Hemorphins—From Discovery to Functions and Pharmacology
Abstract
:1. Introduction
2. Proteolysis of Hemoglobin β Chain
3. How to Identify Endogenous Molecule?
4. Identification and Quantification of Hemorphins
4.1. Endogenous Peptides
4.1.1. Antibody-based Methods
4.1.2. Mass Spectrometry
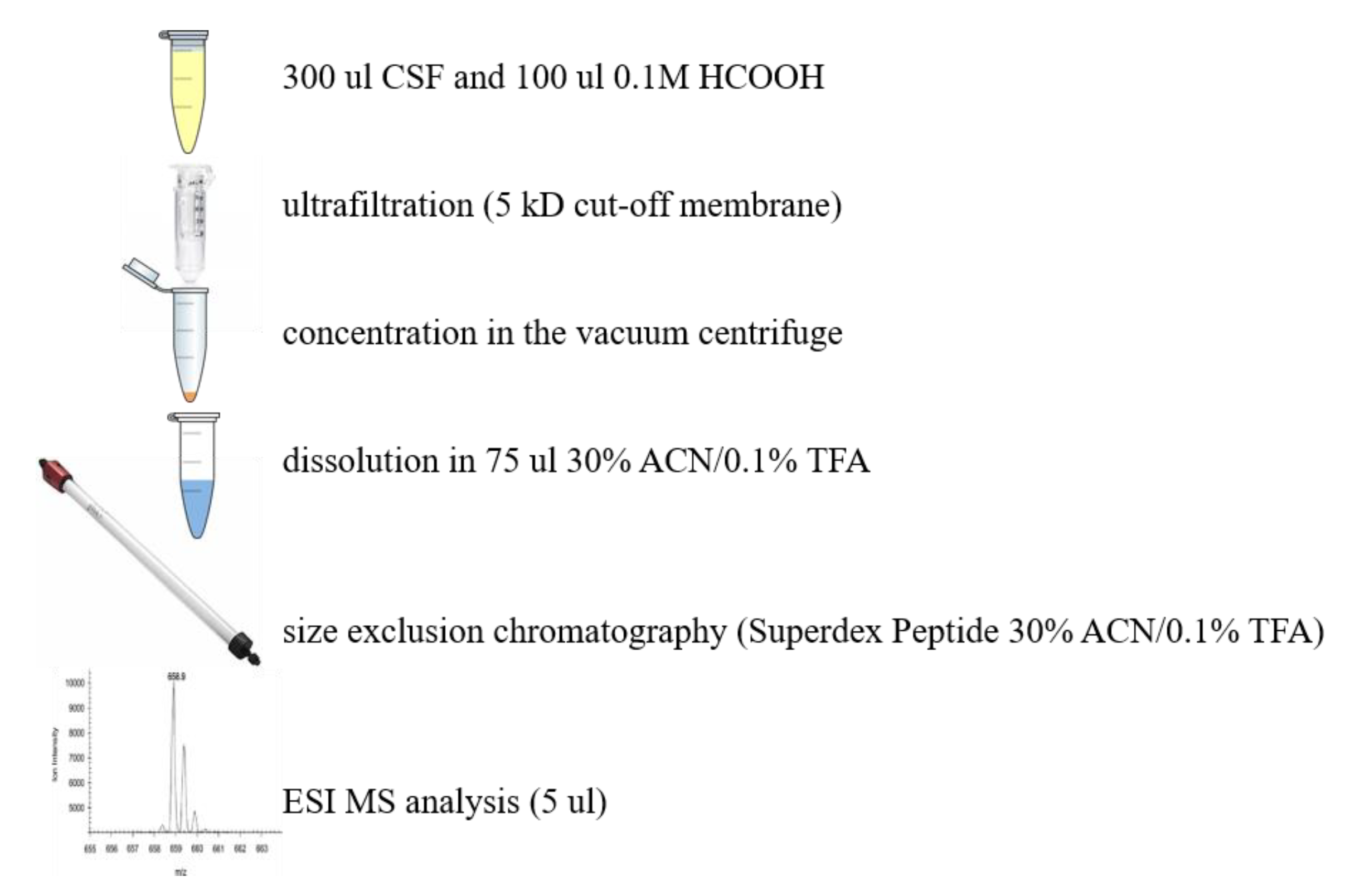
4.1.3. Other Techniques
4.2. Synthetic Hemorphins
5. Pharmacology of Hemorphins
6. Learning
7. Pain
8. Other Effects
9. Hemorphin Analogues
10. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Karelin, A.A.; Philippova, M.M.; Karelina, E.V.; Ivanov, V.T. Isolation of endogenous hemorphin-related hemoglobin fragments from bovine brain. Biochem. Biophys. Res. Commun. 1994, 202, 410–415. [Google Scholar] [CrossRef]
- Sanderson, K.; Nyberg, F.; Khalil, Z. Modulation of peripheral inflammation by locally administered hemorphin-7. Inflamm. Res. 1998, 47, 49–55. [Google Scholar] [CrossRef]
- Nyberg, F.; Sanderson, K.; Glämsta, E.L. The hemorphins: A new class of opioid peptides derived from the blood protein hemoglobin. Biopolymers 1997, 43, 147–156. [Google Scholar] [CrossRef]
- Glämsta, E.L.; Marklund, A.; Hellman, U.; Wernstedt, C.; Terenius, L.; Nyberg, F. Isolation and characterization of a hemoglobin-derived opioid peptide from the human pituitary gland. Regul. Pept. 1991, 34, 169–179. [Google Scholar] [CrossRef]
- Barkhudaryan, N.; Oberthuer, W.; Lottspeich, F.; Galoyan, A. Structure of hypothalamic coronaro-constrictory peptide factors. Neurochem. Res. 1992, 17, 1217–1221. [Google Scholar] [CrossRef] [PubMed]
- Cerpa-Poljak, A.; Lahnstein, J.; Mason, K.E.; Smythe, G.A.; Duncan, M.W. Mass spectrometric identification and quantification of hemorphins extracted from human adrenal and pheochromocytoma tissue. J. Neurochem. 1997, 68, 1712–1719. [Google Scholar] [CrossRef]
- Yatskin, O.N.; Philippova, M.M.; Blishchenko, E.; Karelin, A.A.; Ivanov, V.T. Lvv- and vv-hemorphins: Comparative levels in rat tissues. FEBS Lett. 1998, 428, 286–290. [Google Scholar] [CrossRef] [Green Version]
- Glämsta, E.L.; Meyerson, B.; Silberring, J.; Terenius, L.; Nyberg, F. Isolation of a hemoglobin-derived opioid peptide from cerebrospinal fluid of patients with cerebrovascular bleedings. Biochem. Biophys. Res. Commun. 1992, 184, 1060–1066. [Google Scholar] [CrossRef]
- Brantl, V.; Gramsch, C.; Lottspeich, F.; Mertz, R.; Jaeger, K.H.; Herz, A. Novel opioid peptides derived from hemoglobin: Hemorphins. Eur. J. Pharmacol. 1986, 125, 309–310. [Google Scholar] [CrossRef]
- Fruitier, I.; Garreau, I.; Lacroix, A.; Cupo, A.; Piot, J.M. Proteolytic degradation of hemoglobin by endogenous lysosomal proteases gives rise to bioactive peptides: Hemorphins. FEBS Lett. 1999, 447, 81–86. [Google Scholar] [CrossRef] [Green Version]
- Piot, J.M.; Zhao, Q.; Guillochon, D.; Ricart, G.; Thomas, D. Isolation and characterization of two opioid peptides from a bovine hemoglobin peptic hydrolysate. Biochem. Biophys. Res. Commun. 1992, 189, 101–110. [Google Scholar] [CrossRef]
