Construction of Porous Starch-Based Hydrogel via Regulating the Ratio of Amylopectin/Amylose for Enhanced Water-Retention
Abstract
:1. Introduction
2. Results and Discussion
2.1. Potential Mechanism of Amylose/Amylopectin Synergistic Regulation of Gel Microstructure Interaction
2.2. Structural Characteristics of Starch Gel
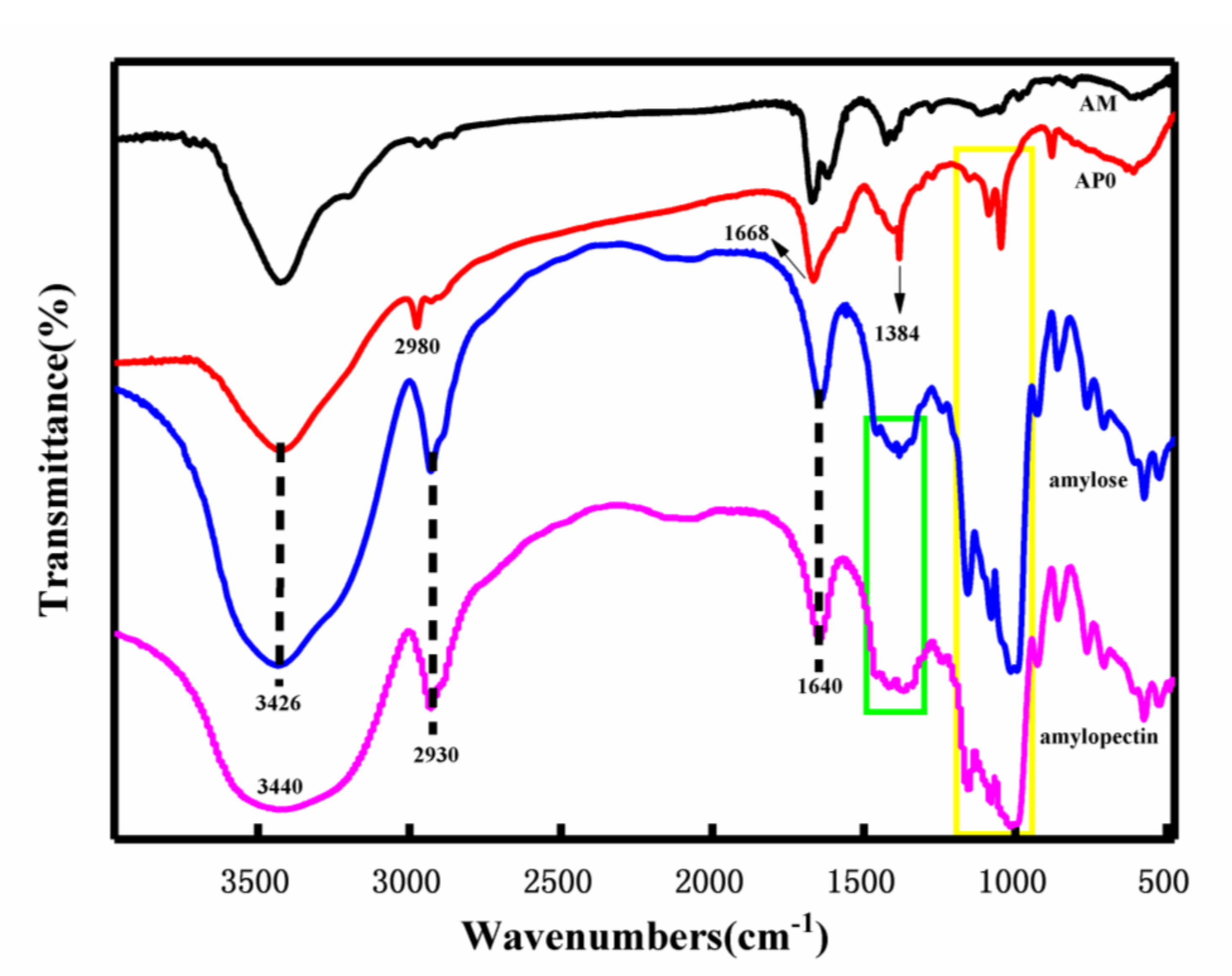
2.2.1. Chemical Structure Analysis
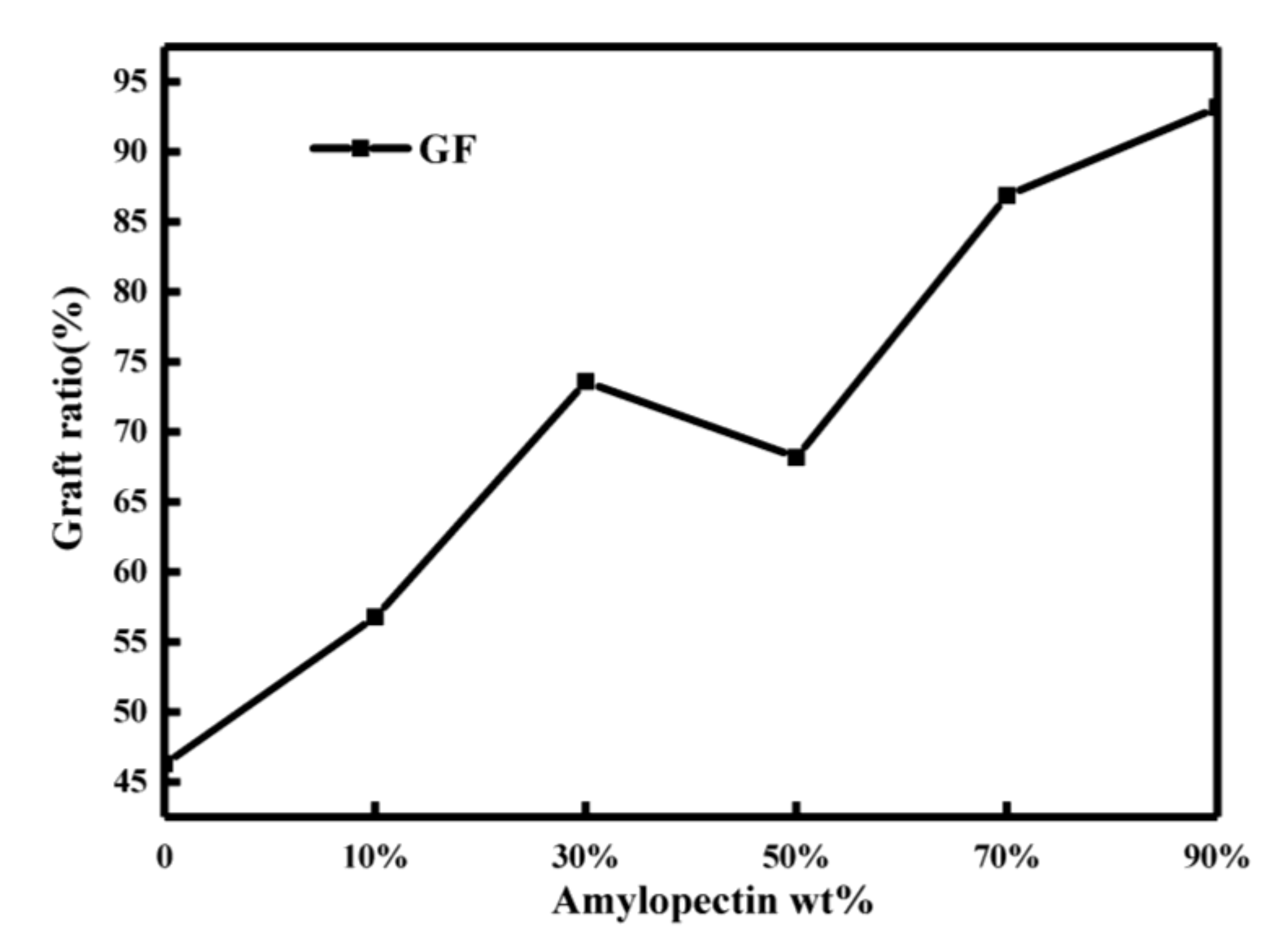
2.2.2. Analysis of Graft Ratio of Starch Gel
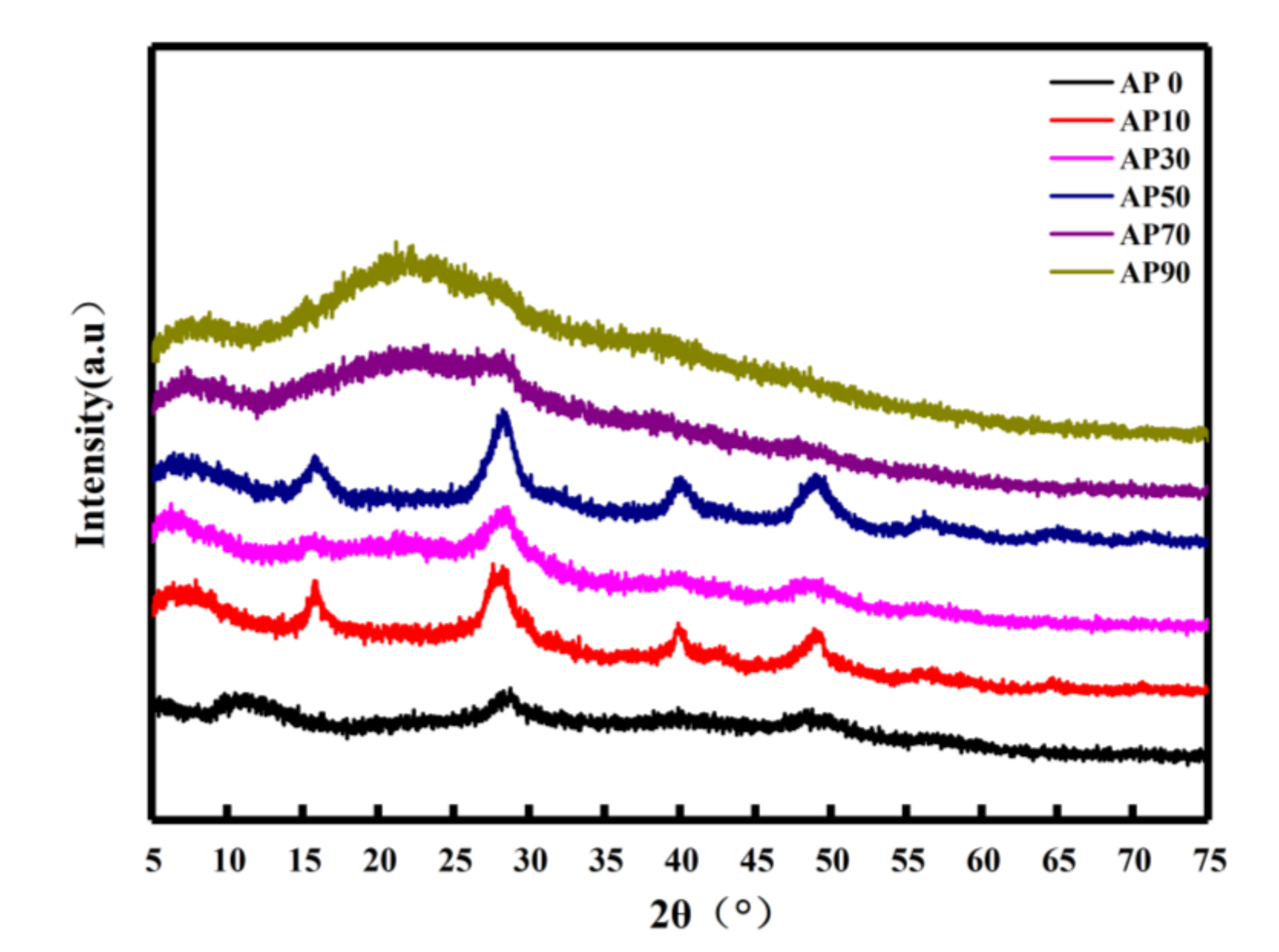
2.2.3. Crystallization Performance Analysis
2.2.4. Thermogravimetric Analysis (TGA)
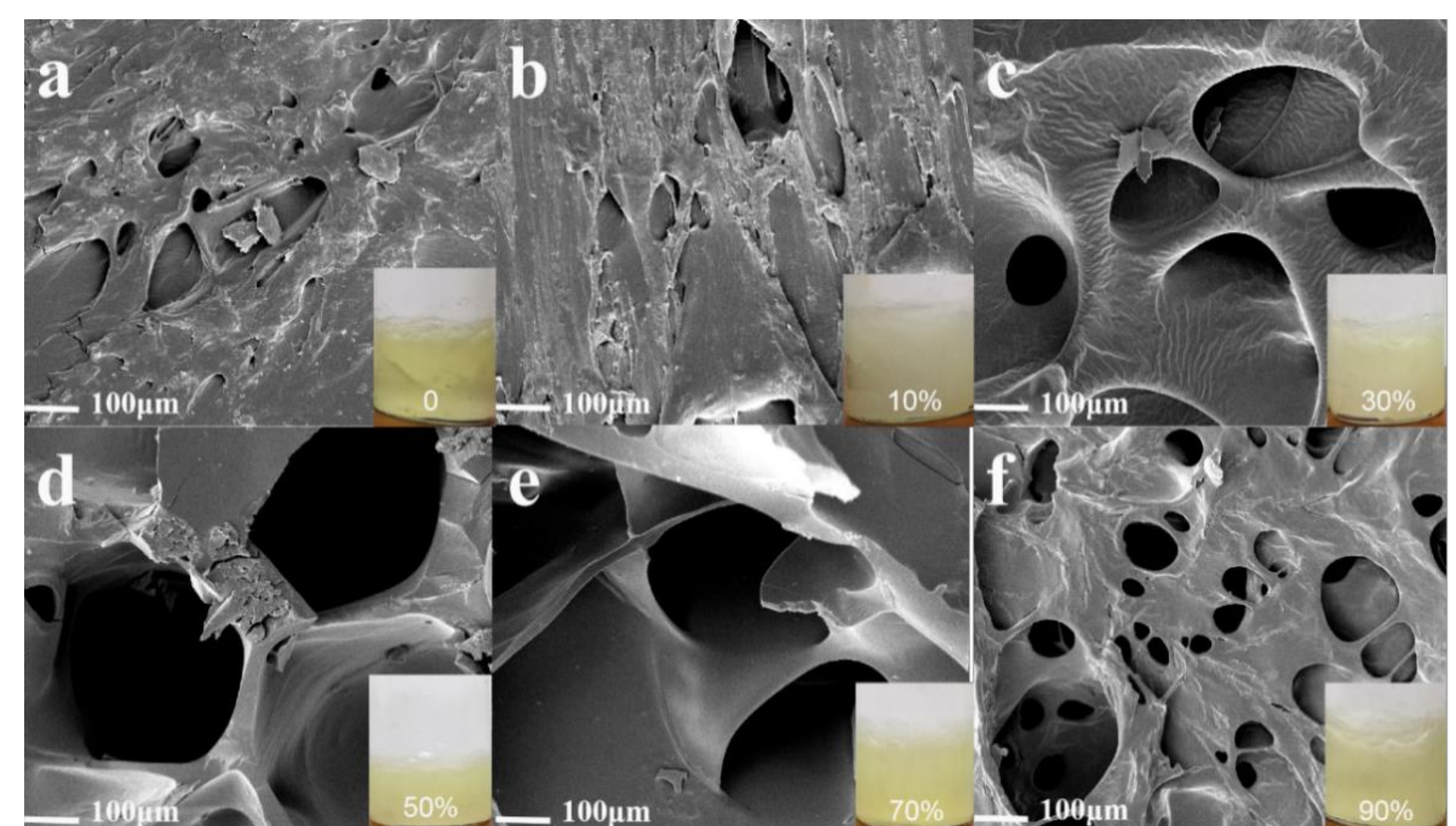
2.2.5. SEM Characterization of Starch Gel Micromorphology
2.3. Water Absorption Properties of Starch Gel
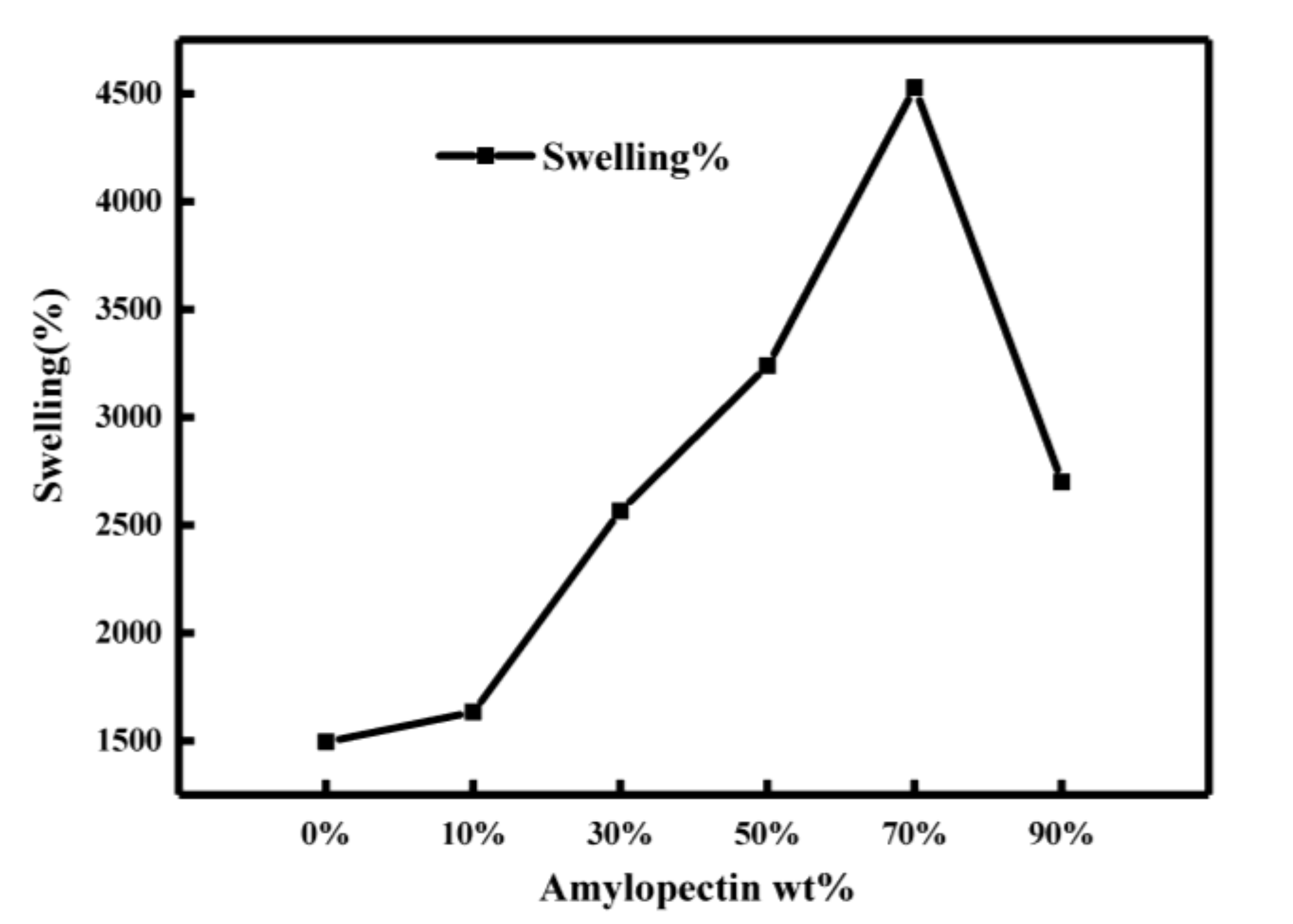
2.3.1. Swelling Performance Analysis
2.3.2. Analysis of Water Retention Performance
3. Materials and Methods
3.1. Materials
3.2. Gel Synthesis
3.3. Characterization of the Gel
3.3.1. Fourier Transform Infrared Spectroscopy (FTIR)
3.3.2. XRD Analysis
3.3.3. TGA Analysis
3.3.4. SEM Analysis
3.3.5. Analysis of Grafting Ratio
3.3.6. Water Absorption Performance Test
Water Swelling Rate (Swelling) Test
Water Retention Performance Test
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Cassardo, C. Global warming and water sustainability[C]//E3S Web of Conferences. EDP Sci. 2014, 2, 02006. [Google Scholar]
- Araújo, B.R.; Romão, L.P.; Doumer, M.E.; Mangrich, A.S. Evaluation of the interac-tions between chitosan and humics in media for the controlled release of nitrogenfertilizer. J. Environ. Manag. 2017, 190, 122–131. [Google Scholar] [CrossRef]
- Wen, P.; Han, Y.; Wu, Z.; He, Y.; Ye, B.C.; Wang, J. Rapid synthesis of a corncob-basedsemi-interpenetrating polymer network slow-release nitrogen fertilizer by micro-wave irradiation to control water and nutrient losses. Arab. J. Chem. 2017, 10, 922–934. [Google Scholar] [CrossRef]
