioxidant Activity of the Lignins Derived from Fluidized-Bed Fast Pyrolysis. Molecules 2017, 22, 372. [Google Scholar] [CrossRef] [Green Version]
- Juttuporn, W.; Thiengkaew, P.; Rodklongtan, A.; Rodprapakorn, M.; Chitprasert, P. Utltrasound-Assisted extraction of antioxidant and antibacterial phenolic compounds from steam-exploded sugarcane bagasse. Sugar Tech. 2018, 20, 599–608. [Google Scholar] [CrossRef]
- Witzler, M.; Alzagameem, A.; Bergs, M.; Khaldi-Hasen, B.; Klein, S.E.; Hielscher, D.; Kamm, B.; Kreyenschmidt, J.; Tobiasch, E.; Schulze, M. Lignin-Derived biomaterials for drug release and tissue engineering. Molecules 2018, 23, 1885. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gordobil, O.; Herrera, R.; Yahyaoui, M.; Ilk, S.; Kaya, M.; Labidi, J. Potential use of kraft and organosolv lignins as a natural additive for healthcare products. RSC Adv. 2018, 8, 24525. [Google Scholar] [CrossRef] [Green Version]
- García, A.; Toledano, A.; Andrés, M.A.; Labidi, J. Study of the antioxidant capacity of Miscanthus sinensis lignins. Process. Biochem. 2010, 45, 935–940. [Google Scholar] [CrossRef]
- Balzadeh, Z.; Arabi, H. Insights into the chemical composition and thermo-oxidative stability of novel polyethylene copolymers containing ancillary phenolic antioxidant groups as non-migrating polyolefin stabilizer. Polym. Degrad. Stab. 2017, 142, 139–149. [Google Scholar] [CrossRef]
- Fernandes, D.M.; Winkler Hechenleitner, A.A.; Job, A.E.; Radovanocic, E.; Gόmez Pineda, E.A. Thermal and photochemical stability of poly(vinyl alcohol)/modified lignin blends. Polym. Degrad. Stab. 2006, 91, 1192–1201. [Google Scholar] [CrossRef]
- Pouteau, C.; Dole, P.; Cathala, B.; Averous, L.; Boquillon, N. Antioxidant properties of lignin in polypropylene. Polym. Degrad. Stab. 2003, 81, 9–18. [Google Scholar] [CrossRef]
- Tao, J.; Li, S.; Ye, F.; Zhou, Y.; Lei, L.; Zhao, G. Lignin—An underutilized, renewable and valuable material for food industry. Crit. Rev. Food Sci. Nutr. 2019, 60, 22011–22033. [Google Scholar] [CrossRef]
- Li, M.F.; Sun, S.N.; Xu, F.; Sun, R.C. Microwave-assisted organic acid extraction of lignin from bamboo: Structure and antioxidant activity investigation. Food Chem. 2012, 134, 1392–1398. [Google Scholar] [CrossRef]
- Gregorova, A.; Cibulkova, Z.; Kosikova, B.; Simon, P. Stabilization effect of lignin in polypropylene and recycled polypropylene. Polym. Degrad. Stab. 2005, 89, 553–558. [Google Scholar] [CrossRef]
- Dasari, A.; Yu, Z.-Z.; Cai, G.-P.; Mai, Y.-W. Recent developments in the fire retardancy of polymeric materials. Prog. Polym. Sci. 2013, 38, 1357–1387. [Google Scholar] [CrossRef]
- Liguori, F.; Moreno-Marrodan, C.; Barbaro, P. Biomass-derived chemical substitutes forbisphenol A: Recent advancements in catalytic synthesis. Chem. Soc. Rev. 2020, 49, 6329–6363. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Qian, M.; Song, P.; Huang, G.; Yu, Y.; Fu, S. Fabrication of Green Lignin-based Flame Retardants for Enhancing the Thermal and Fire Retardancy Properties of Polypropylene/Wood Composites. ACS Sustain. Chem. Eng. 2016, 4, 2422–2431. [Google Scholar] [CrossRef]
- National Renewable Energy Laboratory, 2010. NREL. Chemical Analysis and Testing Laboratory Analytical Procedures. Available online: http://www.eere.energy.gov/biomass/analytical_procedures.html (accessed on 21 March 2021).
