Systematic Exploration of the Synthetic Parameters for the Production of Dynamic VO2(M1)
Abstract
:1. Introduction
2. Results and Discussion
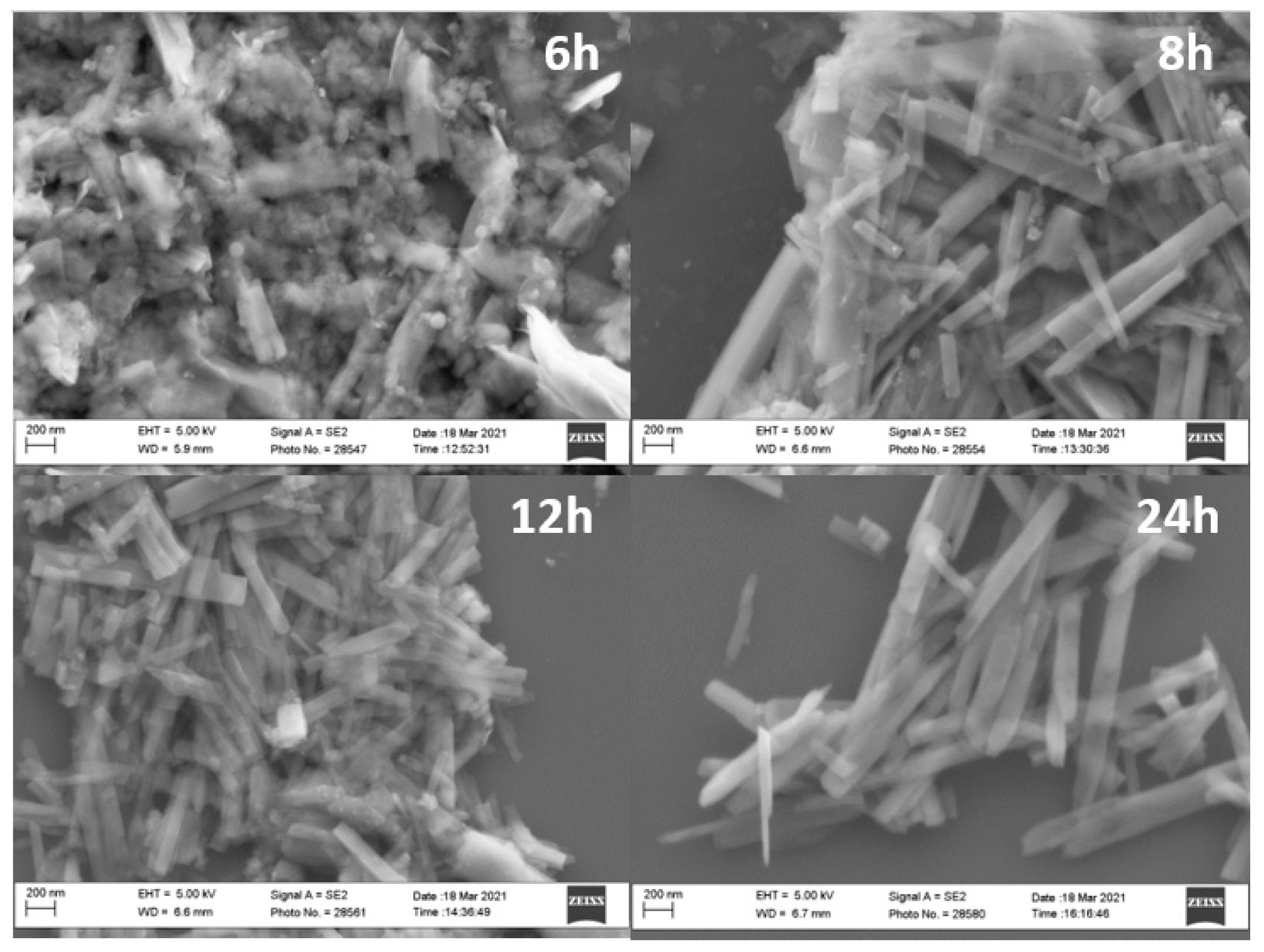
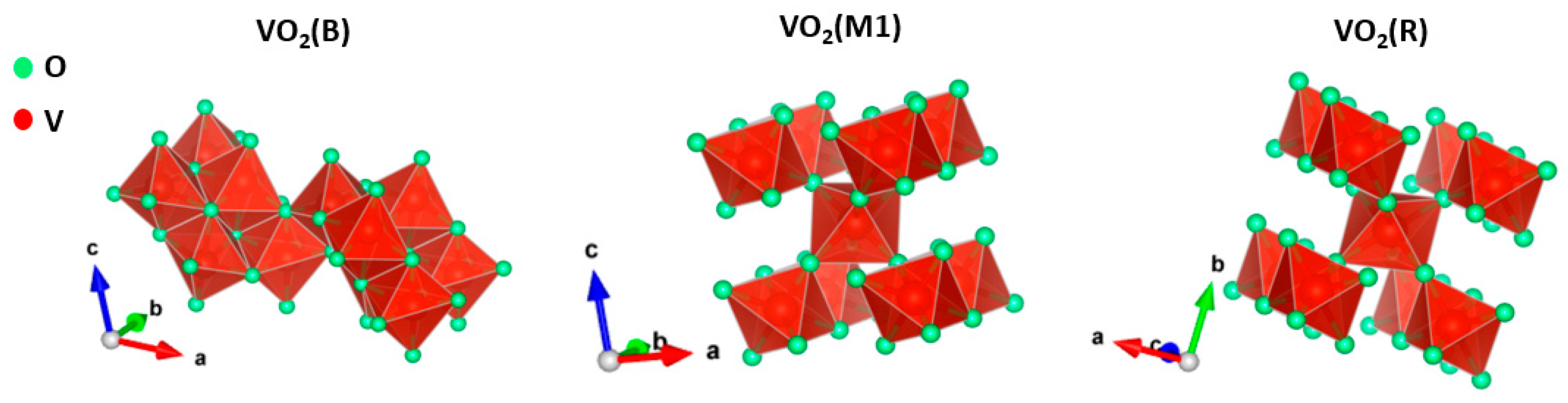
2.1. Synthesis and Characterization of VO2(B) and Its Conversion to VO2(M1)
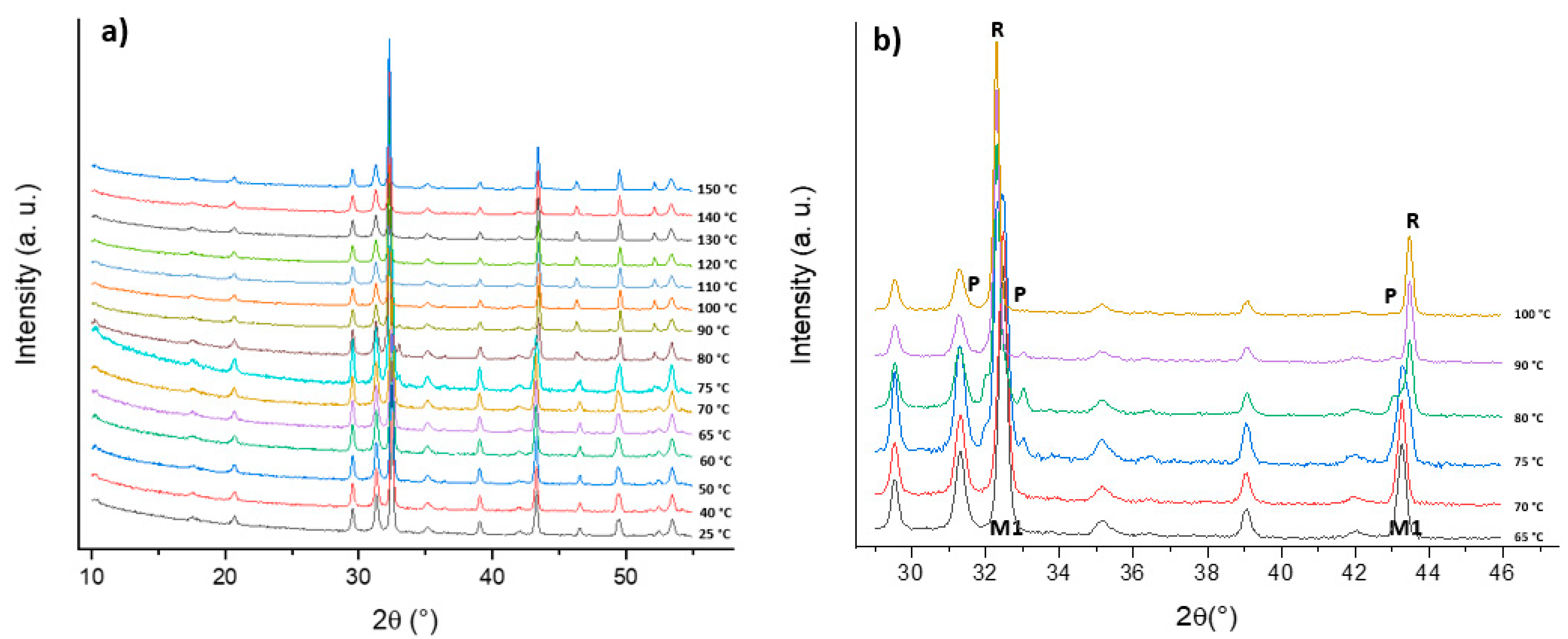
2.2. Conversion of VO2(B) to VO2(M1) Polymorph
2.3. Functionalization of VO2(M1) with a Silica Layer
3. Materials and Methods
3.1. Hydrothermal Synthesis and Scale-Up Process
- Vxy_z, where V = V2O5 precursor, x = number of the synthetized sample, y = V2O5: reducing agent molar ratio, z = reaction time
- Nxy_z, where N = NH4VO3 precursor, x = number of the synthetized sample, y = NH4VO3: reducing agent molar ratio, z = reaction time
3.2. Thermal Conversion of VO2(B) to VO2(M1)
3.3. Functionalization of VO2(M1) with SiO2 Layer
3.4. Stability Tests at Alkaline pH
3.5. Characterizations of VO2(B)
3.5.1. X-ray Diffraction Analysis
3.5.2. SEM-EDX Analysis
3.5.3. TEM Analysis
3.5.4. DSC Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Granqvist, C.G. Solar Energy Materials for Thermal Applications. In Kirk-Othmer Encyclopedia of Chemical Technology; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2017; pp. 1–28. [Google Scholar]
- Baetens, R.; Jelle, B.P.; Gustavsen, A. Properties, requirements and possibilities of smart windows for dynamic daylight and solar energy control in buildings: A state-of-the-art review. Sol. Energy Mater. Sol. Cells 2010, 94, 87–105. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Runnerstrom, E.L.; Milliron, D.J. Switchable Materials for Smart Windows. Annu. Rev. Chem. Biomol. Eng. 2016, 7, 283–304. [Google Scholar] [CrossRef] [PubMed]
- Mott, N.F. Metal-Insulator Transition. Rev. Mod. Phys. 1968, 40, 677–683. [Google Scholar] [CrossRef]
- Kanu, S.S.; Binions, R. Thin films for solar control applications. Proc. R. Soc. A Math. Phys. Eng. Sci. 2010, 466, 19–44. [Google Scholar] [CrossRef] [Green Version]