- Jinsmaa, Y.; Yoshikawa, M. Release of hemorphin-5 from human hemoglobin by pancreatic elastase. Biosci. Biotechnol. Biochem. 2002, 66, 1130–1132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fruitier, I.; Garreau, I.; Piot, J.M. Cathepsin D is a good candidate for the specific release of a stable hemorphin from hemoglobin in vivo: VV-hemorphin-7. Biochem. Biophys. Res. Commun. 1998, 246, 719–724. [Google Scholar] [CrossRef]
- Garreau, I.; Cucumel, K.; Dagouassat, N.; Zhao, Q.; Cupo, A.; Piot, J.M. Hemorphin peptides are released from hemoglobin by cathepsin d. Radioimmunoassay against the c-part of v-v-hemorphin-7: An alternative assay for the cathepsin d activity. Peptides 1997, 18, 293–300. [Google Scholar] [CrossRef]
- Zadina, J.E.; Kastin, A.J.; Kersh, D.; Wyatt, A. Tyr-mif-1 and hemorphin can act as opiate agonists as well as antagonists in the guinea pig ileum. Life Sci. 1992, 51, 869–885. [Google Scholar] [CrossRef]
- Silberring, J.; Nyberg, F. Rapid analysis of endogenous lvv-hemorphin-7 in cerebrospinal fluid by size-exclusion chromatography and electrospray ionization mass spectrometry. J. Chromatogr. A 1997, 777, 41–45. [Google Scholar] [CrossRef]
- Mak, P.; Wójcik, K.; Wicherek, L.; Suder, P.; Dubin, A. Antibacterial hemoglobin peptides in human menstrual blood. Peptides 2004, 25, 1839–1847. [Google Scholar] [CrossRef]
- Ali, A.; Alzeyoudi, S.A.R.; Almutawa, S.A.; Alnajjar, A.N.; Vijayan, R. Molecular basis of the therapeutic properties of hemorphins. Pharmacol. Res. 2020, 158, 104855. [Google Scholar] [CrossRef]
- Altinoz, M.A.; Elmaci, I.; Ince, B.; Ozpinar, A.; Sav, A.M. Hemoglobins, hemorphins, and 11p15.5 chromosomal region in cancer biology and İmmunity with special emphasis for brain tumors. J. Neurol. Surg. A Cent. Eur. Neurosurg. 2016, 77, 247–257. [Google Scholar] [PubMed]
- Altinoz, M.A.; Ozcan, E.M.; Ince, B.; Guloksuz, S. Hemoglobins as new players in multiple sclerosis: Metabolic and immune aspects. Metab. Brain Dis. 2016, 31, 983–992. [Google Scholar] [CrossRef]
- Wei, F.; Zhao, L.; Jing, Y. Hemoglobin-derived peptides and mood regulation. Peptides 2020, 127, 170268. [Google Scholar] [CrossRef] [PubMed]
- Ayoub, M.A.; Vijayan, R. Hemorphins targeting g protein-coupled receptors. Pharmaceuticals 2021, 14, 225. [Google Scholar] [CrossRef] [PubMed]
- Dagouassat, N.; Garreau, I.; Sannier, F.; Zhao, Q.; Piot, J.M. Generation of vv-hemorphin-7 from globin by peritoneal macrophages. FEBS Lett. 1996, 382, 37–42. [Google Scholar] [CrossRef] [Green Version]
- Garreau, I.; Zhao, Q.; Pejoan, C.; Cupo, A.; Piot, J.M. Vv-hemorphin-7 and lvv-hemorphin-7 released during in vitro peptic hemoglobin hydrolysis are morphinomimetic peptides. Neuropeptides 1995, 28, 243–250. [Google Scholar] [CrossRef]
- Jörnvall, H.; Agerberth, B.; Zasloff, M. Viktor mutt: A giant in the field of bioactive peptides. In Comprehensive Biochemistry; Skulachev, V.P., Semenza, G., Eds.; Elsevier: Amsterdam, The Netherlands, 2008; Volume 46, pp. 397–416. [Google Scholar]
- Glämsta, E.L.; Nyberg, F.; Silberring, J. Application of fast-atom bombardment mass spectrometry for sequencing of a hemoglobin fragment, naturally occurring in human cerebrospinal fluid. Rapid Commun. Mass Spectrom. 1992, 6, 777–780. [Google Scholar] [CrossRef]
