Label-Free Electrochemical Sensor Based on Manganese Doped Titanium Dioxide Nanoparticles for Myoglobin Detection: Biomarker for Acute Myocardial Infarction
Abstract
:1. Introduction
2. Experimental Details
2.1. Materials and Chemicals
2.2. Synthesis of TiO2 and Mn-Doped TiO2 Nanoparticles
2.3. Characterizations of TiO2 and Mn-Doped TiO2 Nanoparticles
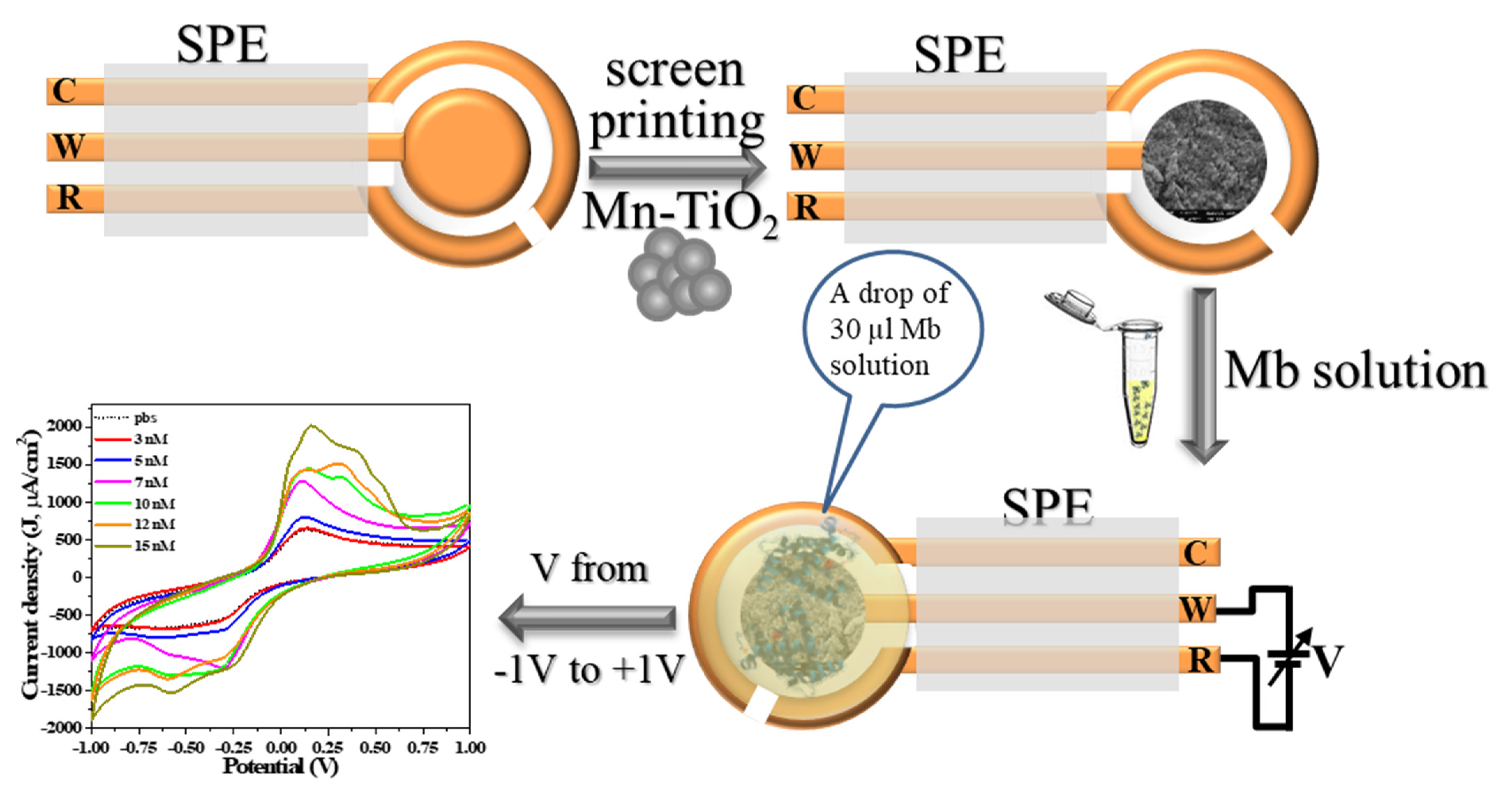
2.4. Fabrication and Characterization of Mb Electrochemical Sensor
3. Results and Discussion
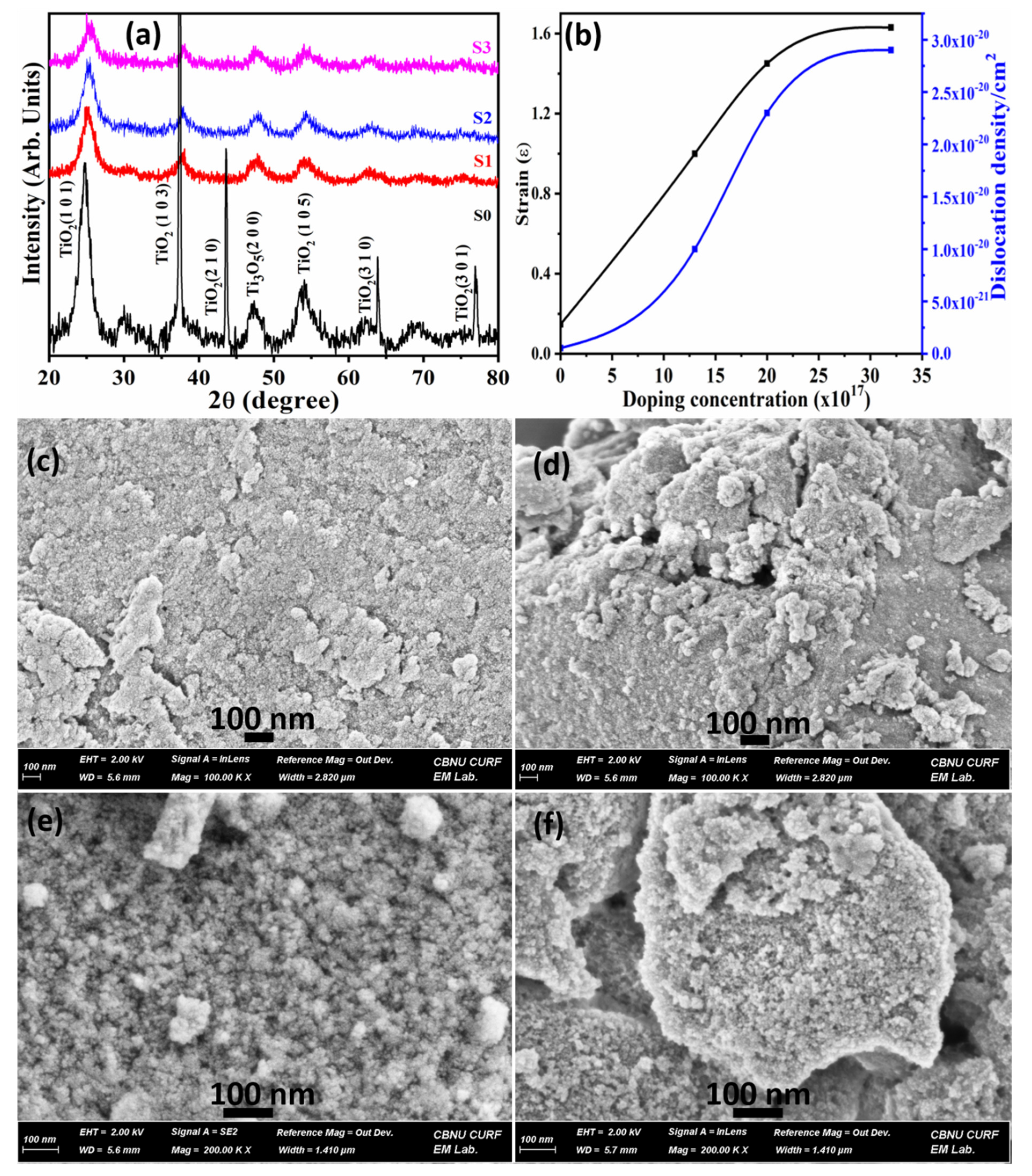
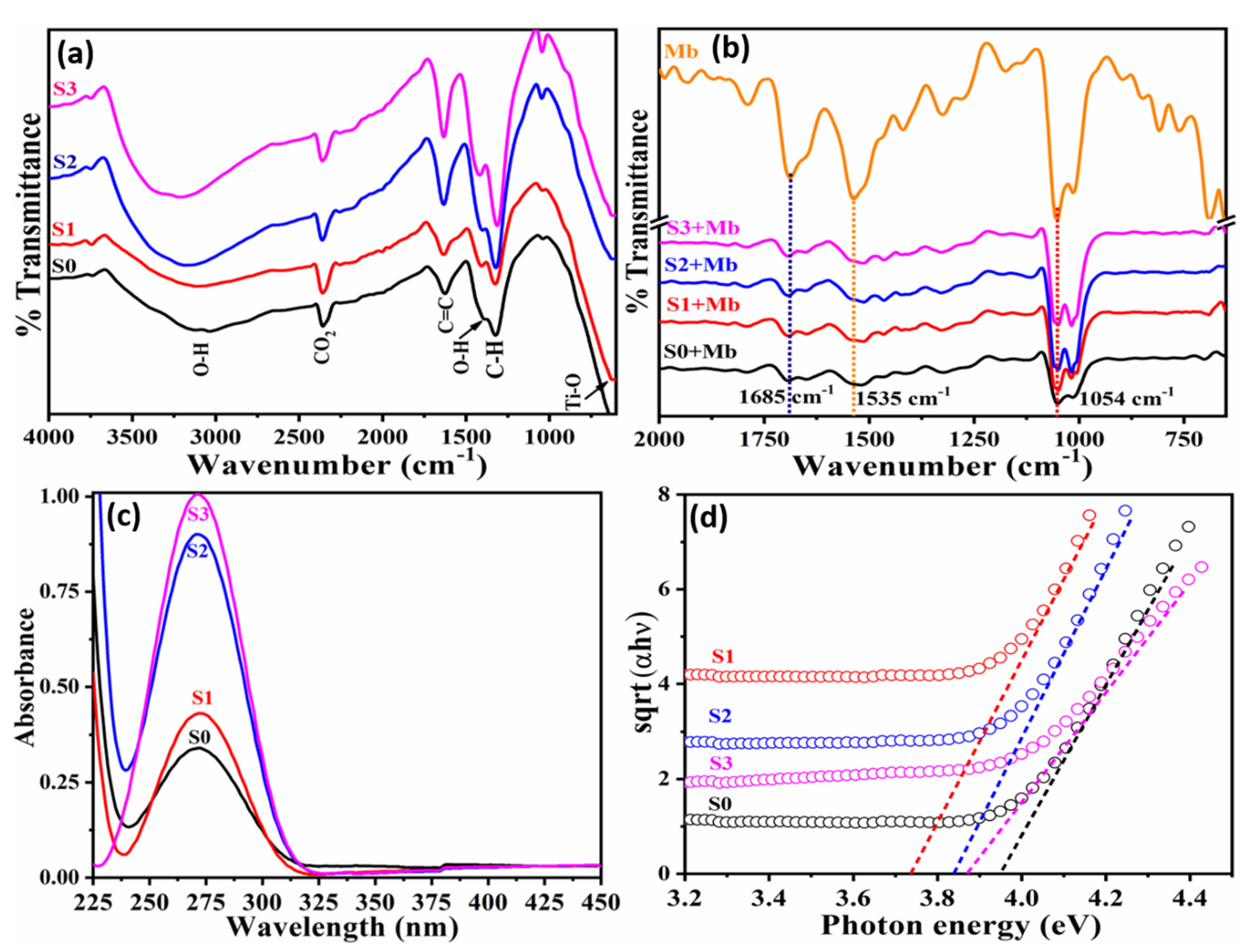
3.1. Characterizations and Properties of Pristine and Doped tio2 Nanoparticles
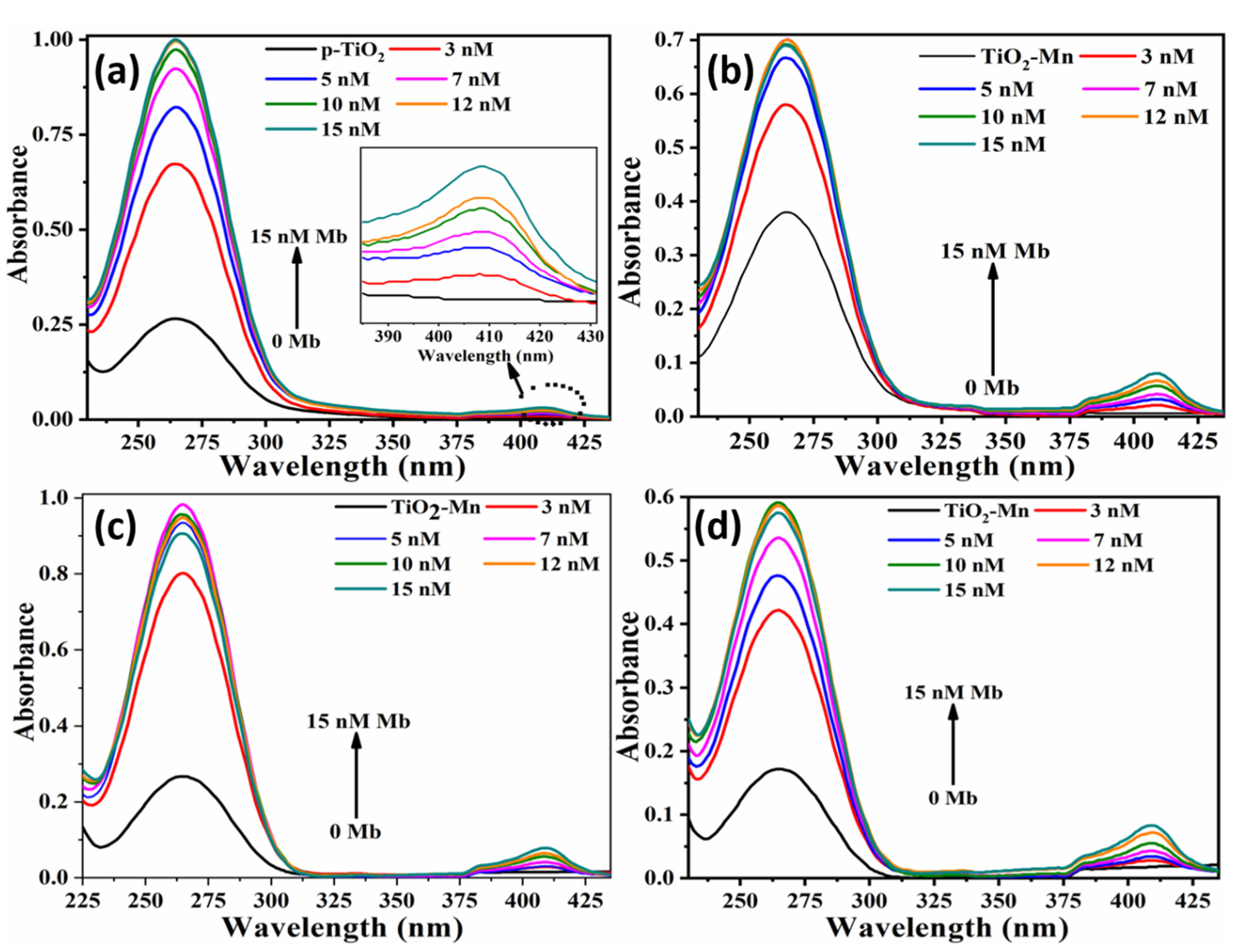
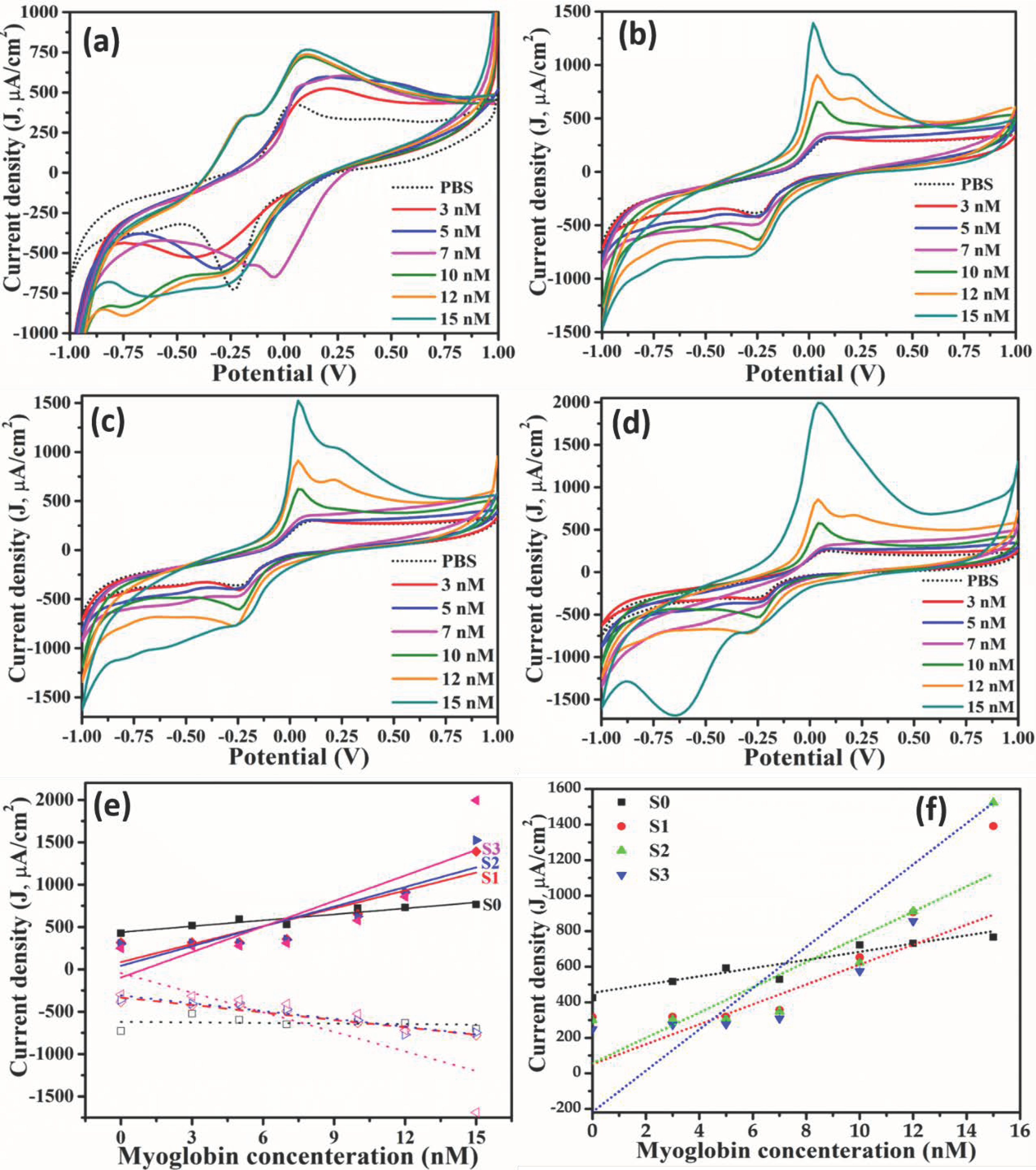
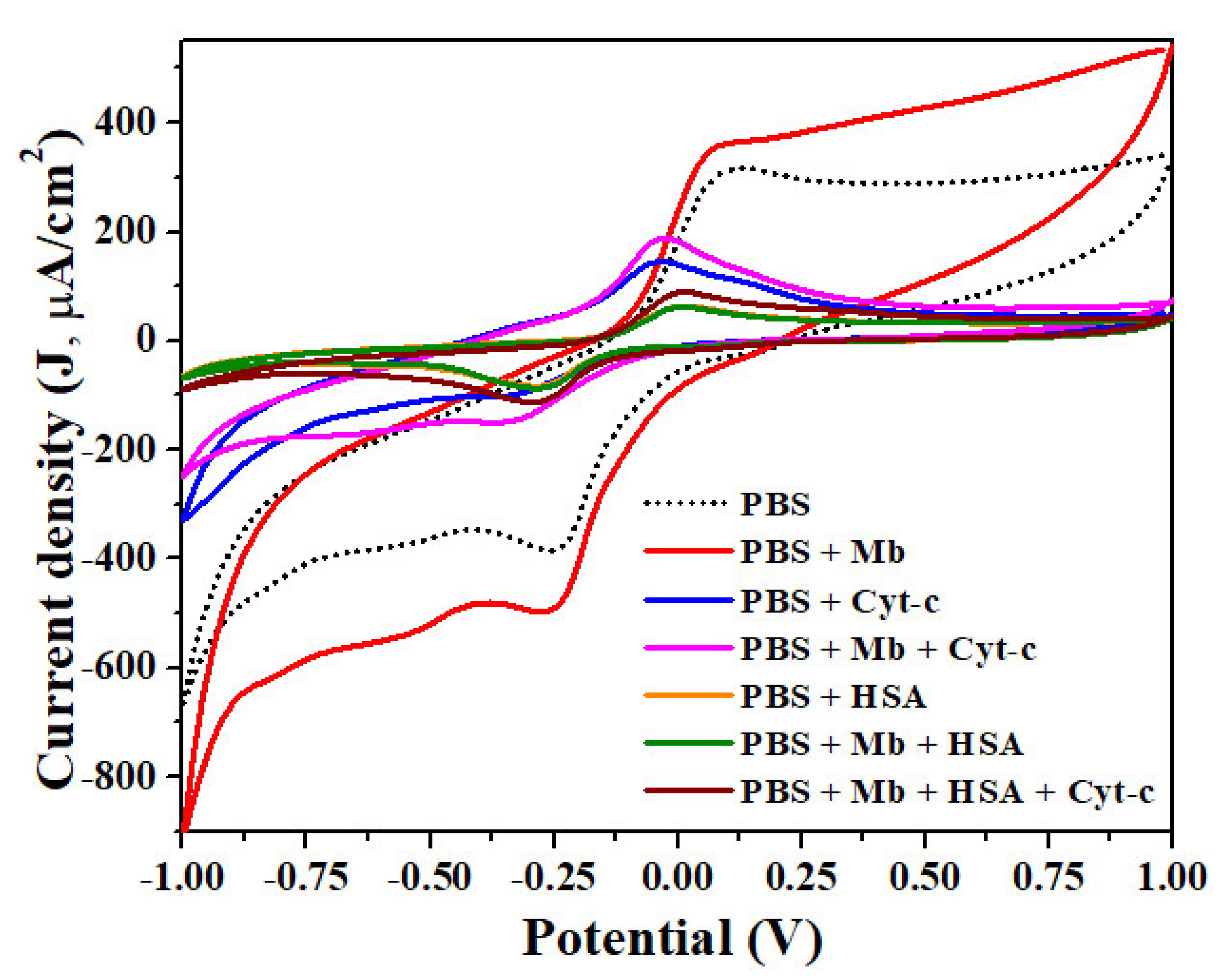
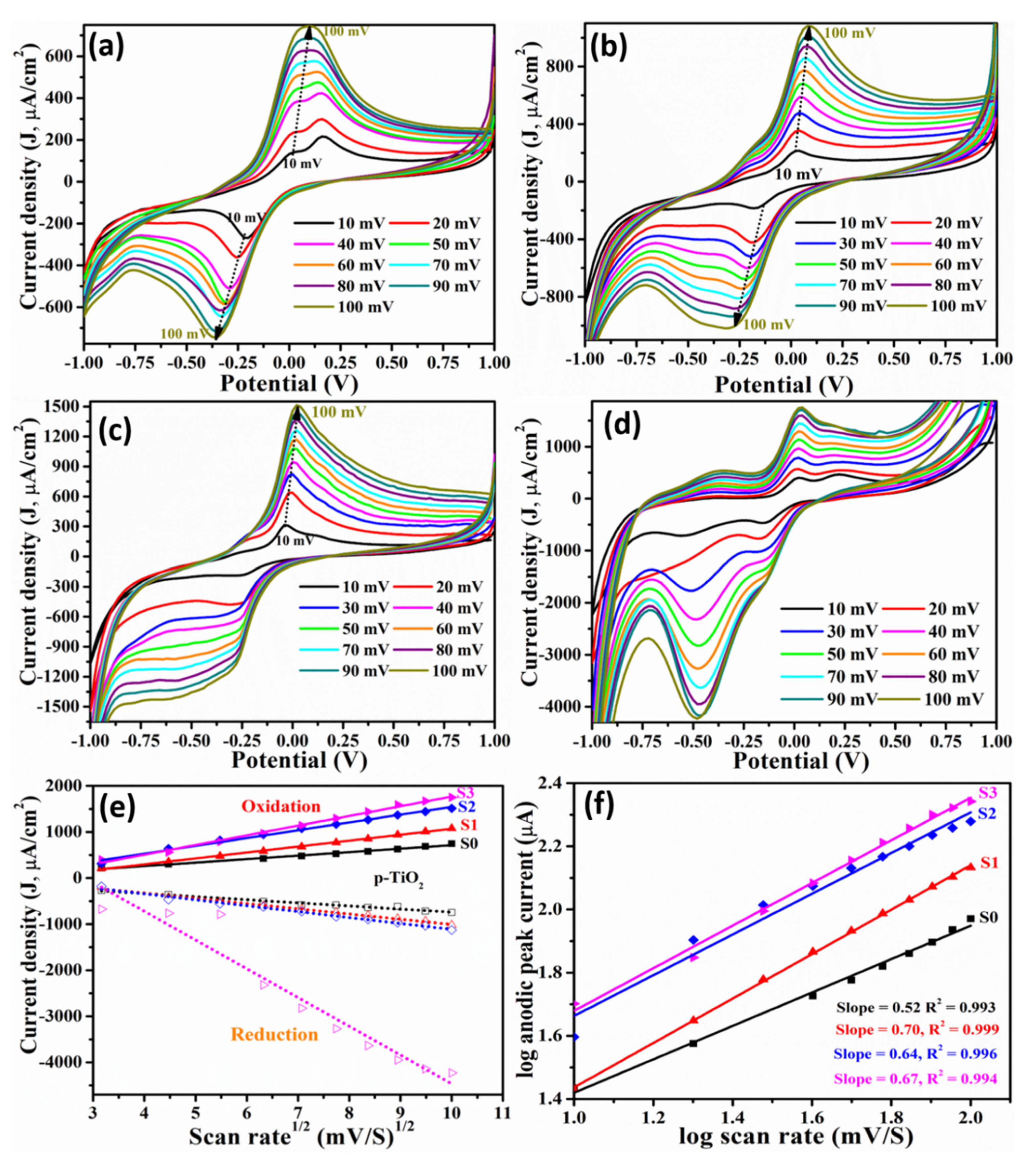
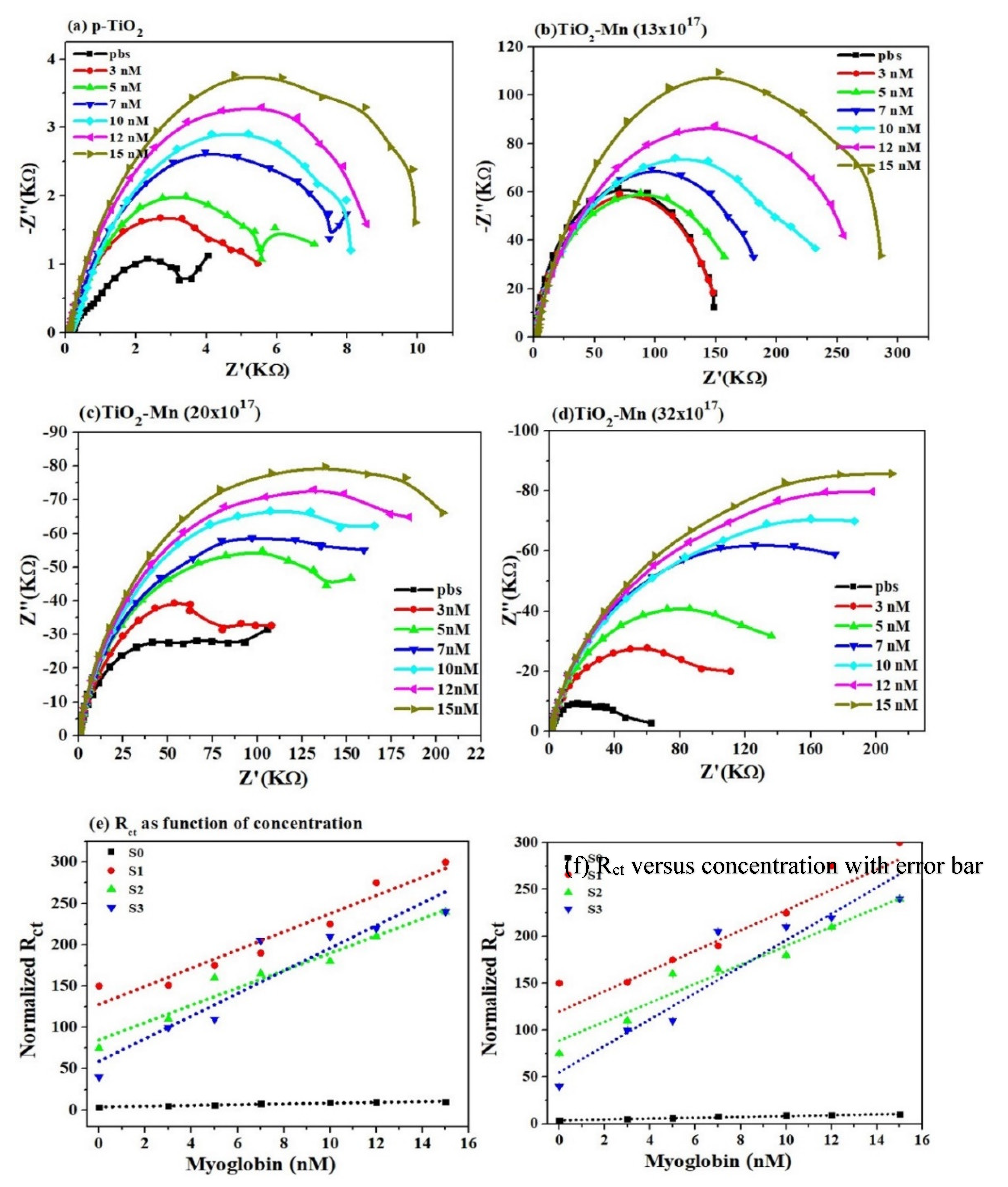
3.2. Label-Free Mb Electrochemical Sensor Based on Pure- and Mn Doped-tio2 Nanoparticles
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Kar, P.; Pandey, A.; Greer, J.J.; Shankar, K. Ultrahigh sensitivity assays for human cardiac troponin I using TiO2 nanotube arrays. Lab Chip 2012, 12, 821. [Google Scholar] [CrossRef] [PubMed]
- Fathil, M.F.M.; Md Arshad, M.K.; Gopinath, S.C.B.; Hashim, U.; Adzhri, R.; Ayub, R.M.; Ruslinda, A.R.; Nuzaihan, M.M.N.; Azman, A.H.; Zaki, M.; et al. Diagnostics on acute myocardial infarction: Cardiac troponin biomarkers. Biosens. Bioelectron. 2015, 70, 209–220. [Google Scholar] [CrossRef]
- Suprun, E.; Bulko, T.; Lisitsa, A.; Gnedenko, O.; Ivanov, A.; Shumyantseva, V.; Archakov, A. Electrochemical nanobiosensor for express diagnosis of acute myocardial infarction in undiluted plasma. Biosens. Bioelectron. 2010, 25, 1694–1698. [Google Scholar] [CrossRef]