- Kamalisarvestani, M.; Saidur, R.; Mekhilef, S.; Javadi, F.S. Performance, materials and coating technologies of thermochromic thin films on smart windows. Renew. Sustain. Energy Rev. 2013, 26, 353–364. [Google Scholar] [CrossRef]
- Lüthi, D.; Le Floch, M.; Bereiter, B.; Blunier, T.; Barnola, J.; Siegenthaler, U.; Raynaud, D.; Jouzel, H.; Kawamura, K.; Stocker, T. High-resolution carbon dioxide concentration record 650,000–800,000 years before present. Nature 2008, 453, 379–382. [Google Scholar] [CrossRef]
- Alley, R.B. Abrupt Climate Change. Science 2003, 299, 2005–2010. [Google Scholar] [CrossRef] [Green Version]
- Granqvist, C.G. Electrochromics and Thermochromics: Towards a New Paradigm for Energy Efficient Buildings. Mater. Today Proc. 2016, 3, S2–S11. [Google Scholar] [CrossRef]
- Adler, D. Mechanisms for Metal-Nonmental Transitions in Transition-Metal Oxides and Sulfides. Rev. Mod. Phys. 1968, 40, 714–736. [Google Scholar] [CrossRef]
- Torrance, J.; Lacorre, P.; Nazzal, A.; Ansaldo, E.; Niedermayer, C. Systematic study of insulator-metal transitions in perovskites RNiO3 (R=Pr,Nd,Sm,Eu) due to closing of charge-transfer gap. Phys. Rev. B 1992, 45, 8209–8212. [Google Scholar] [CrossRef] [Green Version]
- Mjejri, I.; Rougier, A.; Gaudon, M. Low-Cost and Facile Synthesis of the Vanadium Oxides V2O3, VO2, and V2O5 and Their Magnetic, Thermochromic and Electrochromic Properties. Inorg. Chem. 2017, 56, 1734–1741. [Google Scholar] [CrossRef] [Green Version]
- Babulanam, S.M.; Eriksson, T.S.; Niklasson, G.A.; Granqvist, C.G. Thermochromic VO2 films for energy-efficient windows. Sol. Energy Mater. 1987, 16, 347–363. [Google Scholar] [CrossRef]