- An, D.; Liu, B.; Yang, L.; Wang, T.J.; Kan, C. Fabrication of graphene oxide/polymer latex composite film coated on KNO 3 fertilizer to extend its release duration. Chem. Eng. J. 2017, 311, 318–325. [Google Scholar] [CrossRef]
- Xiao, X.; Yu, L.; Xie, F.; Bao, X.; Liu, H.; Ji, Z.; Chen, L. One-step method to prepare starch-based super absorbent polymer for slow release of fertilizer. Chem. Eng. J. 2017, 309, 607–616. [Google Scholar] [CrossRef] [Green Version]
- McClements, D.J. Recent progress in hydrogel delivery systems for improving nutraceutical bioavailability. Food Hydrocoll. 2017, 68, 238–245. [Google Scholar] [CrossRef] [Green Version]
- Wichterle, O.; Lim, D. Hydrophilic gels for biological use. Nature 1960, 185, 117–118. [Google Scholar] [CrossRef]
- Nieuwenhove, I.V.; Salamon, A.; Adam, S.; Dubruel, P.; Vlierberghe, S.V.; Peters, K. Gelatin-and starch-based hydrogels. Part B: In vitro mesenchymal stem cell behaviour on the hydrogels. Carbohydr. Polym. 2017, 161, 295–305. [Google Scholar] [CrossRef] [PubMed]
- García-Astrain, C.; Avérous, L. Synthesis and evaluation of functional alginate hydrogels based on click chemistry for drug delivery applications. Carbohydr. Polym. 2018, 190, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Wei, W.; Li, J.; Su, T.; Pan, X.; Zuo, G.; Zhang, J.; Dong, W. Design of Salecan-containing semi-IPN hydrogel for amoxicillin delivery. Mater. Sci. Eng. C 2017, 75, 487–494. [Google Scholar] [CrossRef]
- Seetapan, N.; Wongsawaeng, J.; Kiatkamjornwong, S. Gel strength and swelling of acrylamide-protic acid superabsorbent copolymers. Polym. Adv. Technol. 2011, 22, 1685–1695. [Google Scholar] [CrossRef]
- Zohuriaan-Mehr, M.J.; Omidian, H.; Doroudiani, S.; Kabiri, K. Advances in non-hy-gienic applications of superabsorbent hydrogel materials. J. Mater. Sci. 2010, 45, 5711–5735. [Google Scholar] [CrossRef]
