Facile and Green Synthesis of Starfruit-Like ZIF-L, and Its Optimization Study
Abstract
:1. Introduction
2. Results and Discussion
2.1. One-Factor-at-a-Time (OFAT) Experiments
2.1.1. Effects of the Molar Ratio of Ligand to Metal
2.1.2. Effects of Reaction Time
2.1.3. Effects of the Temperature
2.2. Response Surface Analysis
2.2.1. Central Composite Design (CCD) and Analysis of Variance (ANOVA)
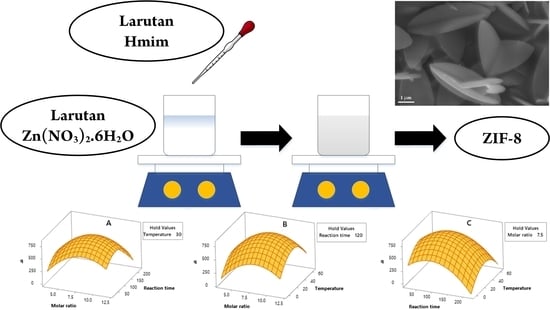
2.2.2. Response Surface Plots
2.2.3. Optimization and Validation
2.3. Characterizations of ZIF-L
2.3.1. Scanning Electron Microscopy (SEM) Analysis
2.3.2. X-ray Diffraction (XRD) Analysis
2.3.3. Fourier-Transform Infrared Spectroscopy (FTIR) Analysis
2.3.4. Thermalgravimetric Analysis (TGA)
3. Materials and Methods
3.1. Materials
3.2. Synthesis of ZIF-L
3.3. Adsorption of Crystal Violet Dye
3.4. Design of the Experiment
3.4.1. One-Factor-at-a-Time (OFAT) Method
3.4.2. Response Surface Methodology
3.4.3. Optimization
3.5. Characterizations
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- He, Y.; Shi, L.; Wang, J.; Yan, J.; Chen, Y.; Wang, X.; Song, Y.; Han, G. UiO-66-NDC (1,4-naphthalenedicarboxilic acid) as a novel fluorescent probe for the selective detection of Fe3+. J. Solid State Chem. 2020, 285, 121206. [Google Scholar] [CrossRef]
- Jian, M.; Liu, B.; Liu, R.; Qu, J.; Wang, H.; Zhang, X. Water-based synthesis of zeolitic imidazolate framework-8 with high morphology level at room temperature. RSC Adv. 2015, 5, 48433–48441. [Google Scholar] [CrossRef]
- Sun, S.; Yang, Z.; Cao, J.; Wang, Y.; Xiong, W. Copper-doped ZIF-8 with high adsorption performance for removal of tetracycline from aqueous solution. J. Solid State Chem. 2020, 285, 121219. [Google Scholar] [CrossRef]
- Zhao, H.; Li, Q.; Wang, Z.; Wu, T.; Zhang, M. Synthesis of MIL-101(Cr) and its water adsorption performance. Microporous Mesoporous Mater. 2020, 297, 110044. [Google Scholar] [CrossRef]
- Zhuang, S.; Liu, Y.; Wang, J. Mechanistic insight into the adsorption of diclofenac by MIL-100: Experiments and theoretical calculations. Environ. Pollut. 2019, 253, 616–624. [Google Scholar] [CrossRef] [PubMed]
- Ediati, R.; Dewi, S.K.; Hasan, M.R.; Kahardina, M.; Murwani, I.K.; Nadjib, M. Mesoporous HKUST-1 synthesized using solvothermal method. Rasayan J. Chem. 2019, 12, 1653–1659. [Google Scholar] [CrossRef]
- Masoumi, S.; Tabrizi, F.F.; Sardarian, A.R. Efficient tetracycline hydrochloride removal by encapsulated phosphotungstic acid (PTA) in MIL-53 (Fe): Optimizing the content of PTA and recycling study. J. Environ. Chem. Eng. 2020, 8, 103601. [Google Scholar] [CrossRef]
- Mao, Y.; Qi, H.; Ye, G.; Han, L.; Zhou, W.; Xu, W.; Sun, Y. Green and time-saving synthesis of MIL-100(Cr) and its catalytic performance. Microporous Mesoporous Mater. 2019, 274, 70–75. [Google Scholar] [CrossRef]
