Non-Saccharomyces as Biotools to Control the Production of Off-Flavors in Wines
Abstract
:1. Introduction
2. Bioprotection
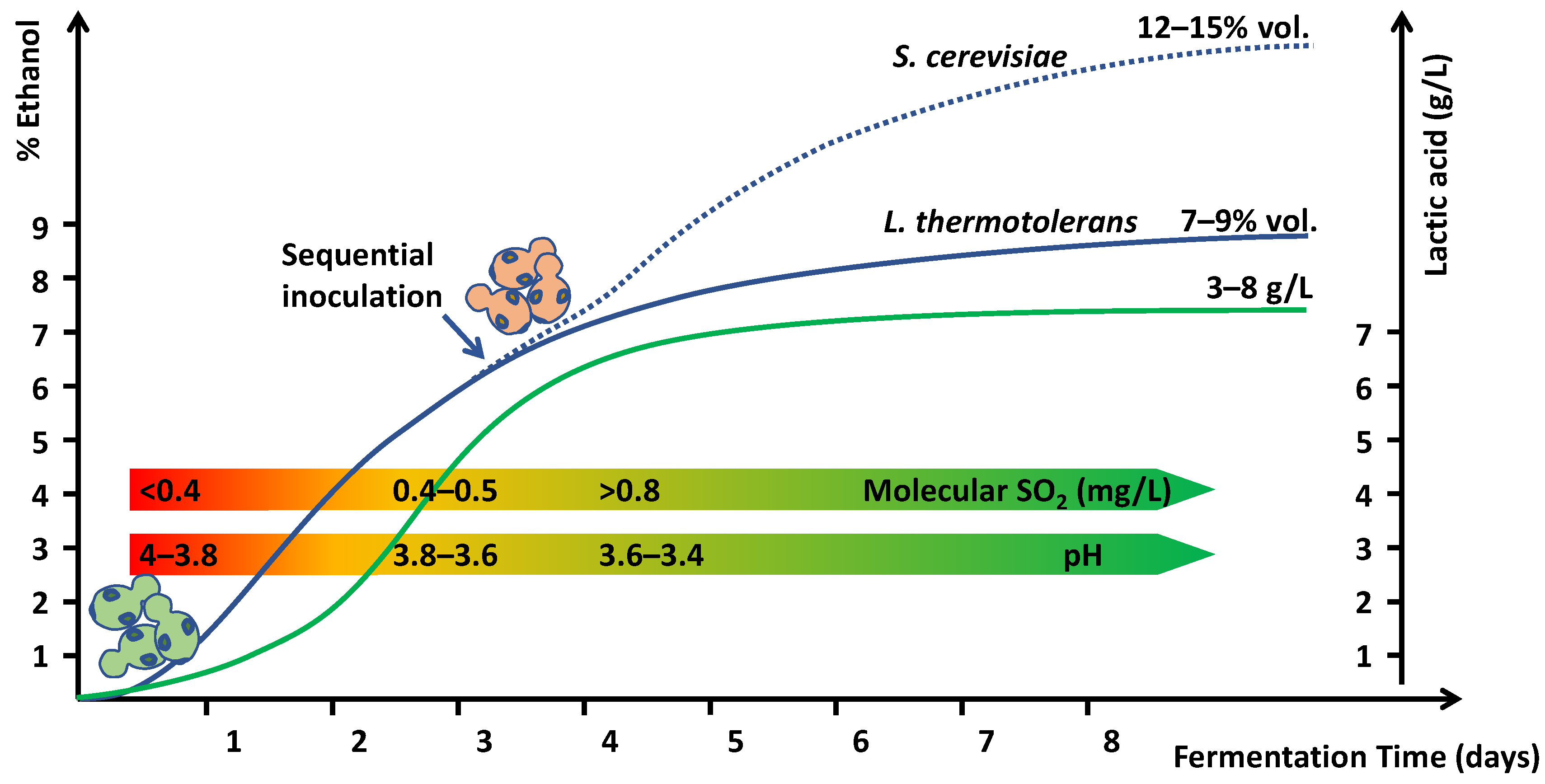
3. Bioacidification by Lachancea thermotolerans (Lt)
4. Apiculate Yeasts and Volatile Acidity
5. Biocompatibility
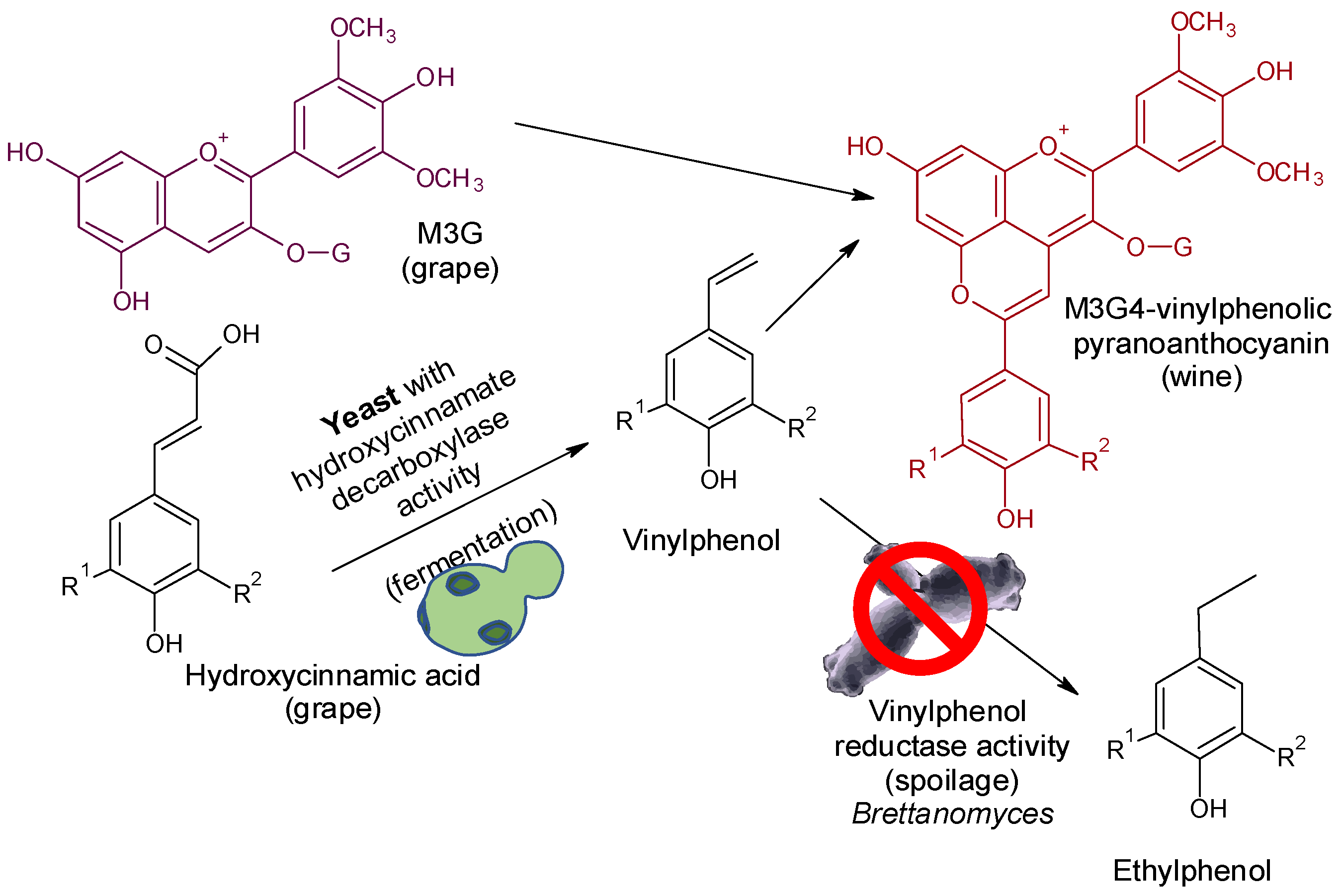
6. Depletion of Off-Flavor Precursors
7. Increasing the Implantation of Non-Saccharomyces as Bioprotective Tools Using Emerging Non-Thermal Technologies
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Swiegers, J.H.; Pretorius, I.S. Modulation of volatile sulfur compounds by wine yeast. Appl. Microbiol. Biotechnol. 2007, 74, 954–960. [Google Scholar] [CrossRef]
- Ugliano, M.; Kolouchova, R.; Henschke, P.A. Occurrence of hydrogen sulfide in wine and in fermentation: Influence of yeast strain and supplementation of yeast available nitrogen. J. Ind. Microbiol. Biotechnol. 2011, 38, 423–429. [Google Scholar] [CrossRef]
- Bely, M.; Rinaldi, A.; Dubourdieu, D. Influence of assimilable nitrogen on volatile acidity production by Saccharomyces cerevisiae during high sugar fermentation. J. Biosci. Bioeng. 2003, 96, 507–512. [Google Scholar] [CrossRef]
