Dramatic Effect of A Ring Size of Alicyclic α-Dioximate Ligand Synthons on Kinetics of the Template Synthesis and of the Acidic Decomposition of the Methylboron-Capped Iron(II) Clathrochelates
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis and Spectra
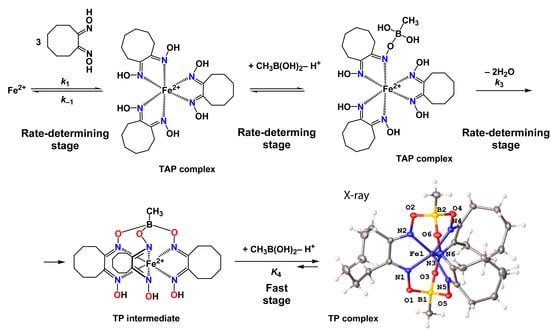
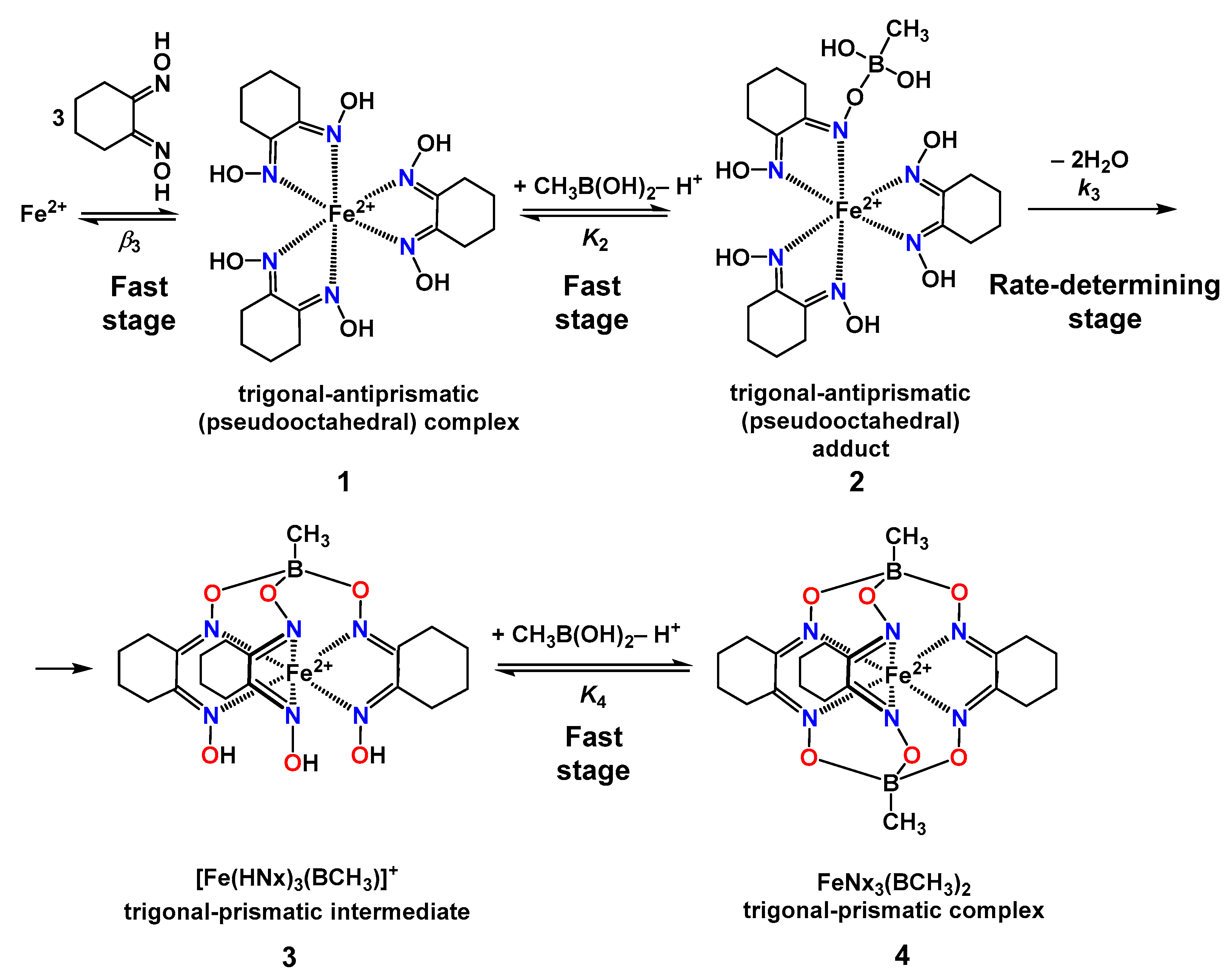
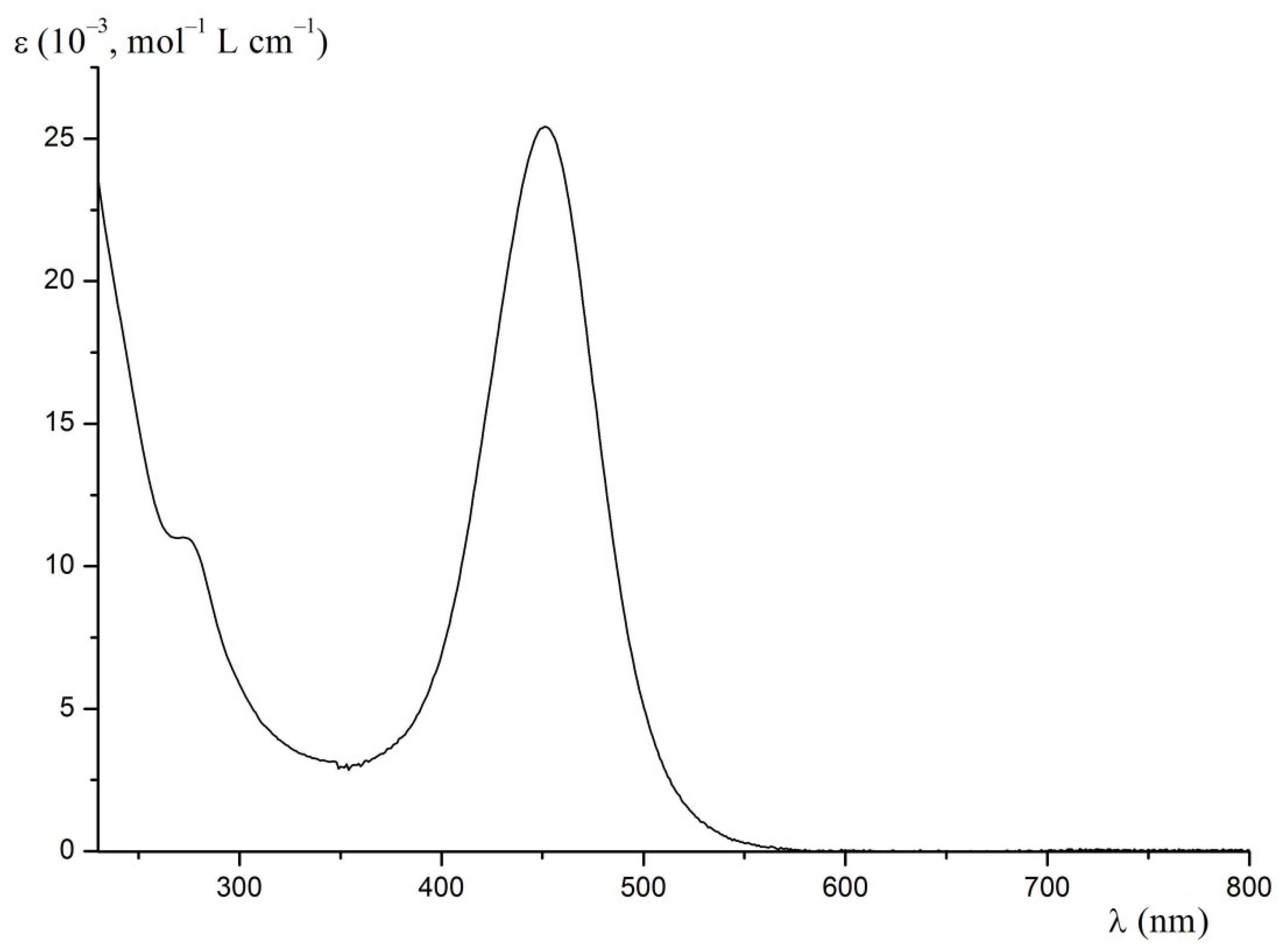
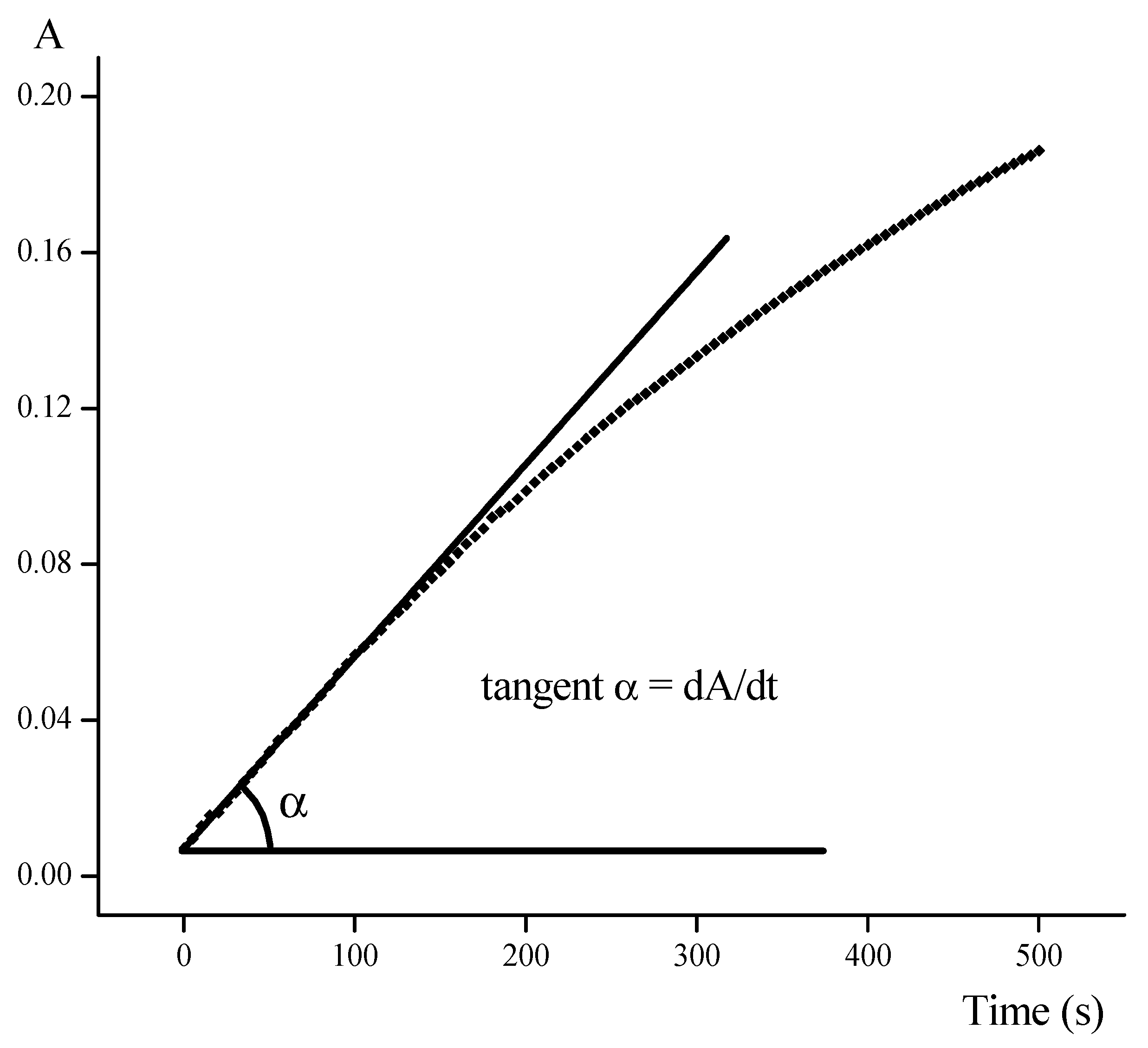
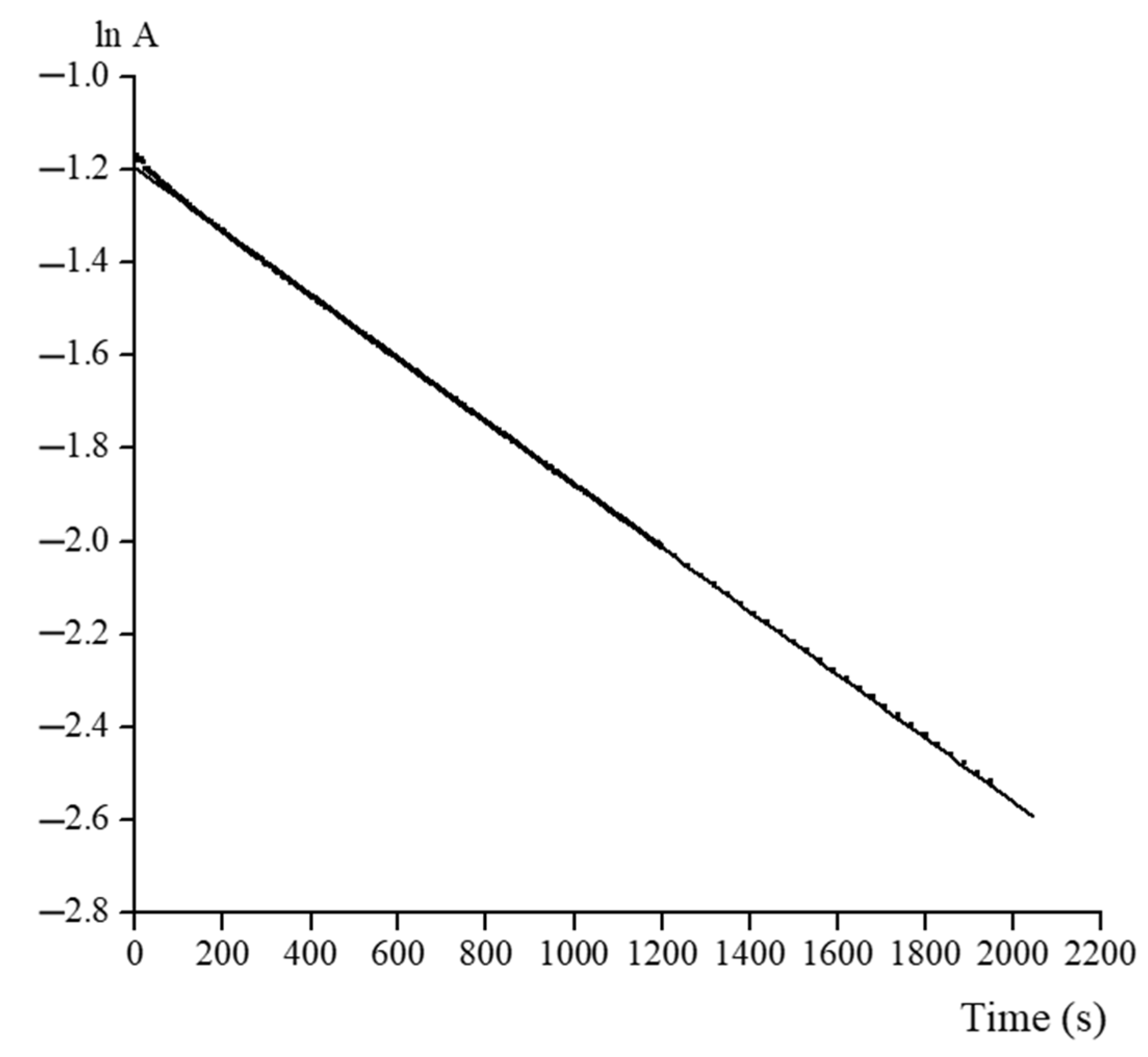
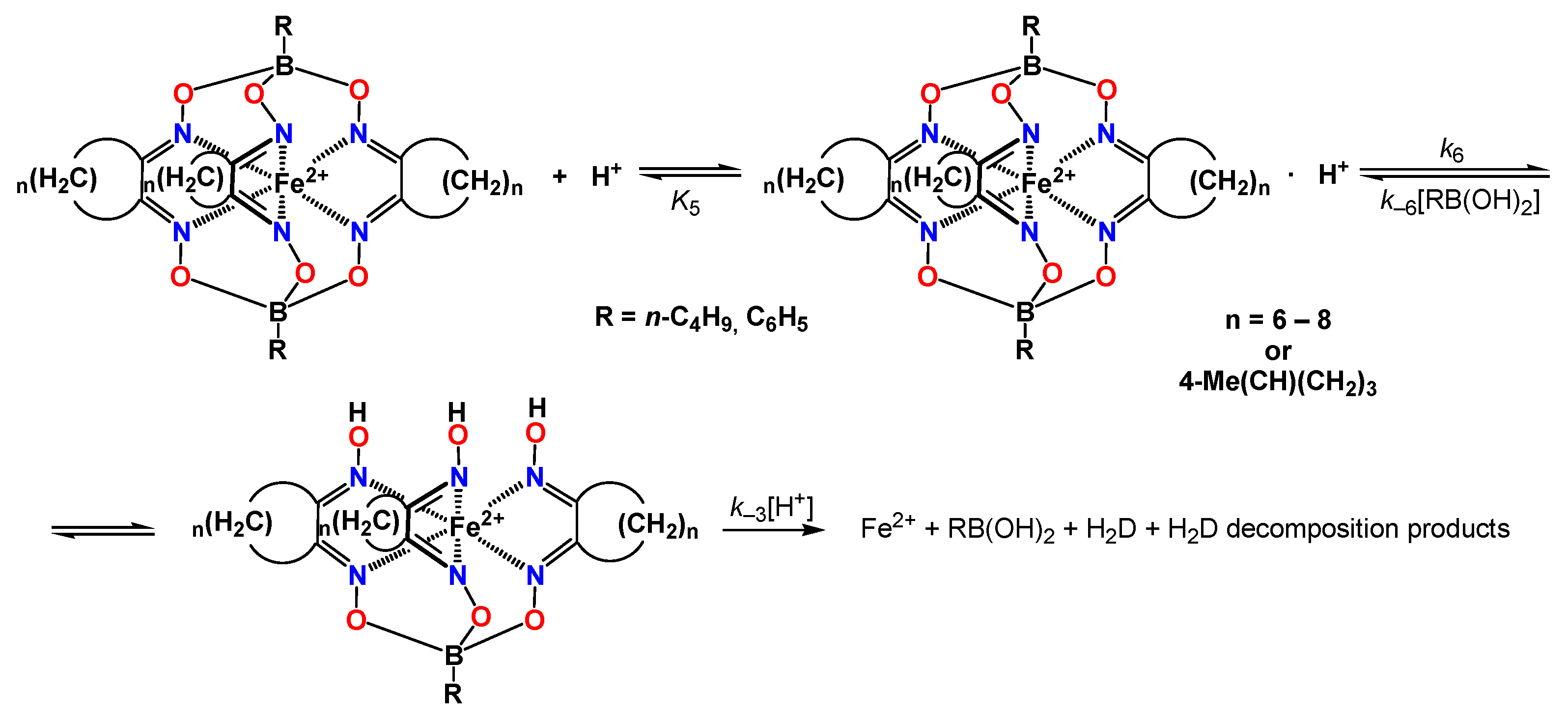
2.2. Kinetics of the Template Synthesis of FeOx3(BCH3)2
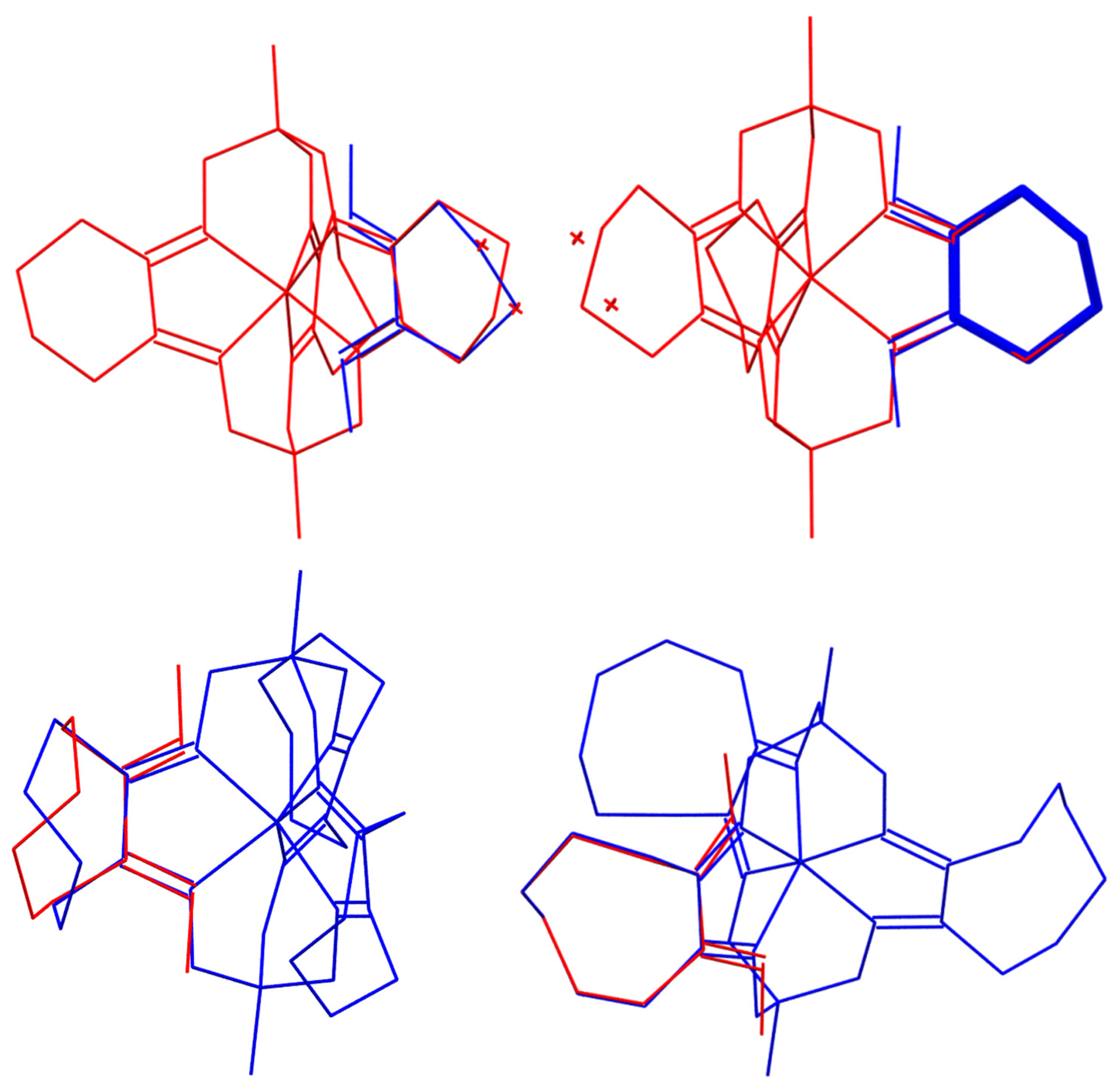
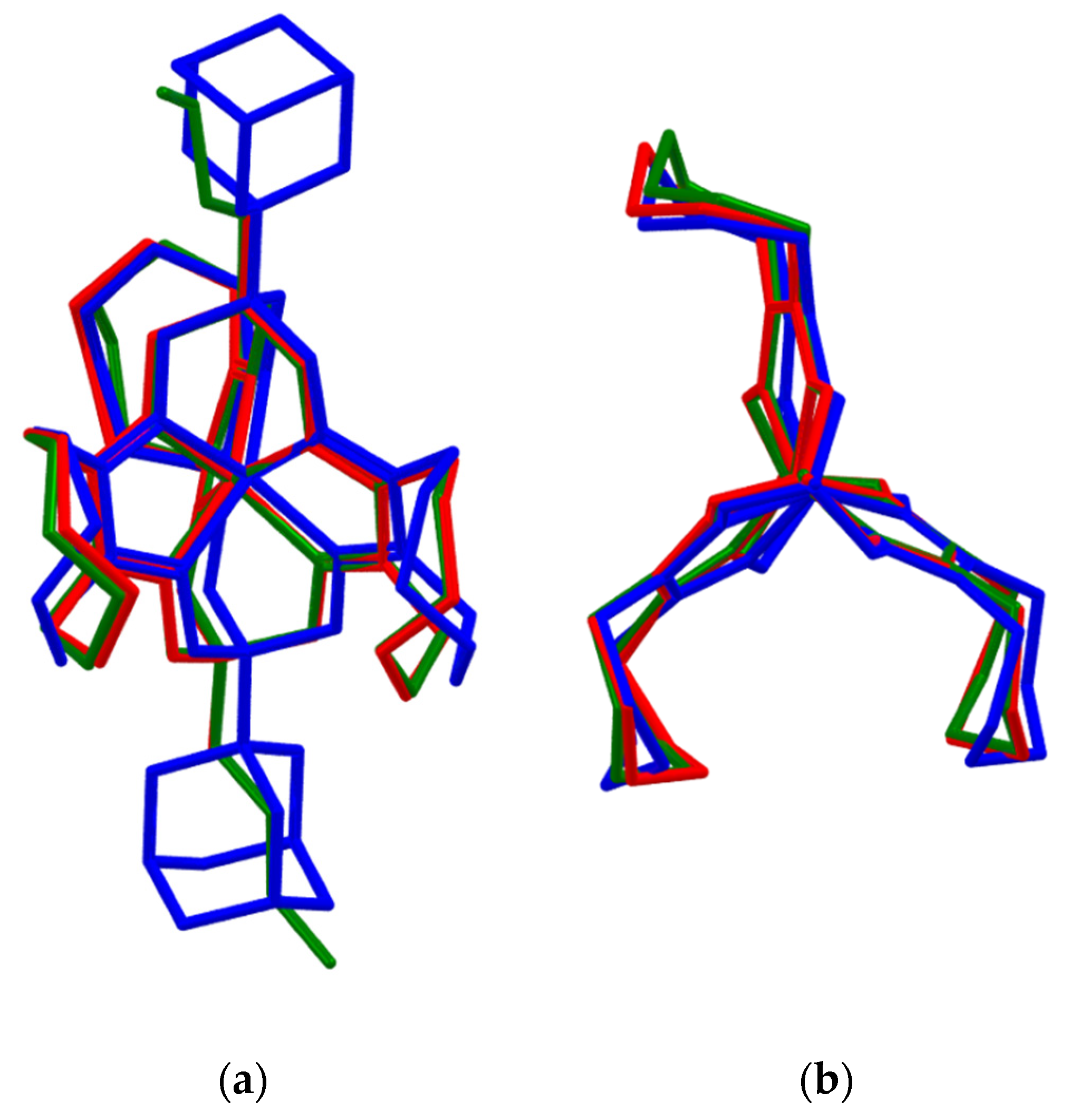
2.3. X-ray Structures
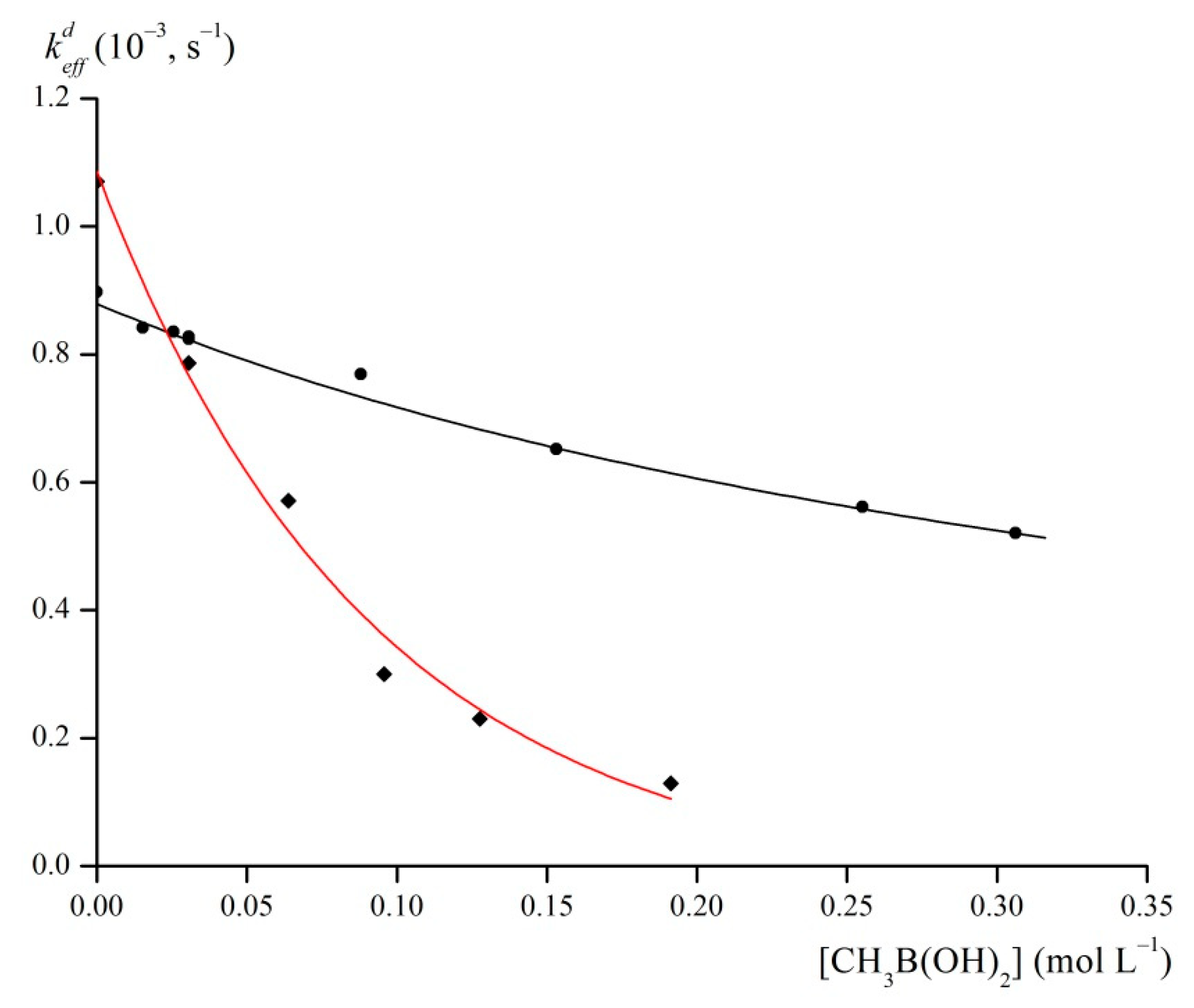
2.4. Acidic Decomposition of FeOx3(BCH3)2 in Its Diluted Solutions
3. Experimental
3.1. Synthesis
3.1.1. Materials and Methods
3.1.2. Synthesis and Spectral Characteristics
3.1.3. Single Crystal X-ray Diffraction Experiments
3.2. Kinetic Studies
3.2.1. Materials and Methods
3.2.2. Kinetic Experiments
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Voloshin, Y.Z.; Kostromina, N.A.; Krämer, R. Clathrochelates: Synthesis, Structure and Properties; Elsevier: Amsterdam, The Netherlands, 2002. [Google Scholar]
- Voloshin, Y.; Belaya, I.; Krämer, R. Cage Metal Complexes: Clathrochelates Revisited; Springer: Berlin/Heidelberg, Germany, 2017. [Google Scholar]
- Voloshin, Y.Z.; Noskov, Y.G. Kinetics of the template synthesis and decomposition of iron(II) macrobicyclic hydroxy-boron-containing cyclooctandione-1,2-dioximate. Ukr. Khim. Zh. 1993, 59, 347–352. [Google Scholar]
- Voloshin, Y.Z.; Noskov, Y.G.; Terekhova, M.I.; Kron, T.E. The influence of the substituent and ring size on the kinetic and thermodynamic parameters of template synthesis and decomposition reaction of clathrochelate alkyl- and aryl-boron-containing iron(ll) tris-dioximates. Pol. J. Chem. 1996, 70, 1229–1235. [Google Scholar]
- Voloshin, Y.Z.; Terekhova, M.I.; Noskov, Y.G.; Zavodnik, V.E.; Belsky, V.K. Structure aspects of kinetics and thermodynam-ics of the template synthesis and decomposition reactions of boron-containing clathrochelate iron(ll) dioximates. An. Quim. Int. 1998, 94, 142–147. [Google Scholar]