- Kong, T.; Su, R.; Zhang, B.; Zhang, Q.; Cheng, G. CMOS-compatible, label-free silicon-nanowire biosensors to detect cardiac troponin I for acute myocardial infarction diagnosis. Biosens. Bioelectron. 2012, 34, 267–272. [Google Scholar] [CrossRef]
- Silva, B.V.; Cavalcanti, I.T.; Mattos, A.B.; Moura, P.; Sotomayor, M.D.P.T.; Dutra, R.F. Disposable immunosensor for human cardiac troponin T based on streptavidin-microsphere modified screen-printed electrode. Biosens. Bioelectron. 2010, 26, 1062–1067. [Google Scholar] [CrossRef]
- Gondal, A.Z.; Foris, L.A.; Richards, J.R. Serum Myoglobin; StatPearls Publishing: St. Petersburg, FL, USA, 2019. [Google Scholar]
- Aydin, S.; Ugur, K.; Aydin, S.; Sahin, İ.; Yardim, M. Biomarkers in acute myocardial infarction: Current perspectives. Vasc. Health Risk Manag. 2019, 15, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Garg, P.; Morris, P.; Fazlanie, A.L.; Vijayan, S.; Dancso, B.; Dastidar, A.G.; Plein, S.; Mueller, C.; Haaf, P. Cardiac biomarkers of acute coronary syndrome: From history to high-sensitivity cardiac troponin. Intern. Emerg. Med. 2017, 12, 147–155. [Google Scholar] [CrossRef] [Green Version]
- Danese, E.; Montagnana, M. An historical approach to the diagnostic biomarkers of acute coronary syndrome. Ann. Transl. Med. 2016, 4, 194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klocke, F.J.; Copley, D.P.; Krawczyk, J.A.; Reichlin, M. Rapid renal clearance of immunoreactive canine plasma myoglobin. Circulation 1982, 65, 1522–1528. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mair, J.; Artner-Dworzak, E.; Lechleitner, P.; Morass, B.; Smidt, J.; Wagner, I.; Dienstl, F.; Puschendorf, B. Early diagnosis of acute myocardial infarction by a newly developed rapid immunoturbidimetric assay for myoglobin. Br. Heart J. 1992, 68, 462–468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Puri, N.; Niazi, A.; Srivastava, A.K. Rajesh Biointerfacial impedance characterization of reduced graphene oxide supported carboxyl pendant conducting copolymer-based electrode. Electrochim. Acta 2014, 123, 211–218. [Google Scholar] [CrossRef]