- Mon, M.; Bruno, R.; Tiburcio, E.; Casteran, P.E.; Ferrando-Soria, J.; Armentano, D.; Pardo, E. Efficient Capture of Organic Dyes and Crystallographic Snapshots by a Highly Crystalline Amino-Acid-Derived Metal–Organic Framework. Chem. A Eur. J. 2018, 24, 17712–17718. [Google Scholar] [CrossRef] [PubMed]
- Alivand, M.S.; Tehrani, N.H.M.H.; Shafiei-Alavijeh, M.; Rashidi, A.; Kooti, M.; Pourreza, A.; Fakhraie, S. Synthesis of a modified HF-free MIL-101(Cr) nanoadsorbent with enhanced H2S/CH4, CO2/CH4, and CO2/N2 selectivity. J. Environ. Chem. Eng. 2019, 7, 102946. [Google Scholar] [CrossRef]
- Cui, X.; Sun, X.; Liu, L.; Huang, Q.; Yang, H.; Chen, C. In-situ fabrication of cellulose foam HKUST-1 and surface modi fi cation with polysaccharides for enhanced selective adsorption of toluene and acidic dipeptides. Chem. Eng. J. 2019, 369, 898–907. [Google Scholar] [CrossRef]
- Mon, M.; Bruno, R.; Ferrando-Soria, J.; Armentano, D.; Pardo, E. Metal-organic framework technologies for water remediation: Towards a sustainable ecosystem. J. Mater. Chem. A 2018, 6, 4912–4947. [Google Scholar] [CrossRef]
- Chaturvedi, G.; Kaur, A.; Umar, A.; Khan, M.A.; Algarni, H.; Kansal, S.K. Removal of fluoroquinolone drug, levofloxacin, from aqueous phase over iron based MOFs, MIL-100(Fe). J. Solid State Chem. 2020, 281, 121029. [Google Scholar] [CrossRef]
- He, Y.; Zeng, L.; Feng, Z.; Zhang, Q.; Zhao, X.; Ge, S.; Hu, X.; Lin, H. Preparation, characterization, and photocatalytic activity of novel AgBr/ZIF-8 composites for water purification. Adv. Powder Technol. 2020, 31, 439–447. [Google Scholar] [CrossRef]
- Chu, F.; Zheng, Y.; Wen, B.; Zhou, L.; Yan, J.; Chen, Y. Adsorption of toluene with water on zeolitic imidazolate framework-8/graphene oxide hybrid nanocomposites in a humid atmosphere. RSC Adv. 2018, 8, 2426–2432. [Google Scholar] [CrossRef] [Green Version]
- Elhussein, E.A.A.; Şahin, S.; Bayazit, Ş.S. Removal of carbamazepine using UiO-66 and UiO-66/graphene nanoplatelet composite. J. Environ. Chem. Eng. 2020, 8, 2–9. [Google Scholar] [CrossRef]
- Fan, C.; Dong, H.; Liang, Y.; Yang, J.; Tang, G.; Zhang, W.; Cao, Y. Sustainable synthesis of HKUST-1 and its composite by biocompatible ionic liquid for enhancing visible-light photocatalytic performance. J. Clean. Prod. 2019, 208, 353–362. [Google Scholar] [CrossRef]
- Pangestu, T.; Kurniawan, Y.; Soetaredjo, F.E.; Santoso, S.P.; Irawaty, W.; Yuliana, M.; Hartono, S.B.; Ismadji, S. The synthesis of biodiesel using copper based metal-organic framework as a catalyst. J. Environ. Chem. Eng. 2019, 7, 103277. [Google Scholar] [CrossRef] [Green Version]
- Kumar, A.; Chowdhuri, A.R.; Kumari, A.; Sahu, S.K. IRMOF-3: A fluorescent nanoscale metal organic frameworks for selective sensing of glucose and Fe (III) ions without any modification. Mater. Sci. Eng. C 2018, 92, 913–921. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Yu, Y.; Fu, C.; Wang, L.; Li, X. Water-based synthesis of zeolitic imidazolate framework-8 for CO2 capture. RSC Adv. 2017, 7, 29227–29232. [Google Scholar] [CrossRef] [Green Version]