- Pomadchik, A.L.; Belov, A.S.; Lebed, E.G.; Voloshin, Y.Z. Kinetics and Thermodynamics of the Template Synthesis and of an Acidic Decomposition of the Clathrochelate Iron(II) Tris-Cyclohexanedion-1,2-Dioximate (Nioximate) Formed by a Cross-Linking with Methylboronic Acid. Russ. J. Inorg. Chem. 2020, 65, 1503–1512. [Google Scholar] [CrossRef]
- Wang, Y.-M.; Chung, C.-S.; Lo, J.-M.; Wu, Y.-L. Kinetics and mechanism of the complexation reaction of copper(II) with diazadioximes. Polyhedron 1999, 18, 1917–1925. [Google Scholar] [CrossRef]
- Biver, T.; Secco, F.; Tinè, M.R.; Venturini, M. Equilibria and kinetics of complex formation between copper(II) and the polyamine Me2octaen. Polyhedron 2001, 20, 1953–1959. [Google Scholar] [CrossRef]
- Stynes, D.; Vernik, I.; Zobi, F. Iron complexes of borylated vicinal dioxime macrocycles. Coord. Chem. Rev. 2002, 233–234, 273–287. [Google Scholar] [CrossRef]
- Sharma, V.; Millero, F.J.; Homonnay, Z. The kinetics of the complex formation between iron(III)–ethylenediaminetetraacetate and hydrogen peroxide in aqueous solution. Inorg. Chim. Acta 2004, 357, 3583–3587. [Google Scholar] [CrossRef]
- Biver, T.; Secco, F.; Tiné, M.R.; Venturini, M. Kinetics and equilibria for the formation of a new DNA metal-intercalator: The cyclic polyamine Neotrien/copper(II) complex. J. Inorg. Biochem. 2004, 98, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Ozay, H.; Baran, Y. Synthesis, complex formation kinetics and thermodynamic study of some acyclic polyamine and N2O2 ligands with copper(II). J. Coord. Chem. 2010, 63, 4299–4308. [Google Scholar] [CrossRef]
- Nandi, D.; Chattopadhyay, A.; Ray, S.; Acharjee, A.; Sarkar, R.; Laskar, S.; Ghosh, A.K. Kinetic and mechanistic studies on the substitution of aqua ligands from diaquaethylenediamineplatinum(II) ion by vicinal dioximes. Mon. Chem. Chem. Mon. 2015, 147, 1015–1022. [Google Scholar] [CrossRef]
- Parker, B.F.; Zhang, Z.; Rao, L.; Arnold, J. An overview and recent progress in the chemistry of uranium extraction from seawater. Dalton Trans. 2017, 47, 639–644. [Google Scholar] [CrossRef]
- Jackels, S.C.; Rose, N.J. Encapsulation reactions. Synthesis and characterization of clathro chelates derived from iron(II), dimethylglyoxime and boron compounds. Inorg. Chem. 1973, 12, 1232–1237. [Google Scholar] [CrossRef]