- Sameni, J.; Krigstin, S.; dos Santos Rosa, D.; Leao, A.; Mohini, S. Thermal characteristics of lignin residue from industrial processes. Bioresources 2014, 9, 725–737. [Google Scholar] [CrossRef] [Green Version]
- Re, R.; Pannala, A.; Pellegrini, N.; Proteggente, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
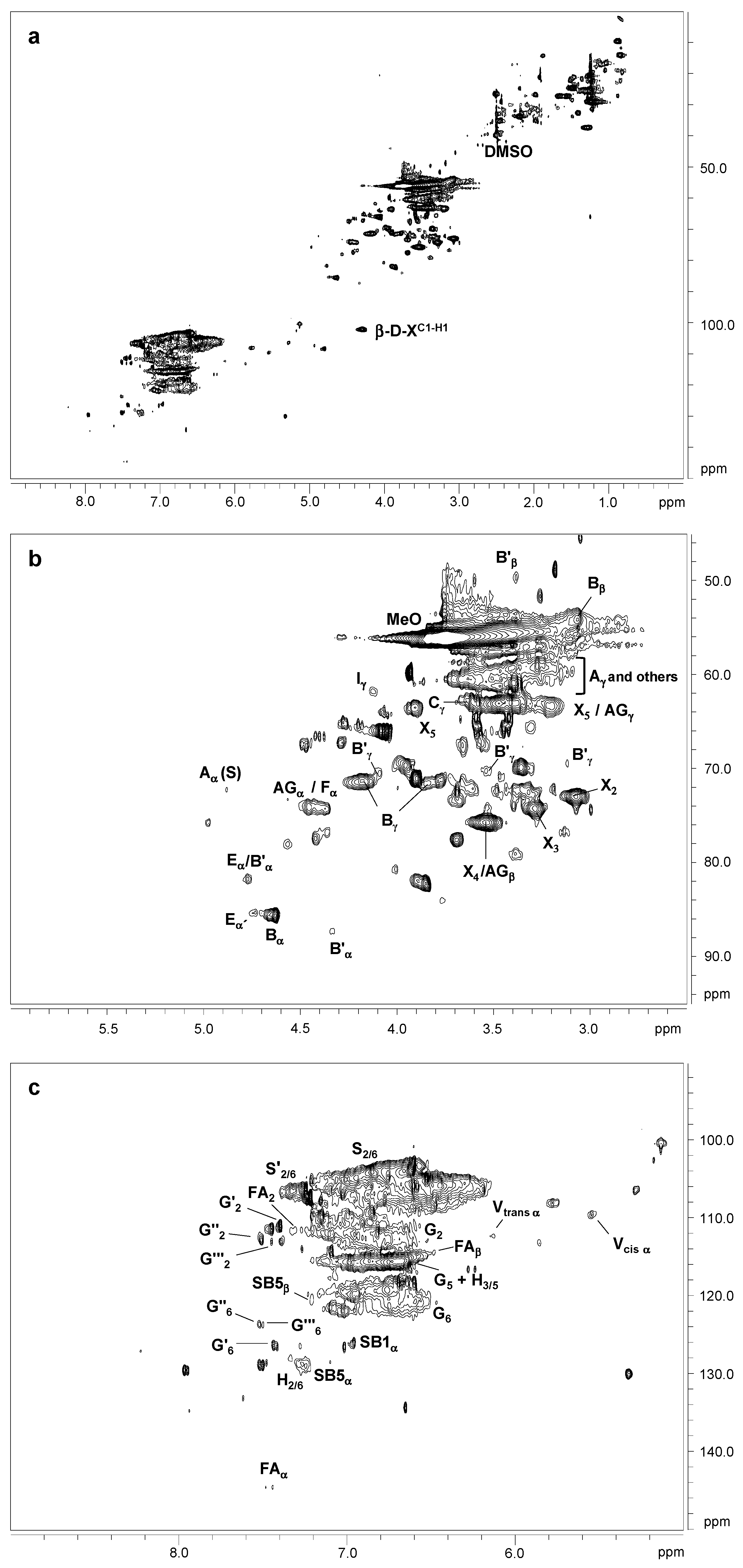
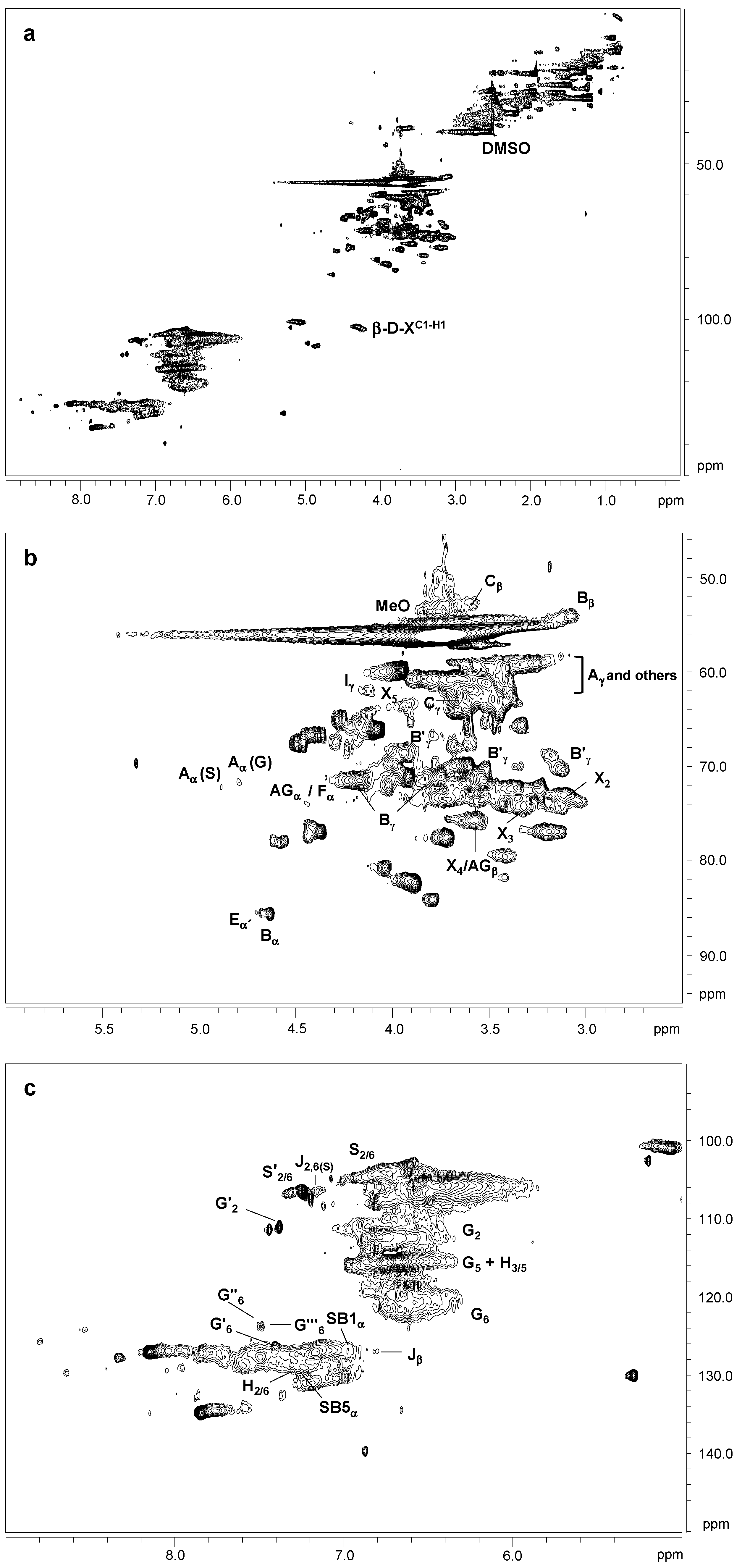
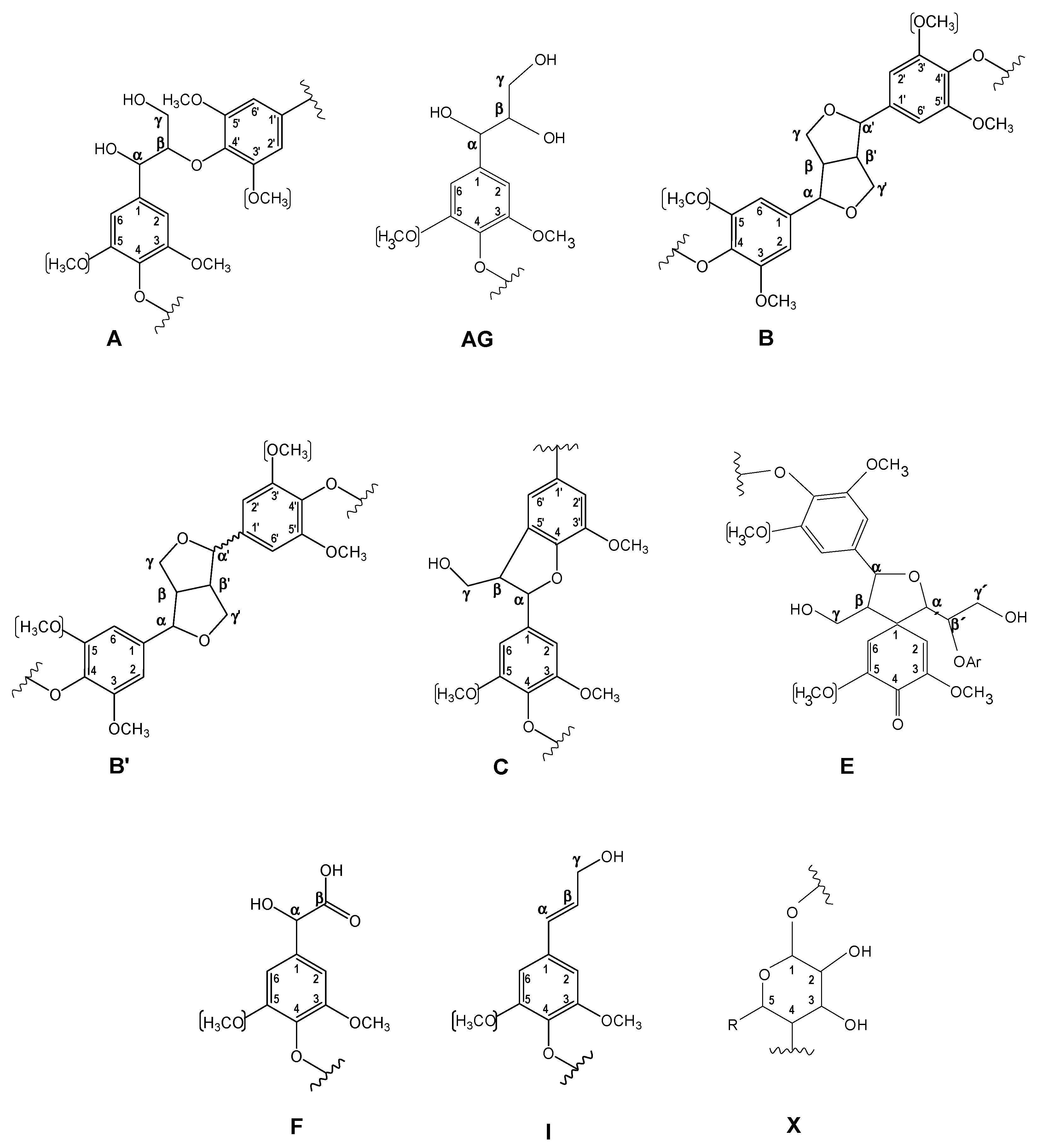
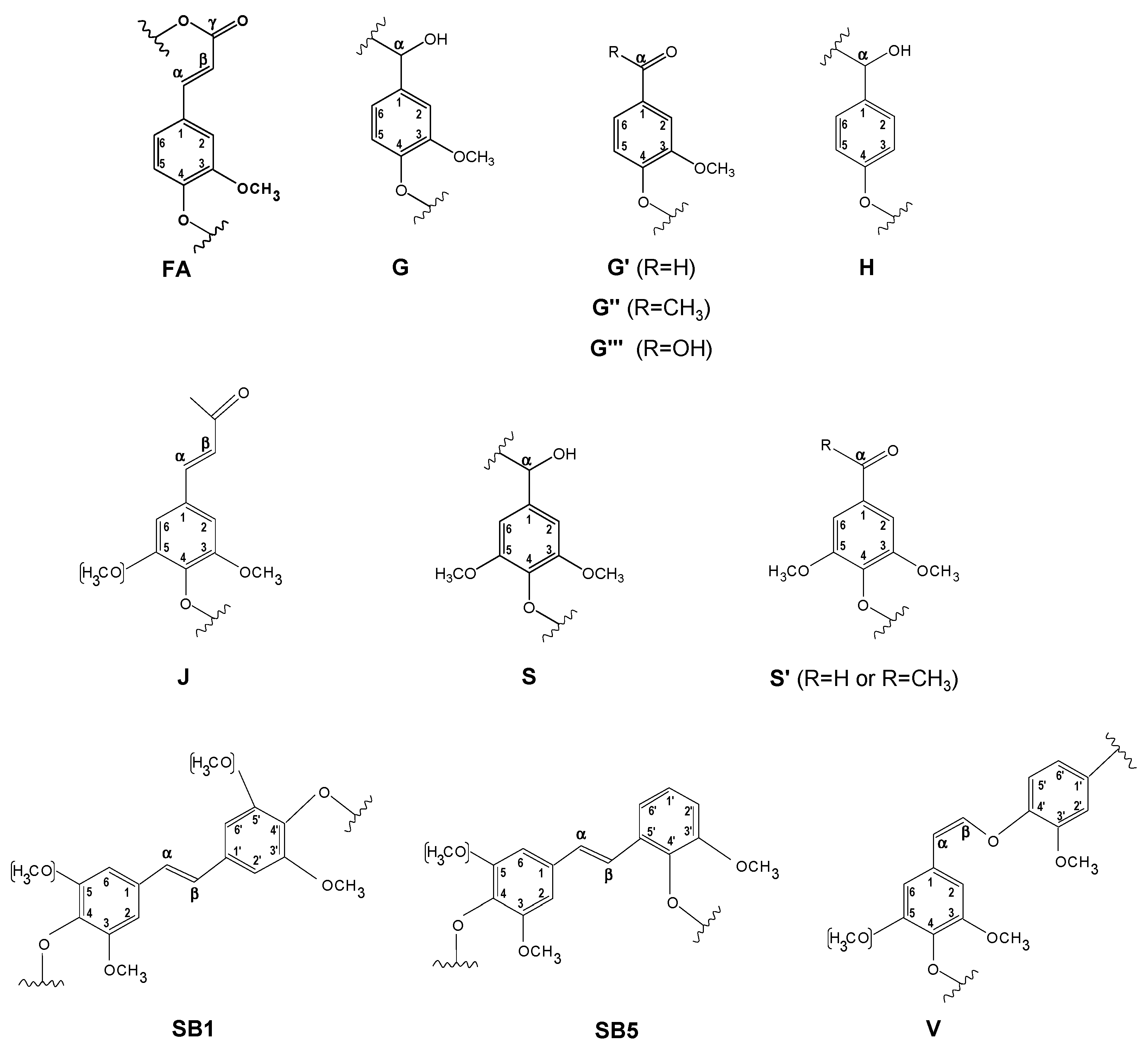
| δC/δH (ppm) | Assignment |
|---|---|
| 49.7/3.38 | Cβ–Hβ, epiresinol substructures (B’) |
| 53.3/3.49 | Cβ–Hβ, phenylcoumaran substructures (C) |
| 54.2/3.08 | Cβ–Hβ, resinol substructures (B) |
| 56.1/3.72 | C–H, methoxyls (MeO) |
| 60.6/3.38–3.64 | Cγ–Hγ, β-O-4’ substructures (A) |
| 61.8/4.11 | Cγ–Hγ, cinnamyl alcohol end groups (I) |
| 62.7/3.65 | Cγ–Hγ, phenylcoumaran substructures (C) |
| 63.3/3.23–3.90 | C5–H5, xylan |