- Nyberg, G.; Sanderson, K.; Andren, P.; Thornwall, M.; Einarsson, M.; Danielson, B.; Nyberg, F. Isolation of haemorphin-related peptides from filter membranes collected in connection with haemofiltration of human subjects. J. Chromatogr. A 1996, 723, 43–49. [Google Scholar] [CrossRef]
- Cohen, M.; Fruitier-Arnaudin, I.; Sauvan, R.; Birnbaum, D.; Piot, J.M. Serum levels of hemorphin-7 peptides in patients with breast cancer. Clin. Chim. Acta 2003, 337, 59–67. [Google Scholar] [CrossRef]
- Ianzer, D.; Konno, K.; Xavier, C.H.; Stöcklin, R.; Santos, R.A.; de Camargo, A.C.; Pimenta, D.C. Hemorphin and hemorphin-like peptides isolated from dog pancreas and sheep brain are able to potentiate bradykinin activity in vivo. Peptides 2006, 27, 2957–2966. [Google Scholar] [CrossRef]
- Murillo, L.; Piot, J.M.; Coitoux, C.; Fruitier-Arnaudin, I. Brain processing of hemorphin-7 peptides in various subcellular fractions from rats. Peptides 2006, 27, 3331–3340. [Google Scholar] [CrossRef]
- Elagli, A.; Belhacene, K.; Dhulster, P.; Froidevaux, R. Sustainable efficient way for opioid peptide lvv-h7 preparation from enzymatic proteolysis in a microfluidic-based reaction-extraction process with solvent recycling. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2016, 1020, 24–28. [Google Scholar] [CrossRef]
- Teschemacher, H. Opioid receptor ligands derived from food proteins. Curr. Pharm. Des. 2003, 9, 1331–1344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kapel, R.; Froidevaux, R.; Nedjar-Arroume, N.; Fertin-Bazus, A.; Dhulster, P.; Guillochon, D. Continuous production of a peptidic fraction containing the intermediate opioid peptide lvv-haemorphin-7 (lvvh-7) by peptic hydrolysis of bovine haemoglobin in a continuous membrane reactor. Biotechnol. Appl. Biochem. 2003, 37, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Glämsta, E.L.; Mørkrid, L.; Lantz, I.; Nyberg, F. Concomitant increase in blood plasma levels of immunoreactive hemorphin-7 and beta-endorphin following long distance running. Regul. Pept. 1993, 49, 9–18. [Google Scholar] [CrossRef]
- Sanderson, K.; Thörnwall, M.; Nyberg, G.; Glämsta, E.L.; Nyberg, F. Reversed-phase high-performance liquid chromatography for the determination of haemorphin-like immunoreactivity in human cerebrospinal fluid. J. Chromatogr. A 1994, 676, 155–160. [Google Scholar] [CrossRef]
- Cohen, M.; Fruitier-Arnaudin, I.; Garreau-Balandier, I.; Piot, J.M. Quantification of hemorphin-7 peptides by enzyme linked immunosorbent assay with secondary antibody. Anal. Chim. Acta 2002, 461, 229–233. [Google Scholar] [CrossRef]
- Fricker, L.D. Analysis of mouse brain peptides using mass spectrometry-based peptidomics: Implications for novel functions ranging from non-classical neuropeptides to microproteins. Mol. Biosyst. 2010, 6, 1355–1365. [Google Scholar] [CrossRef]
- Domenger, D.; Cudennec, B.; Kouach, M.; Touche, V.; Landry, C.; Lesage, J.; Gosselet, F.; Lestavel, S.; Goossens, J.F.; Dhulster, P.; et al. Food-derived hemorphins cross intestinal and blood-brain barriers. Front. Endocrinol. (Lausanne) 2018, 9, 159. [Google Scholar] [CrossRef]