- Voloshin, Y.Z.; Polshin, E.V.; Nazarenko, A.Y. Prediction of the Geometry of Low-Spin Iron(II) Complexes Using a Modified Concept of Partial Quadrupole Splitting (PQS): Advantages and Limitations. Hyperfine Interact. 2002, 141/142, 309–320. [Google Scholar] [CrossRef]
- Asher, R.L.; Stevens, J.G. A Mössbauer spectroscopic investigation of a series of boron-capped iron II clathrochelates. J. Solid State Chem. 1990, 87, 408–414. [Google Scholar] [CrossRef]
- Nagy, L.; Zsakó, J.; Novak, C.; Várhelyi, C.; Vanko, G.; Liptay, G. On the Oximine Complexes of Transition Metals: Part 110: Spectroscopic and DSC study on some [Fe(Diox·H)2L2] and [Fe(Diox)3(BOR)2] type chelates and clathrochelates. J. Therm. Anal. Calorim. 1999, 57, 433–445. [Google Scholar] [CrossRef]
- Lever, A.B.P. Inorganic Electronic Spectroscopy, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 1984. [Google Scholar]
- Kepert, D.L. Inorganic Stereochemistry; Springer: Berlin/Heidelberg, Germany, 1982. [Google Scholar]
- Bancroft, G. Partial quadrupole splittings in inorganic chemistry. Coord. Chem. Rev. 1973, 11, 247–262. [Google Scholar] [CrossRef]
- Bancroft, G.M. Mössbauer Spectroscopy—An Introduction for Inorganic Chemists and Geochemists; McGraw-Hill: New York, NY, USA, 1973. [Google Scholar]
- Dabrowiak, J.C.; Merrell, P.H.; Stone, J.A.; Busch, D.H. Moessbauer spectra of iron complexes with macrocyclic ligands. Partial center shifts and partial quadrupole splittings. J. Am. Chem. Soc. 1973, 95, 6613–6622. [Google Scholar] [CrossRef]
- Kuzmann, E.; Lengyel, A.; Homonnay, Z.; Várhelyi, C.; Klencsár, Z.; Kubuki, S.; Szalay, R. Mössbauer study of novel iron(II)-dioxime complexes with branched alkyl chains. Hyperfine Interact. 2014, 226, 181–185. [Google Scholar] [CrossRef]
- Larsen, E.; LaMar, G.N.; Wagner, B.E.; Parks, J.E.; Holm, R.H. Three-dimensional macrocyclic encapsulation reactions. III. Geometrical and electronic features of tris(diimine) complexes of trigonal-prismatic, antiprismatic, and intermediate stereochemistry. Inorg. Chem. 1972, 11, 2652–2668. [Google Scholar] [CrossRef]
- Sato, H.; Tominaga, T. Mössbauer Studies of the Thermal Decomposition of Tris(2,2′-bipyridine)iron(II) Chloride and the Structures of the Isomers of 2,2′-Bipyridineiron(II) Chloride. Bull. Chem. Soc. Jpn. 1976, 49, 697–700. [Google Scholar] [CrossRef] [Green Version]