- Li, M.; Magdassi, S.; Gao, Y.; Long, Y. Hydrothermal Synthesis of VO2 Polymorphs: Advantages, Challenges and Prospects for the Application of Energy Efficient Smart Windows. Small 2017, 13, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhu, J.; Yang, W.; Wen, T.; Pravica, M.; Liu, Z.; Hou, M.; Fei, Y.; Kang, L.; Lin, Z.; et al. Reversible switching between pressure-induced amorphization and thermal-driven recrystallization in VO2(B) nanosheets. Nat. Commun. 2016, 7, 12214. [Google Scholar] [CrossRef]
- Wang, Q.; Jing, P.; Li, M.; Luo, Y.; Wu, H.; Zhong, L.; Li, G. VO2 (B) Nanosheets as a Cathode Material for Li-ion Battery. J. Mater. Sci. Technol. 2015, 31, 630–633. [Google Scholar] [CrossRef]
- Qu, B.Y.; Liu, L.; Xie, Y.; Pan, B.C. Theoretical study of the new compound VO2 (D). Phys. Lett. A 2011, 375, 3474–3477. [Google Scholar] [CrossRef]
- Liu, L.; Cao, F.; Yao, T.; Xu, Y.; Zhou, M.; Qu, B.; Pan, B.; Wu, C.; Wei, S.; Xie, Y. New-phase VO2 micro/nanostructures: Investigation of phase transformation and magnetic property. New J. Chem. 2012, 36, 619–625. [Google Scholar] [CrossRef]
- Zhang, Y.; Fan, M.; Niu, F.; Zhong, Y.; Huang, C.; Liu, X.; Wang, B.; Li, H. Hydrothermal synthesis of VO2(A) nanobelts and their phase transition and optical switching properties. Micro Nano Lett. 2011, 6, 888. [Google Scholar] [CrossRef]
- Wu, C.; Hu, Z.; Wang, W.; Zhang, M.; Yang, J.; Xie, Y. Synthetic paramontroseite VO2 with good aqueous lithium–ion battery performance. Chem. Commun. 2008, 5, 3891. [Google Scholar] [CrossRef] [PubMed]
- Wentzcovitch, R.M.; Schulz, W.W.; Allen, P.B. VO2: Peierls or Mott-Hubbard? A view from band theory. Phys. Rev. Lett. 1994, 72, 3389–3392. [Google Scholar] [CrossRef] [PubMed]
- Shao, Z.; Cao, X.; Luo, H.; Jin, P. Recent progress in the phase-transition mechanism and modulation of vanadium dioxide materials. NPG Asia Mater. 2018, 10, 581–605. [Google Scholar] [CrossRef] [Green Version]
- Bretos, I.; Diodati, S.; Jiméenez, R.; Tajoli, F.; Ricote, J.; Bragaggia, G.; Franca, M.; Calzada, M.L.; Gross, S. Low-Temperature Solution Crystallization of Nanostructured Oxides and Thin Films. Chem. Eur. J. 2020, 26, 9157–9179. [Google Scholar] [CrossRef]