- Guilherme, M.R.; Reis, A.V.; Paulino, A.T.; Moia, T.A.; Mattoso, L.H.; Tambourgi, E.B. Tambourgi, Pectin-based polymer hydrogel as a carrier for release of agricultural nutrients and removal of heavy metals from wastewater. J. Appl. Polym. Sci. 2010, 117, 3146–3154. [Google Scholar]
- Saraydın, D.; Karadağ, E.; Güven, O. Relationship between the swelling process and the releases of water soluble agrochemicals from radiation crosslinked acrylamide/itaconic acid copolymers. Polym. Bull. 2000, 45, 287–294. [Google Scholar] [CrossRef]
- Bouranis, D.L.; Theodoropoulos, A.G.; Drossopoulos, J.B. Designing synthetic polymers as soil conditioners. Commun. Soil Sci. Plant Anal. 1995, 26, 1455–1480. [Google Scholar] [CrossRef]
- El-Rehim, H.A.; Hegazy, E.S.; El-Mohdy, H.A. Radiation synthesis of hydrogels to en-hance sandy soils water retention and increase plant performanc. J. Appl. Polym. Sci. 2004, 93, 1360–1371. [Google Scholar] [CrossRef]
- Zohuriaan-Mehr, M.J.; Kabiri, K. Superabsorbent polymer materials: A review. Iran. Polym. J. 2008, 17, 451–477. [Google Scholar]
- Zou, W.; Yu, L.; Liu, X.; Chen, L.; Zhang, X.; Qiao, D.; Zhang, R. Effects of amylose/amylopectin ratio on starch-based superabsorbent polymers. Carbohydr. Polym. 2012, 87, 1583–1588. [Google Scholar] [CrossRef]
- Ali, A.; Ahmed, S. Recent advances in edible polymer based hydrogels as a sustainable alternative to conventional polymers. J. Agric. Food Chem. 2018, 66, 6940–6967. [Google Scholar] [CrossRef]
- Mun, S.; Kim, Y.-R.; McClements, D.J. Control of β-carotene bioaccessibility using starch-based filled hydrogels. Food Chem. 2015, 173, 454–461. [Google Scholar] [CrossRef]
- Caló, E.; Khutoryanskiy, V. Biomedical applications of hydrogels: A review patents and commercial products. Eur. Polym. J. 2015, 65, 252–267. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Wang, W.; Wang, A. Synthesis, characterization and swelling behaviors of hydroxyethyl cellulose-g-poly(acrylic acid)/attapulgite superabsorbent composite. J. Polym. Eng. Sci. 2010, 50, 1019–1027. [Google Scholar] [CrossRef]