- Linert, W.; Gutmann, V.; Wiesinger, G.; Perkins, P.G. CNDO/2-MO Calculations and Mössbauer Spectroscopy on Tris-(1,10-Phenanthroline)iron-Complexes. Z. Phys. Chem. 1984, 142, 221–238. [Google Scholar] [CrossRef]
- Vargas, A.; Hauser, A.; Daku, L.M.L. Influence of Guest−Host Interactions on the Structural, Energetic, and Mössbauer Spectroscopy Properties of Iron(II)tris(2,2′-bipyridine) in the Low-Spin and High-Spin States: A Density-Functional Theory Study of the Zeolite-Y Embedded Complex. J. Chem. Theory Comput. 2009, 5, 97–115. [Google Scholar] [CrossRef] [Green Version]
- Schmid, R.; Sapunov, V.N. Non-Formal Kinetics; Verlag Chemie: Weinheim, Germany, 1982. [Google Scholar]
- Babcock, L.; Pizer, R. Dynamics of boron acid complexation reactions. Formation of 1:1 boron acid-ligand complexes. Inorg. Chem. 1980, 19, 56–61. [Google Scholar] [CrossRef]
- Haines, R.A.; Ryan, D.E.; Cheney, G.E. 5-methyl-1,2,3-cyclohexanetrionetrioxime: A comparative study with some vic-dioximes. Can. J. Chem. 1962, 40, 1149–1159. [Google Scholar] [CrossRef]
- Finta, Z.; Mitrache, I.; Várhelyi, C.; Zsako, J.; Horák, J. Acidity constants of 1,2-cycloheptane- and 1,2-cyclo-octanedione dioximes. Microchim. Acta 1979, 71, 405–413. [Google Scholar] [CrossRef]
- Banks, C.V.; Carlson, A.B. Determination of acidic dissociation constants of several vic-dioximes. Anal. Chim. Acta 1952, 7, 291–301. [Google Scholar] [CrossRef]
- Gómez-Tagle, P.; Lugo-González, J.C.; Yatsimirsky, A.K. Oximate metal complexes breaking the limiting esterolytic reactivity of oximate anions. Chem. Commun. 2013, 49, 7717–7719. [Google Scholar] [CrossRef]
- Streltsova, N.R.; Bel’Sky, V.K.; Voloshin, Y.Z. Structures of three alicyclic α-dioximes with a ring size of six to eight. Acta Crystallogr. Sect. C Cryst. Struct. Commun. 1993, 49, 635–639. [Google Scholar] [CrossRef] [Green Version]
- Voloshin, Y.; Varzatskii, O.A.; Belov, A.; Lebedev, A.Y.; Makarov, I.S.; Gurskii, M.E.; Antipin, M.Y.; Starikova, Z.A.; Bubnov, Y.N. Cage iron(II) complexes with apical and ribbed adamantyl substituents: The creation of second (hydrophobic) shell of an encapsulated metal ion. Inorg. Chim. Acta 2007, 360, 1543–1554. [Google Scholar] [CrossRef]
- Wise, M.D.; Ruggi, A.; Pascu, M.; Scopelliti, R.; Severin, K. Clathrochelate-based bipyridyl ligands of nanoscale dimensions: Easy-to-access building blocks for supramolecular chemistry. Chem. Sci. 2013, 4, 1658–1662. [Google Scholar] [CrossRef]