| 63.3/3.19–3.28 | Cγ–Hγ, aryl-glycerol (AG) |
| 69.5/3.11–3.57 | Cγ–Hγ, epiresinol substructures (B’) |
| 70.5/3.73–4.09 | Cγ–Hγ, epiresinol substructures (B’) |
| 71.3/3.76–4.18 | Cγ–Hγ, resinol substructures (B) |
| 71.6/4.79 | Cα–Hα, β-O-4’ G unit (A) |
| 72.2/4.88 | Cα–Hα, β-O-4’ S unit (A) |
| 72.8/3.08 | C2–H2, xylan |
| 74.0/4.45 | Cα–Hα, aryl-glycerol (AG) |
| 74.1/3.28 | C3–H3, xylan |
| 74.3/4.37 | Cα–Hα, Ar–CHOH–COOH units (F) |
| 75.6/3.49 | Cβ–Hβ, aryl-glycerol (AG) |
| 75.9/3.55 | C4–H4, xylan |
| 81.7/4.74 | Cα–Hα, spirodienone substructures (E) |
| 81.8/4.76 | Cα–Hα, epiresinol substructures (B’) |
| 85.3/4.74 | Cα’ –Hα’, spirodienone substructures (E) |
| 85.5/4.63 | Cα–Hα, resinol substructures (B) |
| 87.4/4.31 | Cα–Hα, epiresinol substructures (B’) |
| 101.6/4.30 | C-1, (1-4) β-D-Xylp |
| 104.3/6.69 | C2,6–H2,6, S units (S) |
| 106.2/7.12 | C2,6–H2,6, in cinamaldehyde end-groups (J) |