- Inserra, I.; Martelli, C.; Cipollina, M.; Cicione, C.; Iavarone, F.; Taranto, G.D.; Barba, M.; Castagnola, M.; Desiderio, C.; Lattanzi, W. Lipoaspirate fluid proteome: A preliminary investigation by lc-ms top-down/bottom-up integrated platform of a high potential biofluid in regenerative medicine. Electrophoresis 2016, 37, 1015–1026. [Google Scholar] [CrossRef]
- Iavarone, F.; Desiderio, C.; Vitali, A.; Messana, I.; Martelli, C.; Castagnola, M.; Cabras, T. Cryptides: Latent peptides everywhere. Crit. Rev. Biochem. Mol. Biol. 2018, 53, 246–263. [Google Scholar] [CrossRef]
- Ner, J.; Kotlinska, J.H.; Silberring, J. Crypteins--an overlooked piece of peptide systems. Curr. Protein Pept. Sci. 2015, 16, 203–218. [Google Scholar] [CrossRef]
- Iavarone, F.; Olianas, A.; Patini, R.; Gallenzi, P.; Di Tonno, L.; Desiderio, C.; Cabras, T.; Manconi, B.; Vincenzoni, F.; Cordaro, M.; et al. Top down proteomic analysis of gingival crevicular fluid in deciduous, exfoliating and permanent teeth in children. J. Proteomics 2020, 226, 103890. [Google Scholar] [CrossRef]
- Hayakari, M.; Satoh, K.; Izumi, H.; Kudoh, T.; Asano, J.; Yamazaki, T.; Tsuchida, S. Kinetic-controlled hydrolysis of leu-val-val-hemorphin-7 catalyzed by angiotensin-converting enzyme from rat brain. Peptides 2003, 24, 1075–1082. [Google Scholar] [CrossRef]
- John, H.; John, S.; Forssmann, W.G. Kinetic studies on aminopeptidase m-mediated degradation of human hemorphin lvv-h7 and its n-terminally truncated products. J. Pept. Sci. 2008, 14, 797–803. [Google Scholar] [CrossRef]
- John, H.; Hierer, J.; Haas, O.; Forssmann, W.G. Quantification of angiotensin-converting-enzyme-mediated degradation of human chemerin 145-154 in plasma by matrix-assisted laser desorption/ionization-time-of-flight mass spectrometry. Anal. Biochem. 2007, 362, 117–125. [Google Scholar] [CrossRef]
- Dejouvencel, T.; Féron, D.; Rossignol, P.; Sapoval, M.; Kauffmann, C.; Piot, J.M.; Michel, J.B.; Fruitier-Arnaudin, I.; Meilhac, O. Hemorphin 7 reflects hemoglobin proteolysis in abdominal aortic aneurysm. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 269–275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bucknall, M.; Fung, K.Y.; Duncan, M.W. Practical quantitative biomedical applications of maldi-tof mass spectrometry. J. Am. Soc. Mass Spectrom. 2002, 13, 1015–1027. [Google Scholar] [CrossRef] [Green Version]
- Zeccola, M.; Longhi, R.; Rossetti, D.V.; D’Angelo, L.; Tamburrini, G.; Di Rocco, C.; Giardina, B.; Castagnola, M.; Desiderio, C. Development and validation of a capillary electrophoresis tandem mass spectrometry analytical method for the determination of leu-val-val- and val-val-hemorphin-7 peptides in cerebrospinal fluid. J. Chromatogr. A 2012, 1267, 170–177. [Google Scholar] [CrossRef] [PubMed]
- John, H.; Schulz, S.; Forssmann, W.G. Validated multi-component cze-uv procedure for the quantification of human hemorphin lvv-h7 in plasma stability studies. Anal. Bioanal. Chem. 2006, 386, 235–243. [Google Scholar] [CrossRef] [PubMed]