- Xia, Y.; Yang, P.; Un, Y.S.; Wu, Y.; Mayers, B.; Gates, B.; Yin, Y.; Kim, F.; Yan, H. One-Dimensional Nanostructures: Synthesis, Characterization, and Applications. Adv. Mater. 2003, 15, 353–389. [Google Scholar] [CrossRef]
- Jiang, W.; Ni, J.; Yu, K.; Zhu, Z. Hydrothermal synthesis and electrochemical characterization of VO2 (B) with controlled crystal structures. Appl. Surf. Sci. 2011, 257, 3253–3258. [Google Scholar] [CrossRef]
- Yang, Z.; Ko, C.; Ramanathan, S. Oxide Electronics Utilizing Ultrafast Metal-Insulator Transitions. Annu. Rev. Mater. Res. 2011, 41, 337–367. [Google Scholar] [CrossRef]
- Popuri, S.R.; Miclau, M.; Artemenko, A.; Labrugere, C.; Villesuzanne, A.; Pollet, M. Rapid hydrothermal synthesis of VO2 (B) and its conversion to thermochromic VO2 (M1). Inorg. Chem. 2013, 52, 4780–4785. [Google Scholar] [CrossRef]
- Guan, S.; Rougier, A.; Viraphong, O.; Denux, D.; Penin, N.; Gaudon, M. Two-Step Synthesis of VO2 (M) with Tuned Crystallinity. Inorg. Chem. 2018, 57, 8857–8865. [Google Scholar] [CrossRef] [PubMed]
- Qazilbash, M.M.; Brehm, M.; Chae, B.; Ho, P.; Andreev, G.; Kim, B.; Yun, S.; Balatsky, A.; Maple, M.; Keilmann, F.; et al. Mott transition in VO2 revealed by infrared spectroscopy and nano-imaging. Science 2007, 318, 1750–1753. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valmalette, J.-C.; Gavarri, J.-R. High efficiency thermochromic VO2(R) resulting from the irreversible transformation of VO2(B). Mater. Sci. Eng. B 1998, 54, 168–173. [Google Scholar] [CrossRef]
- Zhu, J.; Zhou, Y.; Wang, B.; Zheng, J.; Ji, S.; Yao, H.; Luo, H.; Jin, P. Vanadium Dioxide Nanoparticle-based Thermochromic Smart Coating: High Luminous Transmittance, Excellent Solar Regulation Efficiency, and Near Room Temperature Phase Transition. ACS Appl. Mater. Interfaces 2015, 7, 27796–27803. [Google Scholar] [CrossRef]