- Tzitzios, V.; Kostoglou, N.; Giannouri, M.; Basina, G.; Tampaxis, C.; Charalambopoulou, G.; Steriotis, T.; Polychronopoulou, K.; Doumanidis, C.; Mitterer, C.; et al. Solvothermal synthesis, nanostructural characterization and gas cryo-adsorption studies in a metal–organic framework (IRMOF-1) material. Int. J. Hydrog. Energy 2017, 42, 23899–23907. [Google Scholar] [CrossRef]
- Simon, M.A.; Anggraeni, E.; Soetaredjo, F.E.; Santoso, S.P.; Irawaty, W.; Thanh, T.C.; Hartono, S.B.; Yuliana, M.; Ismadji, S. Hydrothermal Synthesize of HF-Free MIL-100(Fe) for Isoniazid-Drug Delivery. Sci. Rep. 2019, 9, 1–11. [Google Scholar] [CrossRef]
- Strzempek, W.; Menaszek, E.; Gil, B. Fe-MIL-100 as drug delivery system for asthma and chronic obstructive pulmonary disease treatment and diagnosis. Microporous Mesoporous Mater. 2019, 280, 264–270. [Google Scholar] [CrossRef]
- Safaei, M.; Foroughi, M.M.; Ebrahimpoor, N.; Jahani, S.; Omidi, A.; Khatami, M. A review on metal-organic frameworks: Synthesis and applications. Trends Anal. Chem. 2019, 118, 401–425. [Google Scholar] [CrossRef]
- Son, Y.R.; Ryu, S.G.; Kim, H.S. Rapid adsorption and removal of sulfur mustard with zeolitic imidazolate frameworks ZIF-8 and ZIF-67. Microporous Mesoporous Mater. 2020, 293, 109819. [Google Scholar] [CrossRef]
- Abdelhamid, H.N.; Zou, X. Template-free and room temperature synthesis of hierarchical porous zeolitic imidazolate framework nanoparticles and their dye and CO2 sorption. Green Chem. 2018, 20, 1074–1084. [Google Scholar] [CrossRef]
- Li, R.; Li, W.; Jin, C.; He, Q.; Wang, Y. Fabrication of ZIF-8@TiO2 micron composite via hydrothermal method with enhanced absorption and photocatalytic activities in tetracycline degradation. J. Alloys Compd. 2020, 825, 154008. [Google Scholar] [CrossRef]
- Lee, Y.; Jang, M.; Cho, H.; Kwon, H.; Kim, S.; Ahn, W. ZIF-8: A comparison of synthesis methods. Chem. Eng. J. 2015, 271, 276–280. [Google Scholar] [CrossRef]
- Santoso, E.; Ediati, R.; Istiqomah, Z.; Sulistiono, D.O.; Nugraha, R.E.; Kusumawati, Y.; Bahruji, H.; Prasetyoko, D. Facile synthesis of ZIF-8 nanoparticles using polar acetic acid solvent for enhanced adsorption of methylene blue. Microporous Mesoporous Mater. 2021, 310, 110620. [Google Scholar] [CrossRef]
- Huang, C.; Zhang, H.; Zheng, K.; Zhang, Z.; Jiang, Q.; Li, J. Two-dimensional hydrophilic ZIF-L as a highly-selective adsorbent for rapid phosphate removal from wastewater. Sci. Total Environ. 2021, 785, 147382. [Google Scholar] [CrossRef]
- Zhang, F.; Dou, J.; Zhang, H. Mixed membranes comprising carboxymethyl cellulose (as capping agent and gas barrier matrix) and nanoporous ZIF-L nanosheets for gas separation applications. Polymers 2018, 10, 1340. [Google Scholar] [CrossRef] [Green Version]