- John, H.; Schulz, S.; Forssmann, W.G. Comparative in vitro degradation of the human hemorphin lvv-h7 in mammalian plasma analysed by capillary zone electrophoresis and mass spectrometry. Biopharm. Drug Dispos. 2007, 28, 73–85. [Google Scholar] [CrossRef]
- Barkhudaryan, N.; Zakaryan, H.; Sarukhanyan, F.; Gabrielyan, A.; Dosch, D.; Kellermann, J.; Lottspeich, F. Hemorphins act as homeostatic agents in response to endotoxin-induced stress. Neurochem. Res. 2010, 35, 925–933. [Google Scholar] [CrossRef]
- Todorov, P.T.; Peneva, P.N.; Georgieva, S.I.; Tchekalarova, J.; Vitkova, V.; Antonova, K.; Georgiev, A. Synthesis, characterization and anticonvulsant activity of new azobenzene-containing vv-hemorphin-5 bio photoswitch. Amino. Acids 2019, 51, 549–563. [Google Scholar] [CrossRef]
- Ali, A.; Palakkott, A.; Ashraf, A.; Al Zamel, I.; Baby, B.; Vijayan, R.; Ayoub, M.A. Positive modulation of angiotensin ii type 1 receptor-mediated signaling by lvv-hemorphin-7. Front. Pharmacol. 2019, 10, 1258. [Google Scholar] [CrossRef]
- Ali, A.; Johnstone, E.K.M.; Baby, B.; See, H.B.; Song, A.; Rosengren, K.J.; Pfleger, K.D.G.; Ayoub, M.A.; Vijayan, R. Insights into the interaction of lvv-hemorphin-7 with angiotensin ii type 1 receptor. Int. J. Mol. Sci. 2020, 22, 209. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Baby, B.; Soman, S.S.; Vijayan, R. Molecular insights into the interaction of hemorphin and its targets. Sci. Rep. 2019, 9, 14747. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fruitier-Arnaudin, I.; Cohen, M.; Coitoux, C.; Piot, J.M. In vitro metabolism of lvv-hemorphin-7 by renal cytosol and purified prolyl endopeptidase. Peptides 2003, 24, 1201–1206. [Google Scholar] [CrossRef]
- Lammerich, H.P.; Busmann, A.; Kutzleb, C.; Wendland, M.; Seiler, P.; Berger, C.; Eickelmann, P.; Meyer, M.; Forssmann, W.G.; Maronde, E. Identification and functional characterization of hemorphins vv-h-7 and lvv-h-7 as low-affinity agonists for the orphan bombesin receptor subtype 3. Br. J. Pharmacol. 2003, 138, 1431–1440. [Google Scholar] [CrossRef] [Green Version]
- Barkhudaryan, N. In vivo microdialysis is a tool to study the mechanism of interaction between lvv-hemorphin-7 and brain serotonergic system. Biotechnol. Health Yerevan 2005, 32–42. [Google Scholar]
- Ivanov, V.T.; Karelin, A.A.; Philippova, M.M.; Nazimov, I.V.; Pletnev, V.Z. Hemoglobin as a source of endogenous bioactive peptides: The concept of tissue-specific peptide pool. Biopolymers 1997, 43, 171–188. [Google Scholar] [CrossRef]
- Moisan, S.; Harvey, N.; Beaudry, G.; Forzani, P.; Burhop, K.E.; Drapeau, G.; Rioux, F. Structural requirements and mechanism of the pressor activity of leu-val-val-hemorphin-7, a fragment of hemoglobin beta-chain in rats. Peptides 1998, 19, 119–131. [Google Scholar] [CrossRef]
- Darvesh, A.S.; Carroll, R.T.; Geldenhuys, W.J.; Gudelsky, G.A.; Klein, J.; Meshul, C.K.; Van der Schyf, C.J. In vivo brain microdialysis: Advances in neuropsychopharmacology and drug discovery. Expert Opin. Drug Discov. 2011, 6, 109–127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jessop, D.S. Neuropeptides in the immune system: Mediators of stress and inflammation. In Handbook of Neurochemistry and Molecular Biology; Edition: Neuroimmunology; Springer: Berlin/Heidelberg, Germany, 2008; pp. 19–35. [Google Scholar]