- Suárez, R.; Suárez-Lepe, J.A.; Morata, A.; Calderón, F. The production of ethylphenols in wine by yeasts of the genera Brettanomyces and Dekkera: A review. Food Chem. 2007, 102, 10–21. [Google Scholar] [CrossRef]
- Malfeito-Ferreira, M. Two decades of “Horse Sweat” taint and Brettanomyces yeasts in wine: Where do we stand now? Beverages 2018, 4, 32. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.Q.; Pilone, G.J. An overview of formation and roles of acetaldehyde in winemaking with emphasis on microbiological implications. Int. J. Food Sci. Technol. 2000, 35, 49–61. [Google Scholar] [CrossRef]
- Culleré, L.; López, R.; Ferreira, V. Chapter 20—The instrumental analysis of aroma-active compounds for explaining the flavor of red wines. In Red Wine Technology; Morata, A., Ed.; Elsevier, Academic Press: Amsterdam, The Netherlands, 2018; pp. 283–307. ISBN 9780128144008. [Google Scholar]
- Ribéreau-Gayon, P.; Glories, Y.; Maujean, A.; Dubourdieu, D. Alcohols and other volatile compounds. In Handbook of Enology: The Chemistry of Wine Stabilization and Treatments (Volume 2); John Wiley & Sons: Chichester, UK, 2006; pp. 51–64. [Google Scholar]
- OIV International Code of Oenological Practice. Available online: https://www.oiv.int/public/medias/3741/e-code-annex-maximum-acceptable-limits.pdf (accessed on 15 June 2021).