- Mandal, S.S.; Narayan, K.K.; Bhattacharyya, A.J. Employing denaturation for rapid electrochemical detection of myoglobin using TiO2 nanotubes. J. Mater. Chem. B 2013, 1, 3051–3056. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Ran, F.; Chen, Q.; Luo, D.; Ma, W.; Han, T.; Wang, C.; Wang, C. A fluorescent biosensor for cardiac biomarker myoglobin detection based on carbon dots and deoxyribonuclease I-aided target recycling signal amplification. RSC Adv. 2019, 9, 4463–4468. [Google Scholar] [CrossRef] [Green Version]
- Gnedenko, O.V.; Mezentsev, Y.; Molnar, A.A.; Lisitsa, A.V.; Ivanov, A.; Archakov, A.I. Highly sensitive detection of human cardiac myoglobin using a reverse sandwich immunoassay with a gold nanoparticle-enhanced surface plasmon resonance biosensor. Anal. Chim. Acta 2013, 759, 105–109. [Google Scholar] [CrossRef]
- Naveena, B.; Faustman, C.; Tatiyaborworntham, N.; Yin, S.; Ramanathan, R.; Mancini, R. Detection of 4-hydroxy-2-nonenal adducts of turkey and chicken myoglobins using mass spectrometry. Food Chem. 2010, 122, 836–840. [Google Scholar] [CrossRef]
- Giaretta, N.; Di Giuseppe, A.M.; Lippert, M.; Parente, A.; Di Maro, A. Myoglobin as marker in meat adulteration: A UPLC method for determining the presence of pork meat in raw beef burger. Food Chem. 2013, 141, 1814–1820. [Google Scholar] [CrossRef]
- Zhang, X.; Kong, X.; Fan, W.; Du, X. Iminodiacetic Acid-Functionalized Gold Nanoparticles for Optical Sensing of Myoglobin via Cu2+ Coordination. Langmuir 2011, 27, 6504–6510. [Google Scholar] [CrossRef]
- Dolak, I.; Keçili, R.; Onat, R.; Ziyadanoğulları, B.; Ersöz, A.; Say, R. Molecularly imprinted affinity cryogels for the selective recognition of myoglobin in blood serum. J. Mol. Struct. 2018, 1174, 171–176. [Google Scholar] [CrossRef]