- Lee, J.; Albiston, A.L.; Allen, A.M.; Mendelsohn, F.A.; Ping, S.E.; Barrett, G.L.; Murphy, M.; Morris, M.J.; McDowall, S.G.; Chai, S.Y. Effect of i.C.V. Injection of at4 receptor ligands, nle1-angiotensin iv and lvv-hemorphin 7, on spatial learning in rats. Neuroscience 2004, 124, 341–349. [Google Scholar] [CrossRef]
- Olson, M.L.; Olson, E.A.; Qualls, J.H.; Stratton, J.J.; Harding, J.W.; Wright, J.W. Norleucine1-angiotensin iv alleviates mecamylamine-induced spatial memory deficits. Peptides 2004, 25, 233–241. [Google Scholar] [CrossRef]
- Pederson, E.S.; Harding, J.W.; Wright, J.W. Attenuation of scopolamine-induced spatial learning impairments by an angiotensin iv analog. Regul. Pept. 1998, 74, 97–103. [Google Scholar] [CrossRef]
- Wright, J.W.; Clemens, J.A.; Panetta, J.A.; Smalstig, E.B.; Weatherly, L.A.; Kramár, E.A.; Pederson, E.S.; Mungall, B.H.; Harding, J.W. Effects of ly231617 and angiotensin iv on ischemia-induced deficits in circular water maze and passive avoidance performance in rats. Brain Res. 1996, 717, 1–11. [Google Scholar] [CrossRef]
- Wiśniewski, K.; Borawska, M.; Car, H. The effect of angiotensin ii and its fragments on post-alcohol impairment of learning and memory. Pol. J. Pharmacol. 1993, 45, 23–29. [Google Scholar]
- Wright, J.W.; Stubley, L.; Pederson, E.S.; Kramár, E.A.; Hanesworth, J.M.; Harding, J.W. Contributions of the brain angiotensin iv-at4 receptor subtype system to spatial learning. J. Neurosci. 1999, 19, 3952–3961. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Albiston, A.L.; Pederson, E.S.; Burns, P.; Purcell, B.; Wright, J.W.; Harding, J.W.; Mendelsohn, F.A.; Weisinger, R.S.; Chai, S.Y. Attenuation of scopolamine-induced learning deficits by lvv-hemorphin-7 in rats in the passive avoidance and water maze paradigms. Behav. Brain Res. 2004, 154, 239–243. [Google Scholar] [CrossRef]
- De Bundel, D.; Smolders, I.; Yang, R.; Albiston, A.L.; Michotte, Y.; Chai, S.Y. Angiotensin iv and lvv-haemorphin 7 enhance spatial working memory in rats: Effects on hippocampal glucose levels and blood flow. Neurobiol. Learn. Mem. 2009, 92, 19–26. [Google Scholar] [CrossRef]
- De Bundel, D.; Demaegdt, H.; Lahoutte, T.; Caveliers, V.; Kersemans, K.; Ceulemans, A.G.; Vauquelin, G.; Clinckers, R.; Vanderheyden, P.; Michotte, Y.; et al. Involvement of the at1 receptor subtype in the effects of angiotensin iv and lvv-haemorphin 7 on hippocampal neurotransmitter levels and spatial working memory. J. Neurochem. 2010, 112, 1223–1234. [Google Scholar] [CrossRef]
- Moeller, I.; Lew, R.A.; Mendelsohn, F.A.; Smith, A.I.; Brennan, M.E.; Tetaz, T.J.; Chai, S.Y. The globin fragment lvv-hemorphin-7 is an endogenous ligand for the at4 receptor in the brain. J. Neurochem. 1997, 68, 2530–2537. [Google Scholar] [CrossRef]
- Albiston, A.L.; Mustafa, T.; McDowall, S.G.; Mendelsohn, F.A.; Lee, J.; Chai, S.Y. At4 receptor is insulin-regulated membrane aminopeptidase: Potential mechanisms of memory enhancement. Trends Endocrinol. Metab 2003, 14, 72–77. [Google Scholar] [CrossRef]