- Zhong, Z.; Yao, J.; Chen, R.; Low, Z.; He, M.; Liu, J.Z.; Wang, H. Oriented two-dimensional zeolitic imidazolate framework-L membranes and their gas permeation properties. J. Mater. Chem. A 2015, 3, 15715–15722. [Google Scholar] [CrossRef]
- Chen, R.; Yao, J.; Gu, Q.; Smeets, S.; Baerlocher, C.; Gu, H.; Zhu, D.; Morris, W.; Yaghi, O.M.; Wang, H. A two-dimensional zeolitic imidazolate framework with a cushion-shaped cavity for CO2 adsorption. Chem. Commun. 2013, 49, 9500–9502. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.U.; Othman, M.H.D.; Ismail, A.F.; Ismail, N.; Jaafar, J.; Hashim, H.; Rahman, M.A.; Jilani, A. Structural transition from two-dimensional ZIF-L to three-dimensional ZIF-8 nanoparticles in aqueous room temperature synthesis with improved CO2 adsorption. Mater. Charact. 2018, 136, 407–416. [Google Scholar] [CrossRef]
- Gross, A.F.; Sherman, E.; Vajo, J.J. Aqueous room temperature synthesis of cobalt and zinc sodalite zeolitic imidizolate frameworks. Dalt. Trans. 2012, 41, 5458–5460. [Google Scholar] [CrossRef] [PubMed]
- Kida, K.; Okita, M.; Fujita, K.; Tanaka, S.; Miyake, Y. Formation of high crystalline ZIF-8 in an aqueous solution. CrystEngComm 2013, 15, 1794–1801. [Google Scholar] [CrossRef]
- Tran, B.L.; Chin, H.Y.; Chang, B.K.; Chiang, A.S.T. Dye adsorption in ZIF-8: The importance of external surface area. Microporous Mesoporous Mater. 2019, 277, 149–153. [Google Scholar] [CrossRef]
- Ding, B.; Wang, X.; Xu, Y.; Feng, S.; Ding, Y.; Pan, Y.; Xu, W.; Wang, H. Hydrothermal preparation of hierarchical ZIF-L nanostructures for enhanced CO2 capture. J. Colloid Interface Sci. 2018, 519, 38–43. [Google Scholar] [CrossRef]
- Valencia, L.; Abdelhamid, H.N. Nanocellulose leaf-like zeolitic imidazolate framework (ZIF-L) foams for selective capture of carbon dioxide. Carbohydr. Polym. 2019, 213, 338–345. [Google Scholar] [CrossRef]
- Mahmoodi, N.M.; Keshavarzi, S.; Oveisi, M.; Rahimi, S.; Hayati, B. Metal-organic framework (ZIF-8)/inorganic nanofiber (Fe2O3) nanocomposite: Green synthesis and photocatalytic degradation using LED irradiation. J. Mol. Liq. 2019, 291, 111333. [Google Scholar] [CrossRef]
- Wijaya, C.J.; Ismadji, S.; Aparamarta, H.W.; Gunawan, S. Hydrophobic modification of cellulose nanocrystals from bamboo shoots using rarasaponins. ACS Omega 2020, 5, 20967–20975. [Google Scholar] [CrossRef] [PubMed]
- Wijaya, C.J.; Ismadji, S.; Aparamarta, H.W.; Gunawan, S. Optimization of cellulose nanocrystals from bamboo shoots using Response Surface Methodology. Heliyon 2019, 5, e02807. [Google Scholar] [CrossRef] [PubMed]
- Yuliana, M.; Sutrisno, R.J.; Hermanto, S.; Ismadji, S.; Wijaya, C.J.; Santoso, S.P.; Soetaredjo, F.E.; Ju, Y.-H. Hydrophobic Cetyltrimethylammonium Bromide-Pillared Bentonite as an Effective Palm Oil Bleaching Agent. ACS Omega 2020, 5, 28844–28855. [Google Scholar] [CrossRef] [PubMed]