- Keçili, R. Selective Recognition of Myoglobin in Biological Samples Using Molecularly Imprinted Polymer-Based Affinity Traps. Int. J. Anal. Chem. 2018, 2018, 1–9. [Google Scholar] [CrossRef]
- Karami, P.; Bagheri, H.; Johari-Ahar, M.; Khoshsafar, H.; Arduini, F.; Afkhami, A. Dual-modality impedimetric immunosensor for early detection of prostate-specific antigen and myoglobin markers based on antibody-molecularly imprinted polymer. Talanta 2019, 202, 111–122. [Google Scholar] [CrossRef]
- Yang, Z.; Wang, H.; Dong, X.; Yan, H.; Lei, C.; Luo, Y. Giant magnetoimpedance based immunoassay for cardiac biomarker myoglobin. Anal. Methods 2017, 9, 3636–3642. [Google Scholar] [CrossRef]
- Yue, Q.; Song, Z. Assay of femtogram level nitrite in human urine using luminol–myoglobin chemiluminescence. Microchem. J. 2006, 84, 10–13. [Google Scholar] [CrossRef]
- Adeel, M.; Rahman, M.; Lee, J.-J. Label-free aptasensor for the detection of cardiac biomarker myoglobin based on gold nanoparticles decorated boron nitride nanosheets. Biosens. Bioelectron. 2019, 126, 143–150. [Google Scholar] [CrossRef]
- Sharma, D.; Lee, J.; Shin, H. An electrochemical immunosensor based on a 3D carbon system consisting of a suspended mesh and substrate-bound interdigitated array nanoelectrodes for sensitive cardiac biomarker detection. Biosens. Bioelectron. 2018, 107, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, A.K.; Mathpal, M.C.; Kumar, P.; Singh, M.K.; Soler, M.; Agarwal, A. Structural, optical and photoconductivity of Sn and Mn doped TiO2 nanoparticles. J. Alloy. Compd. 2015, 622, 37–47. [Google Scholar] [CrossRef]
- Scherrer, P. Bestimmung der inneren Struktur und der Größe von Kolloidteilchen mittels Röntgenstrahlen. In Chemische Technologie in Einzeldarstellungen; Springer: Berlin/Heidelberg, Germany, 1912; pp. 387–409. [Google Scholar]