- Best, P.J.; White, A.M.; Minai, A. Spatial processing in the brain: The activity of hippocampal place cells. Annu. Rev. Neurosci. 2001, 24, 459–486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shapiro, M. Plasticity, hippocampal place cells, and cognitive maps. Arch. Neurol. 2001, 58, 874–881. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kramár, E.A.; Armstrong, D.L.; Ikeda, S.; Wayner, M.J.; Harding, J.W.; Wright, J.W. The effects of angiotensin iv analogs on long-term potentiation within the ca1 region of the hippocampus in vitro. Brain Res. 2001, 897, 114–121. [Google Scholar] [CrossRef]
- Wayner, M.J.; Armstrong, D.L.; Phelix, C.F.; Wright, J.W.; Harding, J.W. Angiotensin iv enhances ltp in rat dentate gyrus in vivo. Peptides 2001, 22, 1403–1414. [Google Scholar] [CrossRef]
- Cheng, B.C.; Tao, P.L.; Cheng, Y.Y.; Huang, E.Y. Lvv-hemorphin 7 and angiotensin iv in correlation with antinociception and anti-thermal hyperalgesia in rats. Peptides 2012, 36, 9–16. [Google Scholar] [CrossRef]
- Chow, L.H.; Chen, Y.H.; Lai, C.F.; Lin, T.Y.; Chen, Y.J.; Kao, J.H.; Huang, E.Y. Sex difference of angiotensin iv-, lvv-hemorphin 7-, and oxytocin-induced antiallodynia at the spinal level in mice with neuropathic pain. Anesth. Analg. 2018, 126, 2093–2101. [Google Scholar] [CrossRef]
- Hung, H.Y.; Chow, L.H.; Kotlinska, J.H.; Drabik, A.; Silberring, J.; Chen, Y.H.; Huang, E.Y. Lvv-hemorphin-7 (lvv-h7) plays a role in antinociception in a rat model of alcohol-induced pain disorders. Peptides 2021, 136, 170455. [Google Scholar] [CrossRef]
- Beyer, C.E.; Dwyer, J.M.; Platt, B.J.; Neal, S.; Luo, B.; Ling, H.P.; Lin, Q.; Mark, R.J.; Rosenzweig-Lipson, S.; Schechter, L.E. Angiotensin iv elevates oxytocin levels in the rat amygdala and produces anxiolytic-like activity through subsequent oxytocin receptor activation. Psychopharmacology 2010, 209, 303–311. [Google Scholar] [CrossRef]
- Da Cruz, K.R.; Turones, L.C.; Camargo-Silva, G.; Gomes, K.P.; Mendonça, M.M.; Galdino, P.; Rodrigues-Silva, C.; Santos, R.A.S.; Costa, E.A.; Ghedini, P.C.; et al. The hemoglobin derived peptide lvv-hemorphin-7 evokes behavioral effects mediated by oxytocin receptors. Neuropeptides 2017, 66, 59–68. [Google Scholar] [CrossRef]
- Huang, E.Y.; Chen, Y.H.; Huang, T.Y.; Chen, Y.J.; Chow, L.H. Chronic administration of nandrolone increases susceptibility to morphine dependence without correlation with lvv-hemorphin 7 in rats. Neuropeptides 2016, 59, 63–69. [Google Scholar] [CrossRef]
- Blishchenko, E.; Sazonova, O.; Surovoy, A.; Khaidukov, S.; Sheikine, Y.; Sokolov, D.; Freidlin, I.; Philippova, M.; Vass, A.; Karelin, A.; et al. Antiproliferative action of valorphin in cell cultures. J. Pept. Sci. 2002, 8, 438–452. [Google Scholar] [CrossRef]
- Petrov, R.V.; Mikhailova, A.A.; Fonina, L.A. Bone marrow immunoregulatory peptides (myelopeptides): Isolation, structure, and functional activity. Biopolymers 1997, 43, 139–146. [Google Scholar] [CrossRef]