- Wise, M.D.; Holstein, J.J.; Pattison, P.; Besnard, C.; Solari, E.; Scopelliti, R.; Bricogne, G.; Severin, K. Large, heterometallic coordination cages based on ditopic metallo-ligands with 3-pyridyl donor groups. Chem. Sci. 2014, 6, 1004–1010. [Google Scholar] [CrossRef] [Green Version]
- Jansze, S.M.; Cecot, G.; Wise, M.D.; Zhurov, K.O.; Ronson, T.K.; Castilla, A.M.; Finelli, A.; Pattison, P.; Solari, E.; Scopelliti, R.; et al. Ligand Aspect Ratio as a Decisive Factor for the Self-Assembly of Coordination Cages. J. Am. Chem. Soc. 2016, 138, 2046–2054. [Google Scholar] [CrossRef] [PubMed]
- Jansze, S.M.; Wise, M.D.; Vologzhanina, A.V.; Scopelliti, R.; Severin, K. PdII2L4-type coordination cages up to three nanometers in size. Chem. Sci. 2016, 8, 1901–1908. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cecot, G.; Marmier, M.; Geremia, S.; De Zorzi, R.; Vologzhanina, A.V.; Pattison, P.; Solari, E.; Tirani, F.F.; Scopelliti, R.; Severin, K. The Intricate Structural Chemistry of MII2nLn-Type Assemblies. J. Am. Chem. Soc. 2017, 139, 8371–8381. [Google Scholar] [CrossRef] [PubMed]
- Jansze, S.M.; Cecot, G.; Severin, K. Reversible disassembly of metallasupramolecular structures mediated by a metastable-state photoacid. Chem. Sci. 2018, 9, 4253–4257. [Google Scholar] [CrossRef] [Green Version]
- Jansze, S.M.; Ortiz, D.; Tirani, F.F.; Scopelliti, R.; Menin, L.; Severin, K. Inflating face-capped Pd6L8 coordination cages. Chem. Commun. 2018, 54, 9529–9532. [Google Scholar] [CrossRef]
- Jansze, S.M.; Severin, K. Palladium-Based Metal–Ligand Assemblies: The Contrasting Behavior upon Addition of Pyridine or Acid. J. Am. Chem. Soc. 2019, 141, 815–819. [Google Scholar] [CrossRef]
- Bila, J.L.; Pijeat, J.; Ramorini, A.; Fadaei-Tirani, F.; Scopelliti, R.; Baudat, E.; Severin, K. Porous networks based on iron(ii) clathrochelate complexes. Dalton Trans. 2019, 48, 4582–4588. [Google Scholar] [CrossRef]
- Cecot, G.; Doll, M.T.; Planes, O.M.; Ramorini, A.; Scopelliti, R.; Fadaei-Tirani, F.; Severin, K. Cages vs. Prisms: Controlling the Formation of Metallosupramolecular Architectures with Ligand Side-Chains. Eur. J. Inorg. Chem. 2019, 2019, 2972–2976. [Google Scholar] [CrossRef]