- Kiatkamjornwong, S.; Mongkolsawat, K.; Sonsuk, M. Synthesis and property characterization of cassava starch grafted poly[acrylamide-co-(maleic acid)] superabsorbent via-irradiation. Polymer 2002, 43, 3915–3924. [Google Scholar] [CrossRef]
- Biduski, B.; da Ferreira Silva, W.M.; Colussi, R.; El Halal, S.L.-M.; Loong-Tak, L.; Guerra Dias, A.R.; Zavareze, E.R. Starch hydrogels: The influence of the amylose content and gelatinization method. Int. J. Biol. Macromol. 2018, 113, 443–449. [Google Scholar] [CrossRef]
- Chan, A.W.; Whitneym, R.A.; Neufeld, R.J. Semisynthesis of a controlled stimuli-responsive alginate hydrogel. Biomacromolecules 2009, 10, 609–616. [Google Scholar] [CrossRef] [PubMed]
- Gattás-Asfura, K.M.; Weisman, E.; Andreopoulos, F.M.; Micic, M.; Muller, B.M.; Sirpal, S.; Pham, S.M.; Leblanc, R. Nitrocinnamate-functionalized gelatin: Synthesis and “smart” hydrogel formation via photo-cross-linking. Biomacromolecules 2005, 6, 1503–1509. [Google Scholar] [CrossRef]
- Li, X.; Xu, S.; Pen, Y.; Wang, J. The swelling behaviors and network parameters of cationic starch-g-acrylic acid/poly (dimethyl diallyl ammonium chloride) semi-interpenetrating polymer networks hydrogels. J. Appl. Polym. Sci. 2008, 110, 1828–1836. [Google Scholar] [CrossRef]
- Moura, M.J.; Figueiredo, M.M.; Gil, M.H. Rheological study of genipin cross-linked chitosan hydrogels. Biomacromolecules 2007, 8, 3823–3829. [Google Scholar] [CrossRef] [Green Version]
- Yamazaki, S.; Takegawa, A.; Kaneko, Y.; Kadokawa, J.; Yamagata, M.; Ishikawa, M. An Acidic Cellulose-Chitin Hybrid Gel as Novel Electrolyte for an Electric Double Layer Capacitor. Electrochem. Commun. 2009, 11, 68–70. [Google Scholar] [CrossRef]
- Varaprasad, K.; Reddy, N.N.; Kumar, N.M.; Vimala, K.; Ravindra, S.; Raju, K.M. Hydroge–silver nanoparticle composites: A new generation of antimicrobials. J. Appl. Polym. Sci. 2010, 115, 1199–1207. [Google Scholar] [CrossRef]