- Poljak, A.; McLean, C.A.; Sachdev, P.; Brodaty, H.; Smythe, G.A. Quantification of hemorphins in alzheimer’s disease brains. J. Neurosci. Res. 2004, 75, 704–714. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, Y.; Kanazawa, T.; Shimamura, M.; Ueki, M.; Hazato, T. Inhibitory effects of spinorphin, a novel endogenous regulator, on chemotaxis, o2- generation, and exocytosis by n-formylmethionyl-leucyl-phenylalanine (fmlp)-stimulated neutrophils. Biochem. Pharmacol. 1997, 54, 695–701. [Google Scholar] [CrossRef]
- Barkhudaryan, N.; Gambarov, S.; Gyulbayazyan, T.; Nahapetyan, K. Lvv-hemorphin-4 modulates ca2+/calmodulin-dependent pathways in the immune system by the same mechanism as in the brain. J. Mol. Neurosci. 2002, 18, 203–210. [Google Scholar] [CrossRef]
- Todorov, P.; Peneva, P.; Pechlivanova, D.; Georgieva, S.; Dzhambazova, E. Synthesis, characterization and nociceptive screening of new vv-hemorphin-5 analogues. Bioorg. Med. Chem. Lett. 2018, 28, 3073–3079. [Google Scholar] [CrossRef]
- Todorov, P.; Rangelov, M.; Peneva, P.; Todorova, N.; Tchekalarova, J. Anticonvulsant evaluation and docking analysis of vv-hemorphin-5 analogues. Drug Dev. Res. 2019, 80, 425–437. [Google Scholar] [CrossRef]
- Todorov, P.; Peneva, P.; Tchekalarova, J.; Georgieva, S. Potential anticonvulsant activity of novel vv-hemorphin-7 analogues containing unnatural amino acids: Synthesis and characterization. Amino Acids 2020, 52, 567–585. [Google Scholar] [CrossRef]
- Todorov, P.; Peneva, P.; Tchekalarova, J.; Georgieva, S.; Rangelov, M.; Todorova, N. Structure-activity relationship study on new hemorphin-4 analogues containing steric restricted amino acids moiety for evaluation of their anticonvulsant activity. Amino Acids 2020, 52, 1375–1390. [Google Scholar] [CrossRef]
- Todorov, P.; Peneva, P.; Tchekalarova, J.; Rangelov, M.; Georgieva, S.; Todorova, N. Synthesis, characterization and anticonvulsant activity of new series of n-modified analogues of vv-hemorphin-5 with aminophosphonate moiety. Amino Acids 2019, 51, 1527–1545. [Google Scholar] [CrossRef]
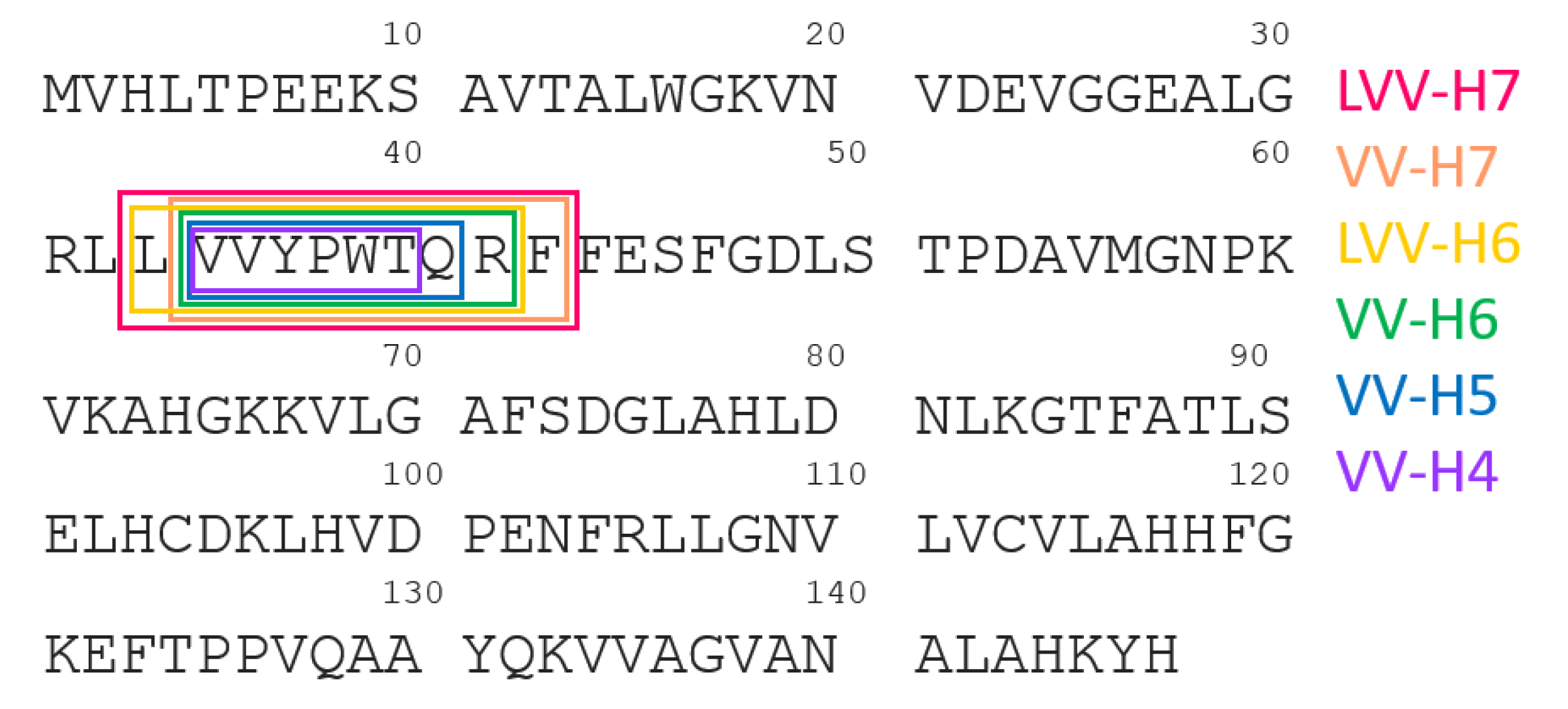

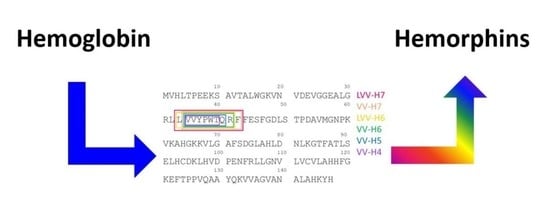
| Hemorphin | Sequence |
|---|---|
| LVV-H7 | LVVYPWTQRF |
| VV-H7 | VVYPWTQRF |
| LVV-H6 | LVVYPWTQR |
| VV-H6 | VVYPWTQR |
| VV-H5 | VVYPWTQ |
| VV-H4 | VVYPWT |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mielczarek, P.; Hartman, K.; Drabik, A.; Hung, H.-Y.; Huang, E.Y.-K.; Gibula-Tarlowska, E.; Kotlinska, J.H.; Silberring, J. Hemorphins—From Discovery to Functions and Pharmacology. Molecules 2021, 26, 3879. https://doi.org/10.3390/molecules26133879
Mielczarek P, Hartman K, Drabik A, Hung H-Y, Huang EY-K, Gibula-Tarlowska E, Kotlinska JH, Silberring J. Hemorphins—From Discovery to Functions and Pharmacology. Molecules. 2021; 26(13):3879. https://doi.org/10.3390/molecules26133879
Chicago/Turabian StyleMielczarek, Przemyslaw, Kinga Hartman, Anna Drabik, Hao-Yuan Hung, Eagle Yi-Kung Huang, Ewa Gibula-Tarlowska, Jolanta H. Kotlinska, and Jerzy Silberring. 2021. "Hemorphins—From Discovery to Functions and Pharmacology" Molecules 26, no. 13: 3879. https://doi.org/10.3390/molecules26133879