- Planes, O.M.; Jansze, S.M.; Scopelliti, R.; Fadaei-Tirani, F.; Severin, K. Two-Step Synthesis of Linear and Bent Dicarboxylic Acid Metalloligands with Lengths of up to 3 nm. Inorg. Chem. 2020, 59, 14544–14548. [Google Scholar] [CrossRef]
- Planes, O.M.; Schouwink, P.A.; Bila, J.L.; Fadaei-Tirani, F.; Scopelliti, R.; Severin, K. Incorporation of Clathrochelate-Based Metalloligands in Metal–Organic Frameworks by Solvent-Assisted Ligand Exchange. Cryst. Growth Des. 2020, 20, 1394–1399. [Google Scholar] [CrossRef]
- Sudan, S.; Li, R.-J.; Jansze, S.M.; Platzek, A.; Rudolf, R.; Clever, G.H.; Fadaei-Tirani, F.; Scopelliti, R.; Severin, K. Identification of a Heteroleptic Pd6L6L′6 Coordination Cage by Screening of a Virtual Combinatorial Library. J. Am. Chem. Soc. 2021, 143, 1773–1778. [Google Scholar] [CrossRef]
- Legrand, V.; Pillet, S.; Weber, H.-P.; Souhassou, M.; Létard, J.-F.; Guionneau, P.; LeComte, C. On the precision and accuracy of structural analysis of light-induced metastable states. J. Appl. Crystallogr. 2007, 40, 1076–1088. [Google Scholar] [CrossRef]
- Pillet, S.; Legrand, V.; Weber, H.-P.; Souhassou, M.; Létard, J.-F.; Guionneau, P.; Lecomte, C. Out-of-equilibrium charge density distribution of spin crossover complexes from steady-state photocrystallographic measurements: Experimental methodology and results. Z. Krist. Cryst. Mater. 2008, 223, 235–249. [Google Scholar] [CrossRef] [Green Version]
- Voloshin, Y.Z.; Varzatskii, O.A.; Belov, A.; Starikova, Z.A.; Strizhakova, N.G.; Dolganov, A.V.; Kochubey, D.I.; Bubnov, Y.N. Synthesis, X-ray structure and redox properties of the macrobicyclic iron(II) N2- and S2-containing vic-dioximates. Inorg. Chim. Acta 2010, 363, 134–146. [Google Scholar] [CrossRef]
- Coropceanu, E.B.; Croitor, L.; Siminel, A.V.; Chumakov, Y.; Fonari, M.S. The luminescence attenuation in the solid state by fluoride anion entrapped in the one-dimensional Zn(II) dioximate and mononuclear Cd(II) dioxime compounds. Polyhedron 2016, 109, 107–114. [Google Scholar] [CrossRef]
- Macrae, C.F.; Sovago, I.; Cottrell, S.J.; Galek, P.T.A.; McCabe, P.; Pidcock, E.; Platings, M.; Shields, G.P.; Stevens, J.S.; Towler, M.; et al. Mercury 4.0: From visualization to analysis, design and prediction. J. Appl. Crystallogr. 2020, 53, 226–235. [Google Scholar] [CrossRef] [Green Version]