- Chatonnet, P.; Dubourdie, D.; Boidron, J.-n.; Pons, M. The origin of ethylphenols in wines. J. Sci. Food Agric. 1992, 60, 165–178. [Google Scholar] [CrossRef]
- Curtin, C.; Bramley, B.; Cowey, G.; Holdstock, M.; Kennedy, E.; Lattey, K.; Coulter, A.; Henschke, P.; Francis, L.; Godden, P. Sensory perceptions of ‘Brett’ and relationship to consumer preference. In Proceedings of the Thirteenth Australian Wine Industry Technical Conference, Adelaide, Australia, 29 July–2 August 2007; Blair, R.J., Williams, P.J., Pretorius, I.S., Eds.; Australian Wine Industry Technical Conference Inc.: Adelaide, Australia, 2007; pp. 207–211. [Google Scholar]
- Mira de Orduña, R. Climate change associated effects on grape and wine quality and production. Food Res. Int. 2010, 43, 1844–1855. [Google Scholar] [CrossRef]
- Sadras, V.O.; Petrie, P.R.; Moran, M.A. Effects of elevated temperature in grapevine. II juice pH, titratable acidity and wine sensory attributes. Aust. J. Grape Wine Res. 2013, 19, 107–115. [Google Scholar] [CrossRef]
- Mozell, M.R.; Thachn, L. The impact of climate change on the global wine industry: Challenges & solutions. Wine Econ. Policy 2014, 3, 81–89. [Google Scholar] [CrossRef] [Green Version]
- Mpelasoka, B.S.; Schachtman, D.P.; Treeby, M.T.; Thomas, M.R. A review of potassium nutrition in grapevines with special emphasis on berry accumulation. Aust. J. Grape Wine Res. 2003, 9, 154–168. [Google Scholar] [CrossRef]
- Rogiers, S.Y.; Coetzee, Z.A.; Walker, R.R.; Deloire, A.; Tyerman, S.D. Potassium in the grape (Vitis vinifera L.) berry: Transport and function. Front. Plant Sci. 2017, 8, 1629. [Google Scholar] [CrossRef] [PubMed]
- Berbegal, C.; Spano, G.; Fragasso, M.; Grieco, F.; Russo, P.; Capozzi, V. Starter cultures as biocontrol strategy to prevent Brettanomyces bruxellensis proliferation in wine. Appl. Microbiol. Biotechnol. 2018, 102, 569–576. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roudil, L.; Russo, P.; Berbegal, C.; Albertin, W.; Spano, G.; Capozzi, V. Non-Saccharomyces commercial starter cultures: Scientific trends, recent patents and innovation in the wine sector. Recent Pat. Food. Nutr. Agric. 2020, 11, 27–39. [Google Scholar] [CrossRef]
- Simonin, S.; Roullier-Gall, C.; Ballester, J.; Schmitt-Kopplin, P.; Quintanilla-Casas, B.; Vichi, S.; Peyron, D.; Alexandre, H.; Tourdot-Maréchal, R. Bio-Protection as an alternative to sulphites: Impact on chemical and microbial characteristics of red wines. Front. Microbiol. 2020, 11, 1308. [Google Scholar] [CrossRef] [PubMed]
- Comitini, F.; Gobbi, M.; Domizio, P.; Romani, C.; Lencioni, L.; Mannazzu, I.; Ciani, M. Selected non-Saccharomyces wine yeasts in controlled multistarter fermentations with Saccharomyces cerevisiae. Food Microbiol. 2011, 28, 873–882. [Google Scholar] [CrossRef] [PubMed]
- Banilas, G.; Sgouros, G.; Nisiotou, A. Development of microsatellite markers for Lachancea thermotolerans typing and population structure of wine-associated isolates. Microbiol. Res. 2016, 193, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Hranilovic, A.; Gambetta, J.M.; Schmidtke, L.; Boss, P.K.; Grbin, P.R.; Masneuf-Pomarede, I.; Bely, M.; Albertin, W.; Jiranek, V. Oenological traits of Lachancea thermotolerans show signs of domestication and allopatric differentiation. Sci. Rep. 2018, 8, 1–13. [Google Scholar] [CrossRef]
- Morata, A.; Loira, I.; Tesfaye, W.; Bañuelos, M.A.; González, C.; Suárez Lepe, J.A. Lachancea thermotolerans applications in wine technology. Fermentation 2018, 4, 53. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Li, B.; Zhang, Z.; Chen, Y.; Tian, S. Antagonistic yeasts: A promising alternative to chemical fungicides for controlling postharvest decay of fruit. J. Fungi 2020, 6, 158. [Google Scholar] [CrossRef]
- Sipiczki, M. Metschnikowia pulcherrima and related pulcherrimin-producing yeasts: Fuzzy species boundaries and complex antimicrobial antagonism. Microorganisms 2020, 8, 1029. [Google Scholar] [CrossRef]