- Peng, Z.; Chen, F. Synthesis and properties of lignin-based polyurethane hydrogels. J. Appl. Polym. Sci. 2011, 60, 674–683. [Google Scholar] [CrossRef]
- Chang, C.; Duan, B.; Cai, J.; Zhang, L. Superabsorbent hydrogels based on cellulose for smart swelling and controllable delivery. Eur. Polym. J. 2010, 46, 92–100. [Google Scholar] [CrossRef]
- Zhou, J.; Chang, C.; Zhang, R.; Zhang, L. Hydrogels prepared from unsubstituted cellulose in NaOH/urea aqueous solution. Macromol. Biosci. 2007, 7, 804–809. [Google Scholar] [CrossRef]
- Ismail, H.; Irani, M.; Ahmad, Z. Starch-based hydrogels: Present status and applications. Int. J. Polym. Mater. Polym. 2013, 62, 411–420. [Google Scholar] [CrossRef]
- Yu, L.; Christov, V.; Christie, G.; Gray, J.; Dutt, U.; Harvey, T.; Halley, P.; Coombs, S.; Jayasekara, R.; Lonergan, G. Effect of additives on gelatinization, rheological properties and biodegradability of thermoplastic starch. Macromol. Symp. 1999, 144, 371–374. [Google Scholar] [CrossRef]
- Chen, P.; Yu, L.; Kealy, T.; Chen, L.; Li, L. Phase transition of starch granules observed by microscope under shearless and shear conditions. Carbohydr. Polym. 2007, 68, 495–501. [Google Scholar] [CrossRef]
- Liu, H.; Yu, L.; Xie, F.; Chen, L. Gelatinization of cornstarch with different amylose/amylopectin content. Carbohydr. Polym. 2006, 65, 357–363. [Google Scholar] [CrossRef]
- Xie, F.; Yu, L.; Su, B.; Liu, P.; Wang, J.; Liu, H.; Chen, L. Rheological properties of starches with different amylose/amylopectin ratios. J. Cereal Sci. 2009, 49, 371–377. [Google Scholar] [CrossRef]
- Xue, T.; Yu, L.; Xie, F.; Chen, L.; Li, L. Rheological properties and phase transition of starch under shear stress. Food Hydrocoll. 2008, 22, 973–978. [Google Scholar] [CrossRef]