- Sheldrick, G.M. SHELXT–Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A Found. Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef] [Green Version]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. 2015, 71, 3–8. [Google Scholar]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Cryst. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Voloshin, Y.Z.; Noskov, Y.G. Kinetics of template synthesis and decomposition of butyl- and phenyl-boron-containing clathrochelate tris-nioximates of iron(II). Ukr. Khim. Zh. 1993, 59, 231–235. [Google Scholar]

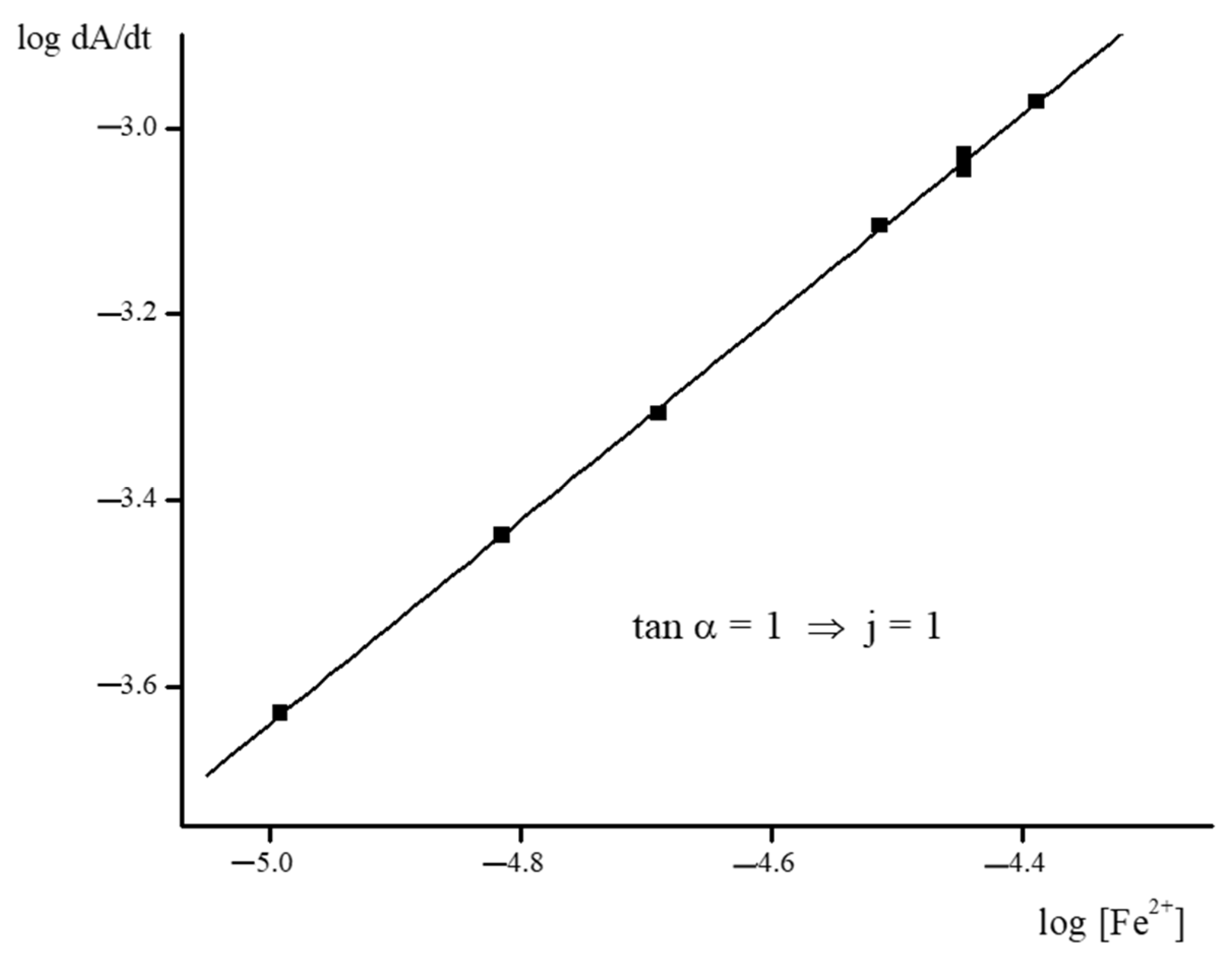


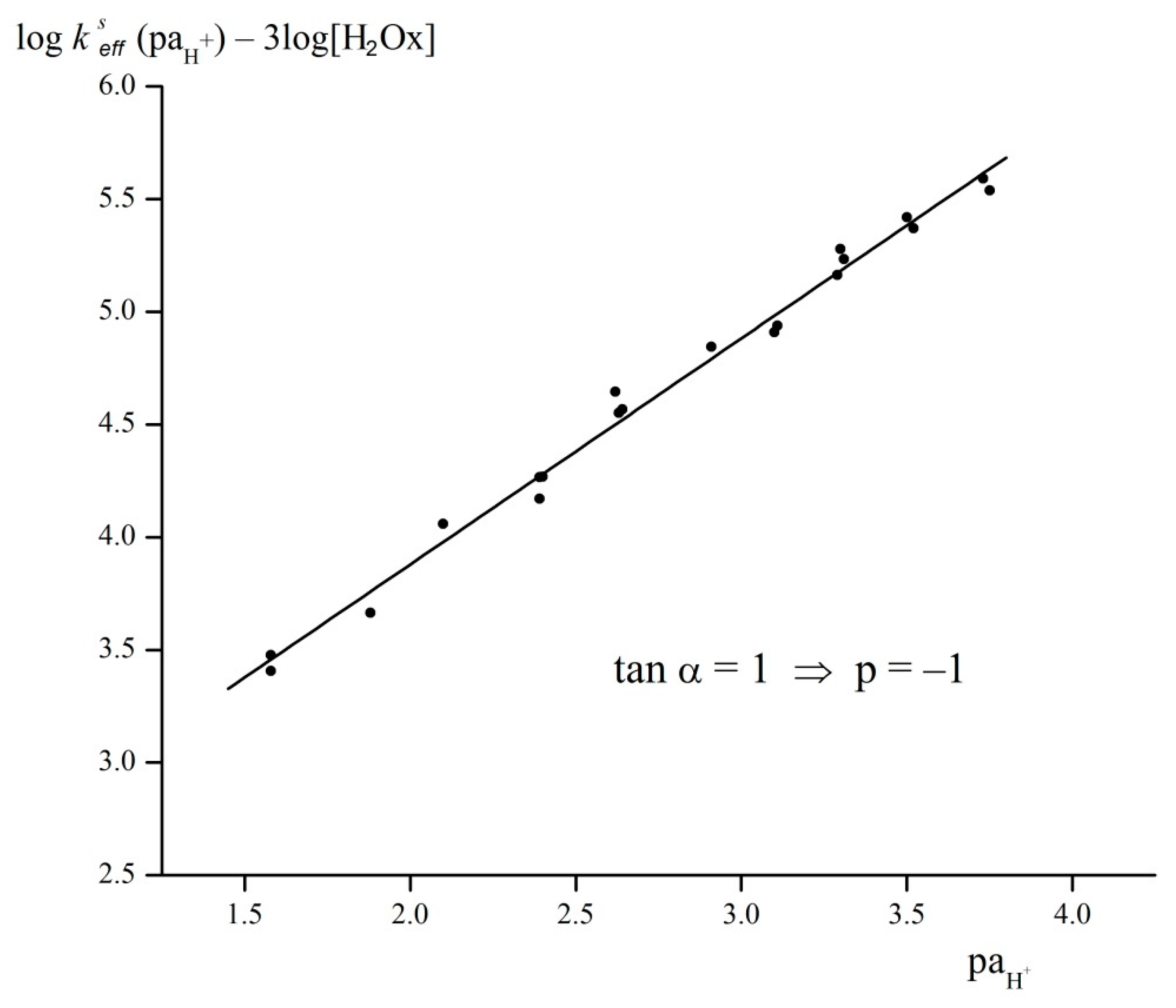

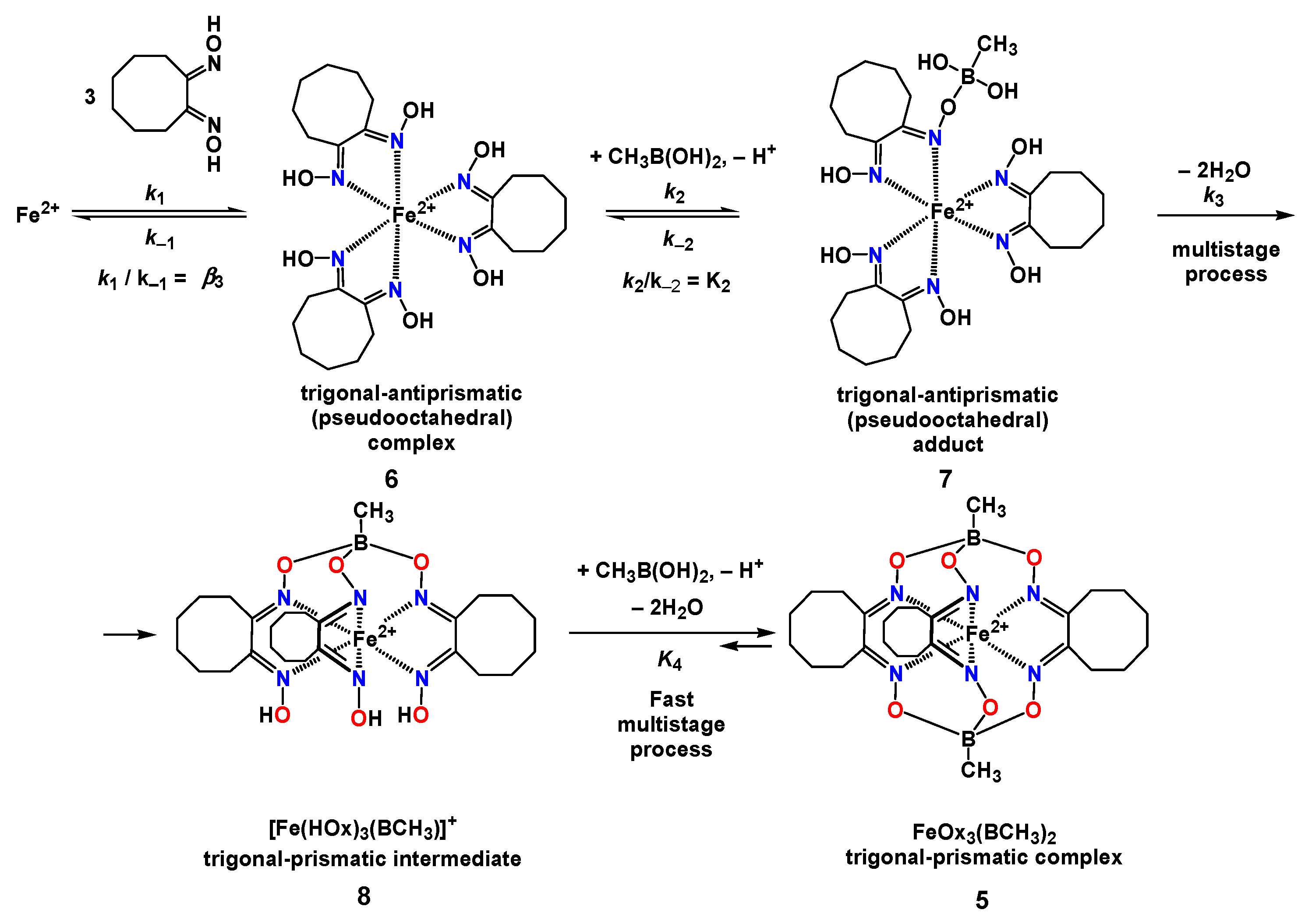


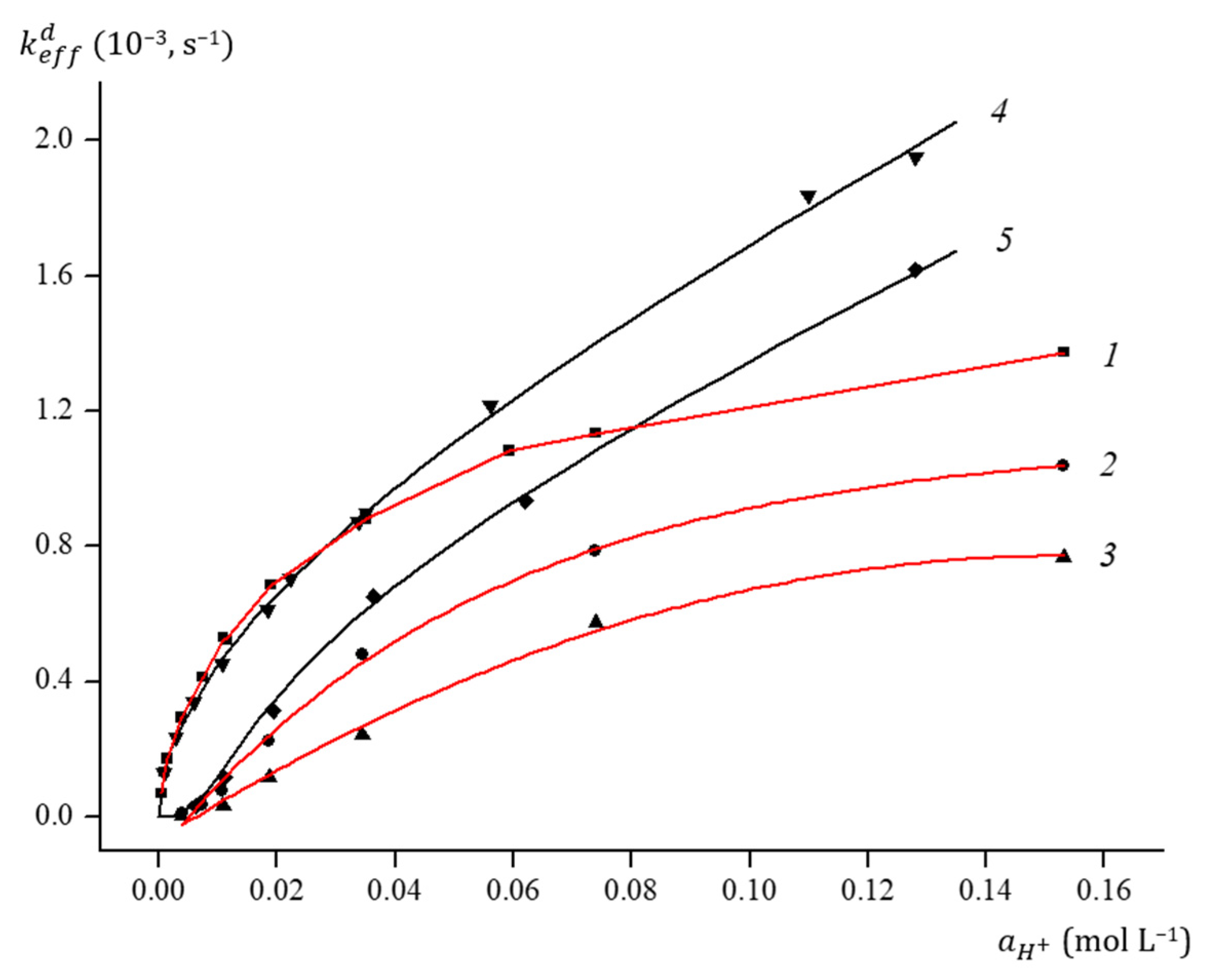
| Complex | b logβ3 | ks s−1 L3 mol−3 | k1 × 10−5 s−1 L3 mol−3 | k−1 s−1 | k2 s−1 L mol−1 | k−2/k3 | k−3 s−1 L2 mol−2 | cK4 | K5 | k6 × 105 s−1 L2 mol−2 | k−6 s−1 L2 mol−2 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| a FeOx3(BCH3)2 | b 5.37 | 4.36 (pH2.38) 5.73 (pH3.18) 6.78 (pH3.75) | 1.86 (pH2.38) 2.45 (pH3.18) 2.89 (pH3.75) | 343 (pH2.38) 451 (pH3.18) 533 (pH3.75) | 8960 | ||||||
| FeOx3(BOH)2 | b 5.37 | 0.445 | 0.19 | 22 | 424 | 0.12 | 129 | ||||
| FeOx3(BC6H5)2 | 1.2 × 104 | 0.09 | 292 | 240 | 5.0 | 3.5 | |||||
| FeOx3(Bn-C4H9)2 | 2.1 × 104 | 0.11 | 147 | 129 | 31.1 | 5.9 | |||||
| FeNx3(BCH3)2 | b 4.94 | 3.2 × 103 |
| Parameter | a FeOx3(BCH3)2 (5) | FeOx3(Bn-C4H9)2 [5] | b FeOx3(BAd)2 [36] | H2Ox [35] |
|---|---|---|---|---|
| Fe–(Å) | 1.910(2)–1.921(2) | 1.868(11)–1.929(11) | 1.895(2)–1.911(2) | - |
| av. 1.916 | av. 1.895 | av. 1.899 | ||
| B–O (Å) | 1.512(3)–1.526(3) | 1.497(19)–1.510(20) | 1.495(4)–1.515(4) | - |
| av. 1.519 | av. 1.505 | av. 1.502 | ||
| N–O (Å) | 1.387(2)–1.394(2) | 1.363(13)–1.394(10) | 1.366(3)–1.383(3) | 1.409(2)–1.413(4) |
| av. 1.390 | av. 1.375 | av. 1.375 | av. 1.411 | |
| C=N (Å) | 1.315(3)–1.323(2) | 1.28(2)–1.34(2) | 1.289(4)–1.313(4) | 1.291(3)–1.293(7) |
| av. 1.318 | av. 1.314 | av. 1.303 | av. 1.292 | |
| chelate C–C (Å) | 1.460(3)–1.463(3) | 1.439(16)–1.459(17) | 1.440(5)–1.454(5) | 1.492(7) |
| av. 1.461 | av. 1.445 | av. 1.447 | ||
| c N…N (Å) | 2.432(2)–2.436(3) | 2.387(12)–2.412(14) | 2.401(4)–2.408(4) | 2.667(3) |
| av. 2.434 | av. 2.397 | av. 2.405 | ||
| N=C–C=N (°) | av. 6.8 | av. 5.7 | av. 6.2 | 26.6(6) |