| 106.7/7.32 | C2,6–H2,6, oxidized (H–Cα=O or H3C–Cα=O) S units (S’) |
| 109.8/5.54 | Cα–Hα, isomer cis of vinil ether (V) |
| 111.0/6.88 | C2–H2, G units (G) |
| 111.0/7.38 | C2–H2, oxidized (H–Cα=O) G units (G’) |
| 111.7/7.31 | C2–H2, ferulate (FA) |
| 112.3/7.51 | C2–H2, oxidized (H3C–Cα=O) G units (G’’) |
| 112.4/6.13 | Cα–Hα, isomer trans of vinyl ether (V) |
| 113.2/7.44 | C2–H2, oxidized (HO–Cα=O) G units (G’’’) |
| 114.3/6.48 | Cβ–Hβ, ferulate (FA) |
| 115.0/6.74 | C3,5–H3,5, p-hydroxyphenyl (H) |
| 115.2/6.42–6.81 | C5–H5, G units (G) |
| 119.6/6.78 | C6–H6, G units (G) |
| 120.6/7.21 | Cβ–Hβ, stilbene (SB5β) |
| 123.7/7.52 | C6–H6, oxidized (H3C–Cα=O) G units (G’’) |
| 123.8/7.49 | C6–H6, oxidized (HO–Cα=O) G units (G’’’) |
| 126.2/6.97 | Cα–Hα, stilbene (SB1α) |
| 126.5/7.43 | C6–H6, oxidized (H–Cα=O) G units (G’) |
| 126.9/6.80 | Cβ–Hβ, cinnamaldehyde end-groups (J) |
| 128.4/7.17 | C2,6–H2,6, p-hydroxyphenyl (H) |