- Liu, X.; Xiao, X.; Liu, P.; Yu, L.; Li, M.; Zhou, S.; Xie, F. Shear degradation of corn starches with different amylose contents. Food Hydrocoll. 2016, 66, 199–205. [Google Scholar] [CrossRef] [Green Version]
- Rath, S.K.; Singh, R.P. Flocculation characteristics of grafted and ungrafted starch, amylose, and amylopectin. J. Appl. Polym. Sci. 1997, 66, 1721–1729. [Google Scholar] [CrossRef]
- Castel, D.; Ricard, A.; Audebert, R. Swelling of Anionic and Cationic Starch-Based Superabsorbents in Water and Saline Solution. J. Appl. Polym. Sci. 1990, 39, 11–29. [Google Scholar] [CrossRef]
- Castel, D.; Ricard, A.; Audebert, R. Preparation of Starch Graft Copolymers: Influence of Several Parameters on Structure and Properties. J. Macromol. Sci. Part A 1988, 25, 235–246. [Google Scholar] [CrossRef]
- Klein, J.M.; Silva de Lima, V.; Couto da Feira, J.M.; Nichele Brandalise, R.; de Camargo Forte, M.M. Chemical modification of cashew gum with acrylamide using an ultrasound-assisted method. J. Appl. Polym. Sci. 2016, 133, 43634. [Google Scholar] [CrossRef]
- Hu, A.; Jiao, S.; Zheng, J.; Li, L.; Fan, Y.; Chen, L.; Zhang, Z. Ultrasonic frequency effect on corn starch and its cavitation. LWT 2015, 60, 941–947. [Google Scholar] [CrossRef]
- Bashari, M.; Abbas, S.; Xu, X.; Jin, Z. Combined of ultrasound irradiation with high hydrostatic pressure (US/HHP) as a new method to improve immobilization of dextranase onto alginate gel. Ultrason. Sonochem. 2014, 21, 1325–1334. [Google Scholar] [CrossRef]
- Erriu, M.; Blus, C.; Szmukler-Moncler, S.; Buogo, S.; Levi, R.; Barbato, G.; Madonnaripa, D.; Denotti, G.; Piras, V.; Orrù, G. Microbial biofilm modulation by ultrasound: Current concepts and Controversies. Ultrason. Sonochem 2014, 21, 15–22. [Google Scholar] [CrossRef]
- Gao, W.; Lin, X.; Lin, X.; Ding, J.; Huang, X.; Wu, H. Preparation of nano-sized flake carboxymethyl cassava starch under ultrasonic irradiation. Carbohydr. Polym. 2011, 84, 1413–1418. [Google Scholar] [CrossRef]