- Morata, A.; Vejarano, R.; Ridolfi, G.; Benito, S.; Palomero, F.; Uthurry, C.; Tesfaye, W.; González, C.; Suárez-Lepe, J.A. Reduction of 4-ethylphenol production in red wines using HCDC+ yeasts and cinnamyl esterases. Enzym. Microb. Technol. 2013, 52, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Chassagne, D.; Guilloux-Benatier, M.; Alexandre, H.; Voilley, A. Sorption of wine volatile phenols by yeast lees. Food Chem. 2005, 91, 39–44. [Google Scholar] [CrossRef]
- Palomero, F.; Ntanos, K.; Morata, A.; Benito, S.; Suárez-Lepe, J.A. Reduction of wine 4-ethylphenol concentration using lyophilised yeast as a bioadsorbent: Influence on anthocyanin content and chromatic variables. Eur. Food Res. Technol. 2011, 232, 971–977. [Google Scholar] [CrossRef]
- Muccilli, S.; Restuccia, C. Bioprotective role of yeasts. Microorganisms 2015, 3, 588–611. [Google Scholar] [CrossRef] [Green Version]
- Escott, C.; Loira, I.; Morata, A.; Bañuelos, M.A.; Suárez-Lepe, J.A. Wine spoilage yeasts: Control strategy. In Yeast—Industrial Applications; Morata, A., Loira, I., Eds.; InTechOpen: London, UK, 2017. [Google Scholar] [CrossRef] [Green Version]
- Nardi, T. Microbial resources as a tool for enhancing sustainability in winemaking. Microorganisms 2020, 8, 507. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morata, A.; Bañuelos, M.A.; López, C.; Song, C.; Vejarano, R.; Loira, I.; Palomero, F.; Suarez Lepe, J.A. Use of fumaric acid to control pH and inhibit malolactic fermentation in wines. Food Addit. Contam.—Part A Chem. Anal. Control. Expo. Risk Assess. 2020, 37, 228–238. [Google Scholar] [CrossRef]
- Woods, D.R.; Bevan, E.A. Studies on the nature of the killer factor produced by Saccharomyces cerevisiae. J. Gen. Microbiol. 1968, 51, 115–126. [Google Scholar] [CrossRef] [Green Version]
- Young, T.W.; Yagiu, M. A comparison of the killer character in different yeasts and its classification. Antonie Leeuwenhoek 1978, 44, 59–77. [Google Scholar] [CrossRef]
- Schmitt, M.J.; Breinig, F. Yeast viral killer toxins: Lethality and self-protection. Nat. Rev. Microbiol. 2006, 4, 212–221. [Google Scholar] [CrossRef] [PubMed]
- Morata, A.; Loira, I.; Escott, C.; del Fresno, J.M.; Bañuelos, M.A.; Suárez-Lepe, J.A. Applications of Metschnikowia pulcherrima in wine biotechnology. Fermentation 2019, 5, 63. [Google Scholar] [CrossRef] [Green Version]
- Comitini, F.; Ciani, M. The zymocidial activity of Tetrapisispora phaffii in the control of Hanseniaspora uvarum during the early stages of winemaking. Lett. Appl. Microbiol. 2010, 50, 50–56. [Google Scholar] [CrossRef]
- Gobert, A.; Tourdot-Maréchal, R.; Sparrow, C.; Morge, C.; Alexandre, H. Influence of nitrogen status in wine alcoholic fermentation. Food Microbiol. 2019, 83, 71–85. [Google Scholar] [CrossRef] [PubMed]
- Chacon-Rodriguez, L.; Joseph, C.M.L.; Nazaris, B.; Coulon, J.; Richardson, S.; Dycus, D.A. Innovative use of non-Saccharomyces in bio-protection: T. delbrueckii and M. pulcherrima applied to a machine harvester. Catal. Discov. Pract. 2020, 4, 82–90. [Google Scholar] [CrossRef]
- Pelonnier-Magimel, E.; Windholtz, S.; Masneuf-Pomarède, I.; Barbe, J.C. Sensory characterisation of wines without added sulfites via specific and adapted sensory profile. Oeno One 2020, 54, 671–685. [Google Scholar] [CrossRef]
- Simonin, S.; Alexandre, H.; Nikolantonaki, M.; Coelho, C.; Tourdot-Maréchal, R. Inoculation of Torulaspora delbrueckii as a bio-protection agent in winemaking. Food Res. Int. 2018, 107, 451–461. [Google Scholar] [CrossRef]
- Rubio-Bretón, P.; Gonzalo-Diago, A.; Iribarren, M.; Garde-Cerdán, T.; Pérez-Álvarez, E.P. Bioprotection as a tool to free additives winemaking: Effect on sensorial, anthocyanic and aromatic profile of young red wines. LWT 2018, 98, 458–464. [Google Scholar] [CrossRef]
- Windholtz, S.; Redon, P.; Lacampagne, S.; Farris, L.; Lytra, G.; Cameleyre, M.; Barbe, J.C.; Coulon, J.; Thibon, J.; Masneuf-Pomarède, I. Non-Saccharomyces yeasts as bioprotection in the composition of red wine and in the reduction of sulfur dioxide. LWT 2021, 149, 111781. [Google Scholar] [CrossRef]
- Johnson, J.; Fu, M.; Qian, M.; Curtin, C.; Osborne, J.P. Influence of select non-Saccharomyces yeast on Hanseniaspora uvarum growth during prefermentation cold maceration. Am. J. Enol. Vitic. 2020, 71, 278–287. [Google Scholar] [CrossRef]