| 128.8/7.13 | Cα–Hα, stilbene (SB5α) |
| 144.7/7.46 | Cα–Hα, ferulate (FA) |
| Soda/AQ-Orange | Soda/AQ-Olive | |
|---|---|---|
| β-O-4’ (A) | 0.8 | 1.3 |
| Resinols (B) | 4.3 | 3.5 |
| Phenylcoumarans (C) | 0.3 | 0.2 |
| Spirodienones (E) | 0.9 | 0.6 |
| Arylglicerol (AG) | 2.1 | 0.5 |
| Ar−CHOH−COOH (F) | 3.1 | 0.5 |
| Ferulates (FA) | 0.5 | - |
| cinnamyl alcohol end-groups (I) | 1.1 | 0.9 |
| cinnamaldehyde end-groups (J) | - | 0.8 |
| Stilbene (SB1) | 0.9 | 2.8 |
| Stilbene (SB5) | 1.5 | 3.0 |
| Vinyl-ether (V) | 1.0 | - |
| H (%) | 0.8 | 0.7 |
| G (%) | 17.7 | 24.1 |
| S (%) | 81.5 | 75.2 |
| S/G ratio | 4.6 | 3.1 |
| Soda/AQ-Orange | Soda/AQ-Olive | |
|---|---|---|
| Mw | 6430 | 6280 |
| Mn | 4890 | 4980 |
| Mw/Mn | 1.31 | 1.26 |
| Soda/AQ-Orange | Soda/AQ-Olive | |
|---|---|---|
| mg TE g−1 lignin | 149.7 ± 1.2 | 598.2 ± 4.7 |
| mM TE g−1 lignin | 202.4 ± 5.1 | 808.9 ± 20.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eugenio, M.E.; Martín-Sampedro, R.; Santos, J.I.; Wicklein, B.; Ibarra, D. Chemical, Thermal and Antioxidant Properties of Lignins Solubilized during Soda/AQ Pulping of Orange and Olive Tree Pruning Residues. Molecules 2021, 26, 3819. https://doi.org/10.3390/molecules26133819
Eugenio ME, Martín-Sampedro R, Santos JI, Wicklein B, Ibarra D. Chemical, Thermal and Antioxidant Properties of Lignins Solubilized during Soda/AQ Pulping of Orange and Olive Tree Pruning Residues. Molecules. 2021; 26(13):3819. https://doi.org/10.3390/molecules26133819
Chicago/Turabian StyleEugenio, María E., Raquel Martín-Sampedro, José I. Santos, Bernd Wicklein, and David Ibarra. 2021. "Chemical, Thermal and Antioxidant Properties of Lignins Solubilized during Soda/AQ Pulping of Orange and Olive Tree Pruning Residues" Molecules 26, no. 13: 3819. https://doi.org/10.3390/molecules26133819