- Yin, N.; Chen, K. Ultrasonically initiated emulsifier-free emulsion copolymerization of n-butyl acrylate and acrylamide. Part I Polym. Mech. Polym. 2004, 45, 3587–3594. [Google Scholar]
- Suslick, K.S. Sonochemistry. Science 1990, 247, 1439–1445. [Google Scholar] [CrossRef]
- Ren, Y.; Rong, L.; Shen, M.; Liu, W.; Xiao, W.; Luo, Y.; Xie, J. Interaction between rice starch and Mesona chinensis Benth polysaccharide gels: Pasting and gelling properties. Carbohydr. Polym. 2020, 240, 116316. [Google Scholar] [CrossRef]
- Qiao, D.L.; Wei, Z.; Liu, X.X.; Yu, L.; Chen, L.; Liu, H.S.; Zhang, N.Z. Starch modification using a twin-roll mixer as a reactor. Starch Strke 2012, 64, 821–825. [Google Scholar] [CrossRef]
- Dobosz, A.; Sikora, M.; Krystyjan, M.; Lach, R.; Borczak, B. Influence of xanthan gum on the short- and long-term retrogradation of potato starches of various amylose content. Food Hydrocoll. 2020, 102, 105618. [Google Scholar] [CrossRef]
- Chang, Y.H.; Lim, S.T.; Yoo, B. Dynamic rheology of corn starch–sugar composites. J. Food Eng. 2004, 64, 521–527. [Google Scholar] [CrossRef]
- Krystyjan, M.; Adamczyk, G.; Sikora, M.; Tomasik, P. Long-term storage stability of selected potato starch-non-starchy hydrocolloid binary gels. Food Hydrocoll. 2013, 31, 270–276. [Google Scholar] [CrossRef]
- Kim, C.; Lee, S.-P.; Yoo, B. Dynamic rheology of rice starch-galactomanna mixtures in the aging process. Starch Stärke 2006, 58, 35–43. [Google Scholar] [CrossRef]
- Wang, S.; Li, C.; Copeland, L.; Niu, Q.; Wang, S. Starch Retrogradation: A Comprehensive Review. Compr. Rev. Food Sci. Food Saf. 2015, 14, 568–585. [Google Scholar] [CrossRef]
- Qiao, D.L.; Liu, H.S.; Yu, L.; Bao, X.Y.; Simon, G.P.; Petinakis, E.; Chen, L. Preparation and characterization of slow-release fertilizer encapsulated by starch-based superabsorbent polymer. Carbohydr. Polym. 2016, 147, 146–154. [Google Scholar] [CrossRef]