- Comuzzo, P.; Battistutta, F. Chapter 2—Acidification and pH Control in Red Wines. In Red Wine Technology; Morata, A., Ed.; Elsevier, Academic Press: Amsterdam, The Netherlands, 2018; pp. 17–34. ISBN 9780128144008. [Google Scholar]
- Yéramian, N.; Chaya, C.; Suárez Lepe, J.A. L-(-)-malic acid production by Saccharomyces spp. during the alcoholic fermentation of wine (1). J. Agric. Food Chem. 2007, 55, 912–919. [Google Scholar] [CrossRef]
- Morata, A.; Escott, C.; Bañuelos, M.A.; Loira, I.; Del Fresno, J.M.; González, C.; Suárez-lepe, J.A. Contribution of non-Saccharomyces yeasts to wine freshness. A review. Biomolecules 2020, 10, 34. [Google Scholar] [CrossRef] [Green Version]
- Gobbi, M.; Comitini, F.; Domizio, P.; Romani, C.; Lencioni, L.; Mannazzu, I.; Ciani, M. Lachancea thermotolerans and Saccharomyces cerevisiae in simultaneous and sequential co-fermentation: A strategy to enhance acidity and improve the overall quality of wine. Food Microbiol. 2013, 33, 271–281. [Google Scholar] [CrossRef]
- Balikci, E.K.; Tanguler, H.; Jolly, N.P.; Erten, H. Influence of Lachancea thermotolerans on cv. Emir wine fermentation. Yeast 2016, 33, 313–321. [Google Scholar] [CrossRef] [Green Version]
- Morata, A.; Bañuelos, M.A.; Vaquero, C.; Loira, I.; Cuerda, R.; Palomero, F.; González, C.; Suárez-Lepe, J.A.; Wang, J.; Han, S.; et al. Lachancea thermotolerans as a tool to improve pH in red wines from warm regions. Eur. Food Res. Technol. 2019, 245, 885–894. [Google Scholar] [CrossRef]
- Vaquero, C.; Loira, I.; Bañuelos, M.A.; Heras, J.M.; Cuerda, R.; Morata, A. Industrial performance of several Lachancea thermotolerans strains for ph control in white wines from warm areas. Microorganisms 2020, 8, 830. [Google Scholar] [CrossRef]
- Hranilovic, A.; Albertin, W.; Capone, D.L.; Gallo, A.; Grbin, P.R.; Danner, L.; Bastian, S.E.P.; Masneuf-Pomarede, I.; Coulon, J.; Bely, M.; et al. Impact of Lachancea thermotolerans on chemical composition and sensory profiles of Merlot wines. Food Chem. 2021, 349, 129015. [Google Scholar] [CrossRef] [PubMed]
- Gatto, V.; Binati, R.L.; Lemos Junior, W.J.F.; Basile, A.; Treu, L.; de Almeida, O.G.G.; Innocente, G.; Campanaro, S.; Torriani, S. New insights into the variability of lactic acid production in Lachancea thermotolerans at the phenotypic and genomic level. Microbiol. Res. 2020, 238, 126525. [Google Scholar] [CrossRef] [PubMed]
- Castro Marín, A.; Colangelo, D.; Lambri, M.; Riponi, C.; Chinnici, F. Relevance and perspectives of the use of chitosan in winemaking: A review. Crit. Rev. Food Sci. Nutr. 2020, 1–15. [Google Scholar] [CrossRef] [PubMed]
- OIV. Treatment with Fumaric Acid in Wine to Inhibit MLF. Resolution OENO-TECHNO 15-581. 2020. Available online: https://www.oiv.int/en/technical-standards-and-documents/resolutions-of-the-oiv/oenology-resolutions (accessed on 28 July 2021).
- Xu, G.; Chen, X.; Liu, L.; Jiang, L. Fumaric acid production in Saccharomyces cerevisiae by simultaneous use of oxidative and reductive routes. Bioresour. Technol. 2013, 148, 91–96. [Google Scholar] [CrossRef]
- Vilela, A. Lachancea thermotolerans, the non-Saccharomyces yeast that reduces the volatile acidity of wines. Fermentation 2018, 4, 56. [Google Scholar] [CrossRef] [Green Version]
- Escott, C.; Morata, A.; Ricardo-Da-Silva, J.M.; Callejo, M.J.; Del Carmen González, M.; Suarez-Lepe, J.A. Effect of Lachancea thermotolerans on the formation of polymeric pigments during sequential fermentation with Schizosaccharosmyces pombe and Saccharomyces cerevisiae. Molecules 2018, 23, 2353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vaquero, C.; Loira, I.; Heras, J.M.; Carrau, F.; González, C.; Morata, A. Biocompatibility in ternary fermentations with Lachancea thermotolerans, other non-Saccharomyces and Saccharomyces cerevisiae to control pH and improve the sensory profile of wines from warm areas. Front. Microbiol. 2021, 12, 656262. [Google Scholar] [CrossRef] [PubMed]
- Escott, C.; Del Fresno, J.M.; Loira, I.; Morata, A.; Tesfaye, W.; del Carmen González, M.; Suárez-Lepe, J.A. Formation of polymeric pigments in red wines through sequential fermentation of flavanol-enriched musts with non-Saccharomyces yeasts. Food Chem. 2018, 239, 975–983. [Google Scholar] [CrossRef] [PubMed]