- Qu, Z.; Yao, L.; Li, J.; He, J.; Mi, J.; Ma, S.; Tang, S.; Feng, L. Bifunctional Template-Induced VO2 @SiO2 Dual-Shelled Hollow Nanosphere-Based Coatings for Smart Windows. ACS Appl. Mater. Interfaces 2019, 11, 15960–15968. [Google Scholar] [CrossRef] [PubMed]






| Samples | Vanadium Precursor (V) | Reducing Agent (R) | Molar Ratio (V:R:S) | Temperature (°C) | Time (h) | XRD Output |
|---|---|---|---|---|---|---|
| V11.2_24h | V2O5 | Citric acid monohydrate | 1:1.2:605 | 160 | 24 | Mixed oxides |
| N11.2_24h | NH4VO3 | Mixed oxides | ||||
| V21.2_24h | V2O5 | Oxalic acid | Mixed oxides | |||
| N21.2_24h | NH4VO3 | Mixed oxides | ||||
| V31.7_24h | V2O5 | Oxalic acid | 1:1.7:605 | 160 | 24 | Mixed oxides |
| V41.7_6h | V2O5 | Oxalic acid | 1:1.7:605 | 180 | 6 | V6O13 |
| V51.7_8h | 8 | V6O13 | ||||
| V61.7_12h | 12 | VO2(B) | ||||
| V71.7_24h | 24 | VO2(B) | ||||
| V81.7_12h | V2O5 | Oxalic acid | 1:1.7:605 | 180 | 12 | VO2(B) |
| V91.7_12h | 1:1.7:509 | |||||
| V101.7_12h | 1:1.7:424 | |||||
| V111.7_12h | 1:1.7:364 | |||||
| V121.7_12h | 1:1.7:318 | |||||
| V131.7_12h | 1:1.7:282 |
| Sample | Temperature (°C) | Time (h) |
|---|---|---|
| V51.7_12h | 550 | 2 |
| 550 | 4.5 | |
| 650 | 4.5 | |
| 700 | 4.5 | |
| 700 | 2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bragaggia, G.; Cacciatore, A.; Poffe, E.; Capone, C.; Zorzi, F.; Causin, V.; Gross, S. Systematic Exploration of the Synthetic Parameters for the Production of Dynamic VO2(M1). Molecules 2021, 26, 4513. https://doi.org/10.3390/molecules26154513
Bragaggia G, Cacciatore A, Poffe E, Capone C, Zorzi F, Causin V, Gross S. Systematic Exploration of the Synthetic Parameters for the Production of Dynamic VO2(M1). Molecules. 2021; 26(15):4513. https://doi.org/10.3390/molecules26154513
Chicago/Turabian StyleBragaggia, Giulia, Andrea Cacciatore, Elisa Poffe, Claudia Capone, Federico Zorzi, Valerio Causin, and Silvia Gross. 2021. "Systematic Exploration of the Synthetic Parameters for the Production of Dynamic VO2(M1)" Molecules 26, no. 15: 4513. https://doi.org/10.3390/molecules26154513