- Horzum, S.; Torun, E.; Serin, T.; Peeters, F.M. Structural, electronic and optical properties of Cu-doped ZnO: Experimental and theoretical investigation. Philos. Mag. 2016, 96, 1743–1756. [Google Scholar] [CrossRef]
- Choudhury, B.; Chetri, P.; Choudhury, A. Annealing temperature and oxygen-vacancy-dependent variation of lattice strain, band gap and luminescence properties of CeO2 nanoparticles. J. Exp. Nanosci. 2015, 10, 103–114. [Google Scholar] [CrossRef] [Green Version]
- Kamnev, A.A. Infrared spectroscopy in studying biofunctionalised gold nanoparticles. In Springer Proceedings in Physics; Springer: Berlin/Heidelberg, Germany, 2013; pp. 35–50. [Google Scholar] [CrossRef]
- Wang, Y.; Ni, Y. New insight into protein–nanomaterial interactions with UV-visible spectroscopy and chemometrics: Human serum albumin and silver nanoparticles. Analyst 2013, 139, 416–424. [Google Scholar] [CrossRef]
- Tai, W.-P.; Oh, J.-H. Humidity sensing behaviors of nanocrystalline Al-doped ZnO thin films prepared by sol–gel process. J. Mater. Sci. Mater. Electron. 2002, 13, 391–394. [Google Scholar] [CrossRef]
- Ren, X.; Zhang, Y.; Sun, Y.; Gao, L. Development of electrochemical impedance immunosensor for sensitive determination of myoglobin. Int. J. Electrochem. Sci. 2017, 12, 7765–7776. [Google Scholar] [CrossRef]
- Li, C.; Li, J.; Yang, X.; Gao, L.; Jing, L.; Ma, X. A label-free electrochemical aptasensor for sensitive myoglobin detection in meat. Sens. Actuators B Chem. 2017, 242, 1239–1245. [Google Scholar] [CrossRef]
- Sun, L.; Li, W.; Wang, M.; Ding, W.; Ji, Y. Development of an electrochemical impedance immunosensor for myoglobin determination. Int. J. Electrochem. Sci. 2017, 12, 6170–6179. [Google Scholar] [CrossRef]