- Morata, A.; Escott, C.; Loira, I.; Manuel Del Fresno, J.; González, C.; Suárez-Lepe, J.A. Influence of Saccharomyces and non-Saccharomyces yeasts in the formation of pyranoanthocyanins and polymeric pigments during red wine making. Molecules 2019, 24, 4490. [Google Scholar] [CrossRef] [Green Version]
- Ciani, M.; Maccarelli, F. Oenological properties of non-Saccharomyces yeasts associated with wine-making. World J. Microbiol. Biotechnol. 1997, 14, 199–203. [Google Scholar] [CrossRef]
- Medina, K.; Boido, E.; Fariña, L.; Gioia, O.; Gomez, M.E.; Barquet, M.; Gaggero, C.; Dellacassa, E.; Carrau, F. Increased flavour diversity of Chardonnay wines by spontaneous fermentation and co-fermentation with Hanseniaspora vineae. Food Chem. 2013, 141, 2513–2521. [Google Scholar] [CrossRef] [PubMed]
- Martin, V.; Valera, M.J.; Medina, K.; Boido, E.; Carrau, F. Oenological impact of the Hanseniaspora/Kloeckera yeast genus on wines—A review. Fermentation 2018, 4, 76. [Google Scholar] [CrossRef] [Green Version]
- Del Fresno, J.M.; Escott, C.; Loira, I.; Herbert-Pucheta, J.E.; Schneider, R.; Carrau, F.; Cuerda, R.; Morata, A. Impact of Hanseniaspora vineae in alcoholic fermentation and ageing on lees of high-quality white wine. Fermentation 2020, 6, 66. [Google Scholar] [CrossRef]
- Zhang, B.; Xu, D.; Duan, C.; Yan, G. Synergistic effect enhances 2-phenylethyl acetate production in the mixed fermentation of Hanseniaspora vineae and Saccharomyces cerevisiae. Process Biochem. 2020, 90, 44–49. [Google Scholar] [CrossRef]
- Del Fresno, J.M.; Escott, C.; Loira, I.; Carrau, F.; Cuerda, R.; Schneider, R.; Bañuelos, M.A.; González, C.; Suárez-Lepe, J.A.; Morata, A. The impact of Hanseniaspora vineae fermentation and ageing on lees on the terpenic aromatic profile of white wines of the albillo variety. Int. J. Mol. Sci. 2021, 22, 2195. [Google Scholar] [CrossRef]
- Valera, M.J.; Boido, E.; Dellacassa, E.; Carrau, F. Comparison of the glycolytic and alcoholic fermentation pathways of Hanseniaspora vineae with Saccharomyces cerevisiae wine yeasts. Fermentation 2020, 6, 78. [Google Scholar] [CrossRef]
- Luan, Y.; Zhang, B.Q.; Duan, C.Q.; Yan, G.L. Effects of different pre-fermentation cold maceration time on aroma compounds of Saccharomyces cerevisiae co-fermentation with Hanseniaspora opuntiae or Pichia kudriavzevii. LWT Food Sci. Technol. 2018, 92, 177–186. [Google Scholar] [CrossRef]
- Feng, C.T.; Du, X.; Wee, J. Microbial and chemical analysis of non-Saccharomyces yeasts from Chambourcin hybrid grapes for potential use in winemaking. Fermentation 2021, 7, 15. [Google Scholar] [CrossRef]
- Seixas, I.; Barbosa, C.; Mendes-Faia, A.; Güldener, U.; Tenreiro, R.; Mendes-Ferreira, A.; Mira, N.P. Genome sequence of the non-conventional wine yeast Hanseniaspora guilliermondii UTAD222 unveils relevant traits of this species and of the Hanseniaspora genus in the context of wine fermentation. DNA Res. 2019, 26, 67–83. [Google Scholar] [CrossRef] [Green Version]
- Steenwyk, J.L.; Opulente, D.A.; Kominek, J.; Shen, X.X.; Zhou, X.; Labella, A.L.; Bradley, N.P.; Eichman, B.F.; Čadež, N.; Libkind, D.; et al. Extensive loss of cell-cycle and DNA repair genes in an ancient lineage of bipolar budding yeasts. PLoS Biol. 2019, 17, e3000255. [Google Scholar] [CrossRef] [Green Version]
- Pinto, L.; Baruzzi, F.; Cocolin, L.; Malfeito-Ferreira, M. Emerging technologies to control Brettanomyces spp. in wine: Recent advances and future trends. Trends Food Sci. Technol. 2020, 99, 88–100. [Google Scholar] [CrossRef]
- Morata, A.; Gómez-Cordovés, M.C.; Calderón, F.; Suárez, J.A. Effects of pH, temperature and SO2 on the formation of pyranoanthocyanins during red wine fermentation with two species of Saccharomyces. Int. J. Food Microbiol. 2006, 106, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Morata, A.; González, C.; Suárez-Lepe, J.A. Formation of vinylphenolic pyranoanthocyanins by selected yeasts fermenting red grape musts supplemented with hydroxycinnamic acids. Int. J. Food Microbiol. 2007, 116, 144–152. [Google Scholar] [CrossRef] [PubMed]