| Run Order | Blocks | Points | Coded Parameters | Uncoded Parameter | as the Response (mg/g) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| (min) | (°C) | Experimental | Predicted | |||||||
| 1 | 1 | Cube | −1 | −1 | −1 | 5 | 60 | 10 | 522.32 | 522.36 |
| 2 | +1 | −1 | −1 | 10 | 60 | 10 | 664.72 | 645.08 | ||
| 3 | −1 | +1 | −1 | 5 | 180 | 10 | 336.47 | 337.96 | ||
| 4 | +1 | +1 | −1 | 10 | 180 | 10 | 408.43 | 424.48 | ||
| 5 | −1 | −1 | +1 | 5 | 60 | 50 | 498.08 | 479.12 | ||
| 6 | +1 | −1 | +1 | 10 | 60 | 50 | 585.69 | 601.84 | ||
| 7 | −1 | +1 | +1 | 5 | 180 | 50 | 350.26 | 353.92 | ||
| 8 | +1 | +1 | +1 | 10 | 180 | 50 | 449.72 | 440.44 | ||
| 9 | Axial | −1.68 | 0 | 0 | 3.30 | 120 | 30 | 437.14 | 439.11 | |
| 10 | +1.68 | 0 | 0 | 11.70 | 120 | 30 | 621.00 | 615.06 | ||
| 11 | 0 | −1.68 | 0 | 7.5 | 19 | 30 | 599.45 | 624.11 | ||
| 12 | 0 | +1.68 | 0 | 7.5 | 221 | 30 | 360.14 | 333.33 | ||
| 13 | 0 | 0 | −1.68 | 7.5 | 120 | −3.6 | 479.18 | 487.93 | ||
| 14 | 0 | 0 | +1.68 | 7.5 | 120 | 63.6 | 477.81 | 464.99 | ||
| 15 | Center | 0 | 0 | 0 | 7.5 | 120 | 30 | 787.09 | 798.02 | |
| 16 | 0 | 0 | 0 | 7.5 | 120 | 30 | 811.31 | 798.02 | ||
| 17 | 0 | 0 | 0 | 7.5 | 120 | 30 | 808.36 | 798.02 | ||
| 18 | 0 | 0 | 0 | 7.5 | 120 | 30 | 802.44 | 798.02 | ||
| 19 | 0 | 0 | 0 | 7.5 | 120 | 30 | 798.52 | 798.02 | ||
| 20 | 0 | 0 | 0 | 7.5 | 120 | 30 | 791.53 | 798.02 | ||
| 21 | 2 | Cube | −1 | −1 | −1 | 5 | 60 | 10 | 547.40 | 522.36 |
| 22 | +1 | −1 | −1 | 10 | 60 | 10 | 647.42 | 645.08 | ||
| 23 | −1 | +1 | −1 | 5 | 180 | 10 | 350.84 | 337.96 | ||
| 24 | +1 | +1 | –1 | 10 | 180 | 10 | 422.30 | 424.48 | ||
| 25 | −1 | −1 | +1 | 5 | 60 | 50 | 489.60 | 479,12 | ||
| 26 | +1 | −1 | +1 | 10 | 60 | 50 | 607.42 | 601.84 | ||
| 27 | −1 | +1 | +1 | 5 | 180 | 50 | 356.08 | 353.92 | ||
| 28 | +1 | +1 | +1 | 10 | 180 | 50 | 452.07 | 440.44 | ||
| 29 | Axial | −1.68 | 0 | 0 | 3.30 | 120 | 30 | 438.61 | 439.11 | |
| 30 | +1.68 | 0 | 0 | 11.70 | 120 | 30 | 632.02 | 615.06 | ||
| 31 | 0 | −1.68 | 0 | 7.5 | 19 | 30 | 627.63 | 624.11 | ||
| 32 | 0 | +1.68 | 0 | 7.5 | 221 | 30 | 327.21 | 333.33 | ||
| 33 | 0 | 0 | −1.68 | 7.5 | 120 | – 3.6 | 490.08 | 487.93 | ||
| 34 | 0 | 0 | +1.68 | 7.5 | 120 | 63.6 | 458.00 | 464.99 | ||
| 35 | Center | 0 | 0 | 0 | 7.5 | 120 | 30 | 795.24 | 798.02 | |
| 36 | 0 | 0 | 0 | 7.5 | 120 | 30 | 798.38 | 798.02 | ||
| 37 | 0 | 0 | 0 | 7.5 | 120 | 30 | 805.92 | 798.02 | ||
| 38 | 0 | 0 | 0 | 7.5 | 120 | 30 | 795.41 | 798.02 | ||
| 39 | 0 | 0 | 0 | 7.5 | 120 | 30 | 816.45 | 798.02 | ||
| 40 | 0 | 0 | 0 | 7.5 | 120 | 30 | 793.29 | 798.02 | ||
| 41 | 3 | Cube | −1 | −1 | −1 | 5 | 60 | 10 | 518.82 | 522.36 |
| 42 | +1 | −1 | −1 | 10 | 60 | 10 | 616.05 | 645.08 | ||
| 43 | −1 | +1 | −1 | 5 | 180 | 10 | 343.07 | 337.96 | ||
| 44 | +1 | +1 | −1 | 10 | 180 | 10 | 423.69 | 424.48 | ||
| 45 | −1 | −1 | +1 | 5 | 60 | 50 | 454.36 | 479,12 | ||
| 46 | +1 | −1 | +1 | 10 | 60 | 50 | 605.29 | 601.84 | ||