- Puri, N.; Mishra, S.K.; Niazi, A.; Srivastava, A.K.; Rajesh, R. Physicochemical characteristics of reduced graphene oxide based Pt-nanoparticles-conducting polymer nanocomposite film for immunosensor applications. J. Chem. Technol. Biotechnol. 2014, 90, 1699–1706. [Google Scholar] [CrossRef]
- Lee, H.Y.; Choi, J.S.; Guruprasath, P.; Lee, B.-H.; Cho, Y.W. An Electrochemical Biosensor Based on a Myoglobin-specific Binding Peptide for Early Diagnosis of Acute Myocardial Infarction. Anal. Sci. 2015, 31, 699–704. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Han, M.; Ye, X.; Wu, K.; Wu, T.; Li, C. Voltammetric myoglobin sensor based on a glassy carbon electrode modified with a composite film consisting of carbon nanotubes and a molecularly imprinted polymerized ionic liquid. Microchim. Acta 2017, 184, 195–202. [Google Scholar] [CrossRef]
- Moreira, F.T.; Dutra, R.A.; Noronha, J.P.; Sales, M.G.F. Electrochemical biosensor based on biomimetic material for myoglobin detection. Electrochim. Acta 2013, 107, 481–487. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; You, Z.; Sha, H.; Sun, Z.; Sun, W. Electrochemical myoglobin biosensor based on carbon ionic liquid electrode modified with Fe3O4@SiO2 microsphere. J. Solid State Electrochem. 2014, 18, 207–213. [Google Scholar] [CrossRef]
- Ji, T.; Xu, X.; Wang, X.; Zhou, Q.; Ding, W.; Chen, B.; Guo, X.; Hao, Y.; Chen, G. Point of care upconversion nanoparticles-based lateral flow assay quantifying myoglobin in clinical human blood samples. Sens. Actuators B Chem. 2019, 282, 309–316. [Google Scholar] [CrossRef]
- Miao, D.; Liu, D.; Zeng, Y.; Zhou, G.; Xie, W.; Yang, Y.; Wang, H.; Zhang, J.; Zhai, Y.; Zhang, Z.; et al. Fluorescent aptasensor based on D-AMA/F-CSC for the sensitive and specific recognition of myoglobin. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 228, 117714. [Google Scholar] [CrossRef] [PubMed]