| φ (°) | 26.0 | 25.1 | 25.2 | |
| α (°) | 78.8 | 78.4 | 78.6 | |
| h (Å) | 2.34 | 2.31 | 2.31 |
| Parameter | a FeNx3(BCH3)2 (4) | FeNx3(Bn-C4H9)2 [52] | b FeNx3(BAd)2 [36] | H2Nx [35] |
|---|---|---|---|---|
| Fe–N (Å) | 1.911(2)–1.918(2) | 1.895(3)–1.923(3) | 1.898(2)–1.906(2) | |
| av. 1.914 | av. 1.911 | av. 1.902 | ||
| B–O (Å) | 1.498(3)–1.513(3) | 1.466(5)–1.532(5) | 1.499(3)–1.506(3) | |
| av. 1.503 | av. 1.504 | av. 1.503 | ||
| N–O (Å) | 1.372(2)–1.379(2) | 1.368(3)–1.386(3) | 1.365(2)–1.374(2) | 1.402(3)–1.410(3) |
| av. 1.376 | av. 1.376 | av. 1.369 | av. 1.406 | |
| C=N (Å) | 1.302(3)–1.314(3) | 1.295(5)–1.325(4) | 1.304(3)–1.308(2) | 1.276(3)–1.280(3) |
| av. 1.308 | av. 1.309 | av. 1.306 | av. 1.278 | |
| C–C (Å) | 1.435(3)–1.446(3) | 1.414(4)–1.441(4) | 1.432(2)–1.439(3) | 1.489(3) |
| av. 1.440 | av. 1.428 | av. 1.436 | ||
| c N…N (Å) | 2.420(2)–2.424(3) | 2.402(3)–2.427(3) | 2.413(3)–2.425(2) | 2.682(3) |
| av. 2.421 | av. 2.414 | av. 2.417 | ||
| N=C–C=N (°) | av. 6.5 | av. 7.6 | av. 6.0 | 26.2(2) |
| φ (°) | 18.9 | 21.1 | 22.9 | |
| α (°) | 78.4 | 78.4 | 79 | |
| h (Å) | 2.37 | 2.35 | 2.34 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pomadchik, A.L.; Belov, A.S.; Lebed, E.G.; Belaya, I.G.; Vologzhanina, A.V.; Voloshin, Y.Z. Dramatic Effect of A Ring Size of Alicyclic α-Dioximate Ligand Synthons on Kinetics of the Template Synthesis and of the Acidic Decomposition of the Methylboron-Capped Iron(II) Clathrochelates. Molecules 2021, 26, 4019. https://doi.org/10.3390/molecules26134019
Pomadchik AL, Belov AS, Lebed EG, Belaya IG, Vologzhanina AV, Voloshin YZ. Dramatic Effect of A Ring Size of Alicyclic α-Dioximate Ligand Synthons on Kinetics of the Template Synthesis and of the Acidic Decomposition of the Methylboron-Capped Iron(II) Clathrochelates. Molecules. 2021; 26(13):4019. https://doi.org/10.3390/molecules26134019
Chicago/Turabian StylePomadchik, Alexander L., Alexander S. Belov, Ekaterina G. Lebed, Irina G. Belaya, Anna V. Vologzhanina, and Yan Z. Voloshin. 2021. "Dramatic Effect of A Ring Size of Alicyclic α-Dioximate Ligand Synthons on Kinetics of the Template Synthesis and of the Acidic Decomposition of the Methylboron-Capped Iron(II) Clathrochelates" Molecules 26, no. 13: 4019. https://doi.org/10.3390/molecules26134019