- Bakker, J.; Timberlake, C.F. Isolation, identification and characterization of new color-stable anthocyanins occurring in some red wines. J. Agric. Food Chem. 1997, 45, 35–43. [Google Scholar] [CrossRef]
- De Freitas, V.; Mateus, N. Formation of pyranoanthocyanins in red wines: A new and diverse class of anthocyanin derivatives. Anal. Bioanal. Chem. 2011, 401, 1467–1477. [Google Scholar] [CrossRef] [PubMed]
- Morata, A.; Loira, I.; Suárez Lepe, J.A. Influence of yeasts in wine colour. In Grape and Wine Biotechnology; InTech: Rijeka, Croatia, 2016. [Google Scholar] [CrossRef] [Green Version]
- Escribano-Bailón, M.T.; Rivas-Gonzalo, J.C.; García-Estévez, I. Wine color evolution and stability. In Red Wine Technology; Elsevier, Academic Press: Amsterdam, The Netherlands, 2018; pp. 195–205. ISBN 9780128144008. [Google Scholar] [CrossRef]
- Escott, C.; Morata, A.; Loira, I.; Tesfaye, W.; Suarez-Lepe, J.A. Characterization of polymeric pigments and pyranoanthocyanins formed in microfermentations of non-Saccharomyces yeasts. J. Appl. Microbiol. 2016, 121, 1346–1356. [Google Scholar] [CrossRef]
- Morata, A.; Loira, I.; Vejarano, R.; González, C.; Callejo, M.J.; Suárez-Lepe, J.A. Emerging preservation technologies in grapes for winemaking. Trends Food Sci. Technol. 2017, 67, 36–43. [Google Scholar] [CrossRef]
- Paniagua-Martínez, I.; Ramírez-Martínez, A.; Serment-Moreno, V.; Rodrigues, S.; Ozuna, C. Non-thermal technologies as alternative methods for Saccharomyces cerevisiae inactivation in liquid media: A review. Food Bioprocess Technol. 2018, 11, 487–510. [Google Scholar] [CrossRef]
- Gómez-López, V.M.; Pataro, G.; Tiwari, B.; Gozzi, M.; Meireles, M.Á.A.; Wang, S.; Guamis, B.; Pan, Z.; Ramaswamy, H.; Sastry, S.; et al. Guidelines on reporting treatment conditions for emerging technologies in food processing. Crit. Rev. Food Sci. Nutr. 2021, 1–25. [Google Scholar] [CrossRef] [PubMed]
- San Martín, M.F.; Barbosa-Cánovas, G.V.; Swanson, B.G. Food processing by high hydrostatic pressure. Crit. Rev. Food Sci. Nutr. 2002, 42, 627–645. [Google Scholar] [CrossRef]
- Morata, A.; Guamis, B. Use of UHPH to obtain juices with better nutritional quality and healthier wines with low levels of SO2. Front. Nutr. 2020, 7, 598286. [Google Scholar] [CrossRef] [PubMed]
- Raso, J.; Calderón, M.L.; Góngora, M.; Barbosa-Cánovas, G.V.; Swanson, B.G. Inactivation of Zygosaccharomyces bailii in fruit juices by heat, high hydrostatic pressure and pulsed electric fields. J. Food Sci. 1998, 63, 1042–1044. [Google Scholar] [CrossRef]
- Santamera, A.; Escott, C.; Loira, I.; Del Fresno, J.M.; González, C.; Morata, A. Pulsed light: Challenges of a non-thermal sanitation technology in the winemaking industry. Beverages 2020, 6, 45. [Google Scholar] [CrossRef]
- Morata, A.; Bañuelos, M.A.; Tesfaye, W.; Loira, I.; Palomero, F.; Benito, S.; Callejo, M.J.; Villa, A.; González, M.C.; Suárez-Lepe, J.A. Electron beam irradiation of wine grapes: Effect on microbial populations, phenol extraction and wine quality. Food Bioprocess Technol. 2015, 8, 1845–1853. [Google Scholar] [CrossRef]
- Sainz-García, E.; López-Alfaro, I.; Múgica-Vidal, R.; López, R.; Escribano-Viana, R.; Portu, J.; Alba-Elías, F.; González-Arenzana, L. Effect of the atmospheric pressure cold plasma treatment on Tempranillo red wine quality in batch and flow systems. Beverages 2019, 5, 50. [Google Scholar] [CrossRef] [Green Version]
- García Martín, J.F.; Sun, D.W. Ultrasound and electric fields as novel techniques for assisting the wine ageing process: The state-of-the-art research. Trends Food Sci. Technol. 2013, 33, 40–53. [Google Scholar] [CrossRef]
- Delfini, C.; Conterno, L.; Carpi, G.; Rovere, P.; Tabusso, A.; Cocito, C.; Amati, A. Microbiological stabilisation of grape musts and wines by High Hydrostatic Pressures. J. Wine Res. 1995, 6, 143–151. [Google Scholar] [CrossRef]
- Bañuelos, M.A.; Loira, I.; Escott, C.; Del Fresno, J.M.; Morata, A.; Sanz, P.D.; Otero, L.; Suárez-Lepe, J.A. Grape processing by High Hydrostatic Pressure: Effect on use of non-Saccharomyces in must fermentation. Food Bioprocess Technol. 2016, 9, 1769–1778. [Google Scholar] [CrossRef] [Green Version]