- Lu, S.J.; Duan, M.L.; Lin, S.B. Synthesis of superabsorbent starch-graft-poly(potassium acrylate-co-acrylamide) and its properties. J. Appl. Polym. Sci. 2003, 88, 1536–1542. [Google Scholar] [CrossRef]
- Singh, N.; Singh, S.; Isono, N.; Noda, T.; Singh, A.M. Diversity in amylopectin structure, thermal and pasting properties of starches from wheat varieties/lines. Int. J. Biol. Macromol. 2009, 45, 298–304. [Google Scholar] [CrossRef]
- Srichuwong, S.; Jane, J. Physicochemical properties of starch affected by molecular composition and structures: A review. Food Sci. Biotechnol. 2007, 16, 663–674. [Google Scholar]
- Flory, P.J. Statistical mechanics of swelling of network structures. J. Chem. Phys. 1950, 18, 108–111. [Google Scholar] [CrossRef]
- Cao, S.; Hu, F.X.; Ma, X.; Yang, K. Development of CR/AA binary graft modified adhesive. J. Xinyang Norm. Univ. (Nat. Sci. Ed.) 2006, 04, 479–482. [Google Scholar]
- Olad, A.; Zebhi, H.; Salari, D.; Mirmohseni, A.; Tabar, A.R. Slow-release NPK fertilizer encapsulated by carboxymethyl cellulose-based nanocomposite with the function of water retention in soil. Mater. Sci. Eng. C 2018, 90, 333–340. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, H.; Dong, F.; Wang, Q.; Li, Y.; Xiong, Y. Construction of Porous Starch-Based Hydrogel via Regulating the Ratio of Amylopectin/Amylose for Enhanced Water-Retention. Molecules 2021, 26, 3999. https://doi.org/10.3390/molecules26133999
Luo H, Dong F, Wang Q, Li Y, Xiong Y. Construction of Porous Starch-Based Hydrogel via Regulating the Ratio of Amylopectin/Amylose for Enhanced Water-Retention. Molecules. 2021; 26(13):3999. https://doi.org/10.3390/molecules26133999
Chicago/Turabian StyleLuo, Huiyuan, Fuping Dong, Qian Wang, Yihang Li, and Yuzhu Xiong. 2021. "Construction of Porous Starch-Based Hydrogel via Regulating the Ratio of Amylopectin/Amylose for Enhanced Water-Retention" Molecules 26, no. 13: 3999. https://doi.org/10.3390/molecules26133999