- Sandford, C.; Edwards, M.A.; Klunder, K.J.; Hickey, D.P.; Li, M.; Barman, K.; Sigman, M.S.; White, H.S.; Minteer, S.D. A synthetic chemist’s guide to electroanalytical tools for studying reaction mechanisms. Chem. Sci. 2019, 10, 6404–6422. [Google Scholar] [CrossRef] [Green Version]
- Haque, M.; Fouad, H.; Seo, H.-K.; Othman, A.Y.; Kulkarni, A.; Ansari, Z.A. Investigation of Mn Doped ZnO Nanoparticles Towards Ascertaining Myocardial Infarction Through an Electrochemical Detection of Myoglobin. IEEE Access 2020, 8, 164678–164692. [Google Scholar] [CrossRef]

| TiO2 with Mn-Doping Concentration (Atoms/cm3) | Optical Band Gap (eV) | Grain Size (nm) * | Dislocation Density (×10−18 m−2) | Strain ε | Sensitivity μA-cm−2/nM | Diffusion Coefficient (D) (×10−9 cm2/s) |
|---|---|---|---|---|---|---|
| 0 (S0) | 3.95 | 39.38 | 0.005 | 0.14 | 23.43 | 1.62 |
| 13 × 1017 (SI) | 3.73 | 61.30 | 0.010 | 1.00 | 70.44 | 3.17 |
| 20 × 1017(SII) | 3.84 | 49.30 | 0.023 | 1.45 | 77.43 | 4.25 |
| 32 × 1017(SIII) | 3.88 | 49.35 | 0.029 | 1.63 | 100.40 | 1.62 |
| Method | Matrix | Sample | Linear Range | LOD | Ref. |
|---|---|---|---|---|---|
| EIS | Ab-MYO/AuNps/APTES /ITO | Serum | 10 ng mL−1–1 μg mL−1 | 2.7 ng mL−1 | [35] |
| EIS | Anti-MYO/4-ATP SAM/Au | Saline, | 350 ng mL−1–17.5 μg L−1 | 5.5 ng mL−1 | [33] |
| DPV | MBA/AuNps/RGD/GRCOOH/GCE | Pork sample | 0.0001–0.2 g L−1 | 26.3 ng mL−1 | [34] |
| EIS | Anti-MYO/PtNP(PPy-PPa)-RGO/ITO | NA | 10 ng mL−1–1 μg mL−1 | 4.0 ng mL−1 | [36] |
| DPV | DSP/SAM/Au | Serum sample | 17.8–1780 ng mL–1 | 9.8 ng mL−1 | [37] |
| DPV | MIP/MWCNT/GCE | Serum | 1 μg mL−1–0.1 mg mL−1 | 0.17 μg mL−1 | [38] |
| SWV | MIP/PVC-COOH/Au-SPE | Serum | 1.1–2.98 μg mL−1 | 2.25 μg mL−1 | [39] |
| CV | Ti-NT/GCE | Serum | 0.001–0.1 mg mL−1 | 1 μg mL−1 | [13] |