- Bañuelos, M.A.; Loira, I.; Guamis, B.; Escott, C.; Del Fresno, J.M.; Codina-Torrella, I.; Quevedo, J.M.; Gervilla, R.; Chavarría, J.M.R.; de Lamo, S.; et al. White wine processing by UHPH without SO2. Elimination of microbial populations and effect in oxidative enzymes, colloidal stability and sensory quality. Food Chem. 2020, 332, 127417. [Google Scholar] [CrossRef] [PubMed]
- Garde-Cerdán, T.; Arias-Gil, M.; Marsellés-Fontanet, A.R.; Ancín-Azpilicueta, C.; Martín-Belloso, O. Effects of thermal and non-thermal processing treatments on fatty acids and free amino acids of grape juice. Food Control 2007, 18, 473–479. [Google Scholar] [CrossRef]
- Vaquero, C.; Loira, I.; Raso, J.; Álvarez, I.; Delso, C.; Morata, A. Pulsed Electric Fields to improve the use of non-Saccharomyces starters in red wines. Foods 2021, 10, 1472. [Google Scholar] [CrossRef] [PubMed]
- Escott, C.; López, C.; Loira, I.; González, C.; Bañuelos, M.A.; Tesfaye, W.; Suárez-Lepe, J.A.; Morata, A. Improvement of must fermentation from late harvest cv. Tempranillo grapes treated with pulsed light. Foods 2021, 10, 1416. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Mittal, G.S.; Griffiths, M.W. Effect of pulsed electric field on the inactivation of microorganisms in grape juices with and without antimicrobials. Biosyst. Eng. 2005, 90, 1–7. [Google Scholar] [CrossRef]
- Corrales, M.; García, A.F.; Butz, P.; Tauscher, B. Extraction of anthocyanins from grape skins assisted by high hydrostatic pressure. J. Food Eng. 2009, 90, 415–421. [Google Scholar] [CrossRef]
- Morata, A.; Loira, I.; Vejarano, R.; Bañuelos, M.A.; Sanz, P.D.; Otero, L.; Suárez-Lepe, J.A. Grape processing by High Hydrostatic Pressure: Effect on microbial populations, phenol extraction and wine quality. Food Bioprocess Technol. 2015, 8, 277–286. [Google Scholar] [CrossRef] [Green Version]
- López, N.; Puértolas, E.; Condón, S.; Álvarez, I.; Raso, J. Effects of pulsed electric fields on the extraction of phenolic compounds during the fermentation of must of Tempranillo grapes. Innov. Food Sci. Emerg. Technol. 2008, 9, 477–482. [Google Scholar] [CrossRef]
- Pérez-Porras, P.; Bautista-Ortín, A.B.; Jurado, R.; Gómez-Plaza, E. Using high-power ultrasounds in red winemaking: Effect of operating conditions on wine physico-chemical and chromatic characteristics. LWT 2021, 138, 110645. [Google Scholar] [CrossRef]

| Compound | Sensory Threshold | Off-Flavor Concentration 1 | Descriptor | Reference |
|---|---|---|---|---|
| H2S | 1.6 μg/L | >1.6 μg/L | Rotten eggs/putrefaction | [2] |
| Volatile acidity | 0.3–0.6 g/L | >0.8 g/L legal limit 1.2 g/L | Vinegar | [7,8,9] |
| Ethyl acetate | 12 mg/L | >150 mg/L | Glue, solvent | [7] |
| 4-Ethylphenol | 230 μg/L | >425 μg/L | Phenolic, stable, leather, horse sweat | [5,10,11] |
| Acetaldehyde | 100–125 mg/L | >125 mg/L | Fruity, rotten apples, nut-like, sherry | [6] |
| Variety (Region) | Inoculation | Lactic Acid (g/L) and Initial→Final pH | Effect of Acidity on the Molecular SO2 (mg/L) * | Reference |
|---|---|---|---|---|
| Tempranillo (Ribera del Duero) | Sequential with S. cerevisiae | 0.91→6.60 g/L 3.90→3.63 | 0.42→0.77 | [50] |
| Tempranillo (Ribera del Duero) | Mixed with O. oeni and sequential with S. cerevisiae | 0.91→7.50 g/L 3.90→3.31 | 0.42→1.56 | [50] |
| Tempranillo (Mancha) | Sequential with S. cerevisiae | 3.8→3.4 | 0.50→1.22 | Unpublished |
| Albariño (Rias Baixas-O Rosal) | Sequential with S. cerevisiae | 0.05→2.7 g/L 3.12→2.85 | 2.07→3.63 | [50] |
| Airén (La Mancha) | Sequential with S. cerevisiae | 0.05→4.20 g/L 3.75→3.35 | 0.51→1.25 | [51] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morata, A.; Loira, I.; González, C.; Escott, C. Non-Saccharomyces as Biotools to Control the Production of Off-Flavors in Wines. Molecules 2021, 26, 4571. https://doi.org/10.3390/molecules26154571
Morata A, Loira I, González C, Escott C. Non-Saccharomyces as Biotools to Control the Production of Off-Flavors in Wines. Molecules. 2021; 26(15):4571. https://doi.org/10.3390/molecules26154571
Chicago/Turabian StyleMorata, Antonio, Iris Loira, Carmen González, and Carlos Escott. 2021. "Non-Saccharomyces as Biotools to Control the Production of Off-Flavors in Wines" Molecules 26, no. 15: 4571. https://doi.org/10.3390/molecules26154571