| 47 | −1 | +1 | +1 | 5 | 180 | 50 | 348.15 | 353.92 | ||
| 48 | +1 | +1 | +1 | 10 | 180 | 50 | 407.54 | 440.44 | ||
| 49 | Axial | −1.68 | 0 | 0 | 3.30 | 120 | 30 | 419.41 | 439.11 | |
| 50 | +1.68 | 0 | 0 | 11.70 | 120 | 30 | 617.88 | 615.06 | ||
| 51 | 0 | −1.68 | 0 | 7.5 | 19 | 30 | 637.00 | 624.11 | ||
| 52 | 0 | +1.68 | 0 | 7.5 | 221 | 30 | 324.36 | 333.33 | ||
| 53 | 0 | 0 | −1.68 | 7.5 | 120 | –3.6 | 486.33 | 487.93 | ||
| 54 | 0 | 0 | +1.68 | 7.5 | 120 | 63.6 | 470.88 | 464.99 | ||
| 55 | Center | 0 | 0 | 0 | 7.5 | 120 | 30 | 780.22 | 798.02 | |
| 56 | 0 | 0 | 0 | 7.5 | 120 | 30 | 780.15 | 798.02 | ||
| 57 | 0 | 0 | 0 | 7.5 | 120 | 30 | 788.73 | 798.02 | ||
| 58 | 0 | 0 | 0 | 7.5 | 120 | 30 | 794.52 | 798.02 | ||
| 59 | 0 | 0 | 0 | 7.5 | 120 | 30 | 810.04 | 798.02 | ||
| 60 | 0 | 0 | 0 | 7.5 | 120 | 30 | 806.20 | 798.02 | ||
| Source | Sum of Squares | Degree of Freedom | Mean Square | F-Value | p-Value |
|---|---|---|---|---|---|
| Model | 1,688,160 | 11 | 153,469 | 894.54 | 0.000 |
| Blocks | 1271 | 2 | 635 | 3.70 | 0.032 |
| 112,111 | 1 | 112,111 | 653.47 | 0.000 | |
| 306,223 | 1 | 306,223 | 1784.91 | 0.000 | |
| 1907 | 1 | 1907 | 11.12 | 0.002 | |
| 396,686 | 1 | 396,686 | 2312.20 | 0.000 | |
| 551,006 | 1 | 551,006 | 3211.70 | 0.000 | |
| 558,801 | 1 | 558,801 | 3257.14 | 0.000 | |
| 1965 | 1 | 1965 | 11.45 | 0.001 | |
| 94 | 1 | 94 | 0.55 | 0.463 * | |
| 5260 | 1 | 5260 | 30.66 | 0.000 | |
| Residual | 8235 | 48 | 172 | ||
| Lack-of-fit | 6585 | 33 | 200 | 1.81 | 0.110 * |
| Pure error | 1650 | 15 | 110 | ||
| Total | 1,696,395 | 59 |
| Runs | Molar Ratio of Ligand to Metal | Reaction Time | Temperature | (mg/g) |
|---|---|---|---|---|
| 1 | 8.2220 | 97 | 29 | 808.21 |
| 2 | 802.30 | |||
| 3 | 820.43 | |||
| Mean value | 810.32 ± 9.25 | |||
| Optimized value | 823.02 | |||
| Error (%) | 1.54 ± 1.12 | |||
| Parameters | Levels | |||
|---|---|---|---|---|
| 2.5 | 5 | 7.5 | 10 | |
| 30 | 60 | 120 | 180 | |
| 10 | 30 | 50 | 70 | |
| Coded Levels | Parameters | ||
|---|---|---|---|
| Molar ratio of Ligand to Metal | Reaction Time | Temperature | |
| −1.68 | 3.30 | 19 | −3.6 |
| −1 | 5 | 60 | 10 |
| 0 | 7.5 | 120 | 30 |
| +1 | 10 | 180 | 50 |
| +1.68 | 11.70 | 221 | 63.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wijaya, C.J.; Ismadji, S.; Aparamarta, H.W.; Gunawan, S. Facile and Green Synthesis of Starfruit-Like ZIF-L, and Its Optimization Study. Molecules 2021, 26, 4416. https://doi.org/10.3390/molecules26154416
Wijaya CJ, Ismadji S, Aparamarta HW, Gunawan S. Facile and Green Synthesis of Starfruit-Like ZIF-L, and Its Optimization Study. Molecules. 2021; 26(15):4416. https://doi.org/10.3390/molecules26154416
Chicago/Turabian StyleWijaya, Christian J., Suryadi Ismadji, Hakun W. Aparamarta, and Setiyo Gunawan. 2021. "Facile and Green Synthesis of Starfruit-Like ZIF-L, and Its Optimization Study" Molecules 26, no. 15: 4416. https://doi.org/10.3390/molecules26154416