| SWV | AuNp/DDAB/Anti- MYO/SPE | Serum sample | 10–1780 ng mL−1 | 10 ng mL−1 | [3] |
| CV | CS/MYOFe3O4@ SiO2/CILE | - | 0.2–11.0 ng mL−1 | 0.18 ng mL−1 | [40] |
| EIS | AuNp-PPy- PPa/RGO/APTES/ITO | Serum sample | 10 ng mL−1–1 μg mL−1 | 1.49 ng mL−1 | [12] |
| EIS | Ab-GO-MWCNT-Fe3O4 | Serum urine | 1–20000 ng mL−1 | 0.83 ng mL−1 | [21] |
| Lateral flow Assay | NaYF4:Yb, Er@NaLuF4 | Blood plasma | 10–400 ng mL−1 | 0.21 ng/mL | [41] |
| Fluorescence | dabcyl [(E)-4-((4-(dimethylamino) phenyl) diazenyl)benzoic acid | PBS | 0.1–5 ng/mL. | 0.07 ng mL−1 | [42] |
| CV/EIS | Mn doped TiO2 nanoparticles | PBS | 3–15 nM | S0: 2.6 ng/mL (0.153 nM) S1:0.63 ng/mL (0.036 nM) S2:0.51 ng/mL (0.029 nM) S3:0.22 ng/mL (0.013 nM) | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al Fatease, A.; Haque, M.; Umar, A.; Ansari, S.G.; Alhamhoom, Y.; Muhsinah, A.B.; Mahnashi, M.H.; Guo, W.; Ansari, Z.A. Label-Free Electrochemical Sensor Based on Manganese Doped Titanium Dioxide Nanoparticles for Myoglobin Detection: Biomarker for Acute Myocardial Infarction. Molecules 2021, 26, 4252. https://doi.org/10.3390/molecules26144252
Al Fatease A, Haque M, Umar A, Ansari SG, Alhamhoom Y, Muhsinah AB, Mahnashi MH, Guo W, Ansari ZA. Label-Free Electrochemical Sensor Based on Manganese Doped Titanium Dioxide Nanoparticles for Myoglobin Detection: Biomarker for Acute Myocardial Infarction. Molecules. 2021; 26(14):4252. https://doi.org/10.3390/molecules26144252
Chicago/Turabian StyleAl Fatease, Adel, Mazharul Haque, Ahmad Umar, Shafeeque G. Ansari, Yahya Alhamhoom, Abdullatif Bin Muhsinah, Mater H. Mahnashi, Wenjuan Guo, and Zubaida A. Ansari. 2021. "Label-Free Electrochemical Sensor Based on Manganese Doped Titanium Dioxide Nanoparticles for Myoglobin Detection: Biomarker for Acute Myocardial Infarction" Molecules 26, no. 14: 4252. https://doi.org/10.3390/molecules26144252








