Antibacterial Polysiloxane Polymers and Coatings for Cochlear Implants
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis of NAC-Modified Dimethyl-Methylvinylsiloxane Copolymers
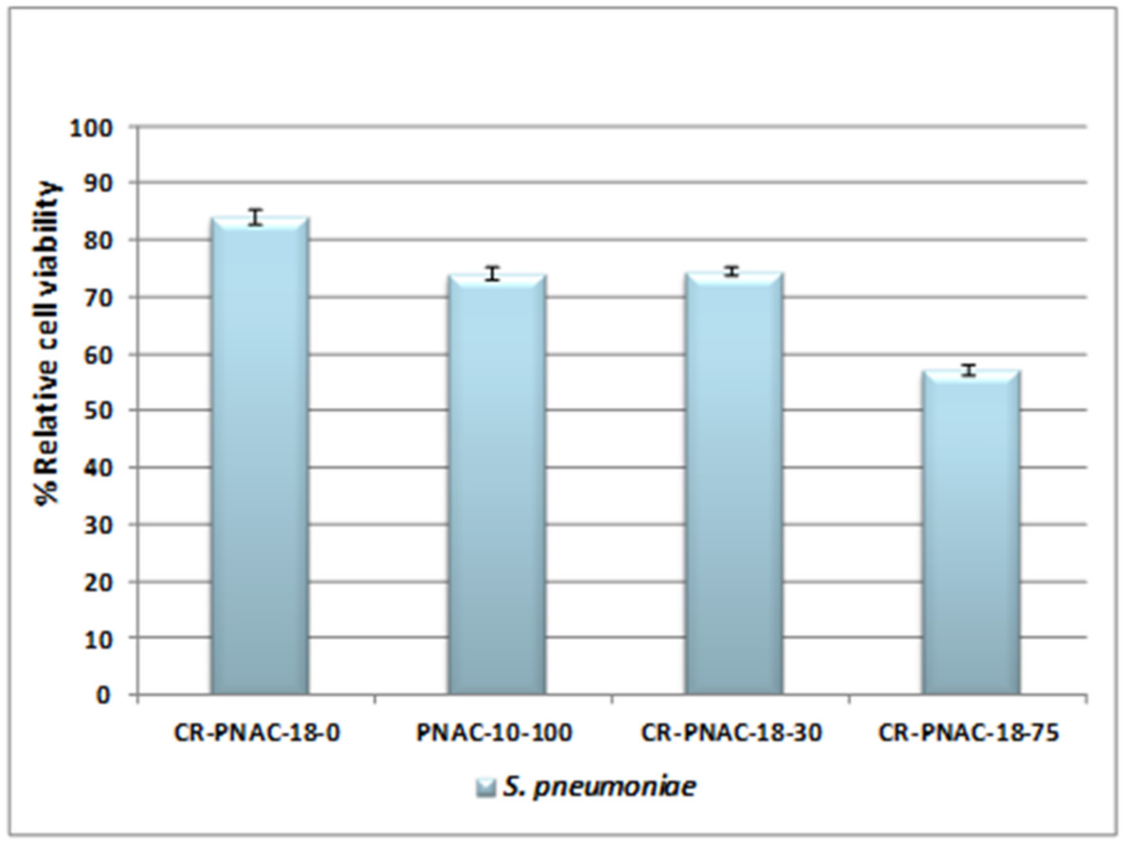
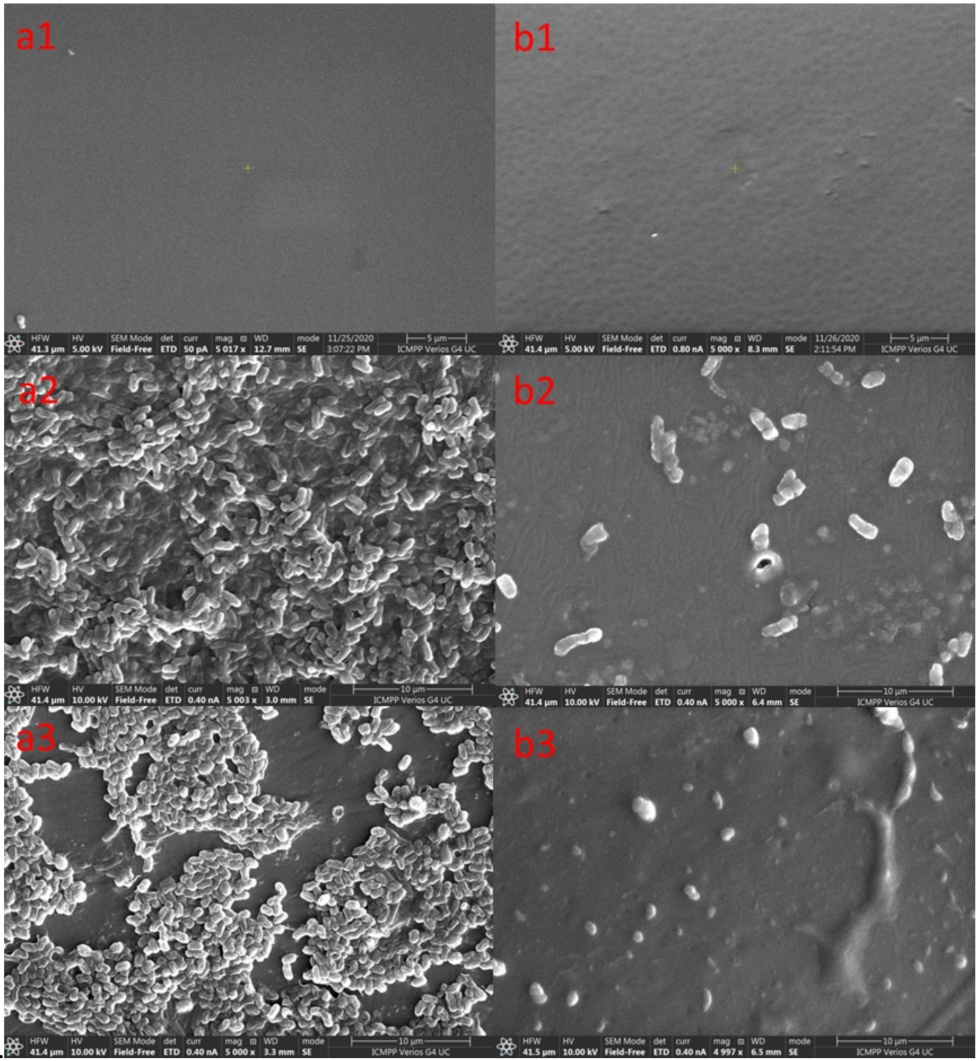
2.2. In Vitro Tests
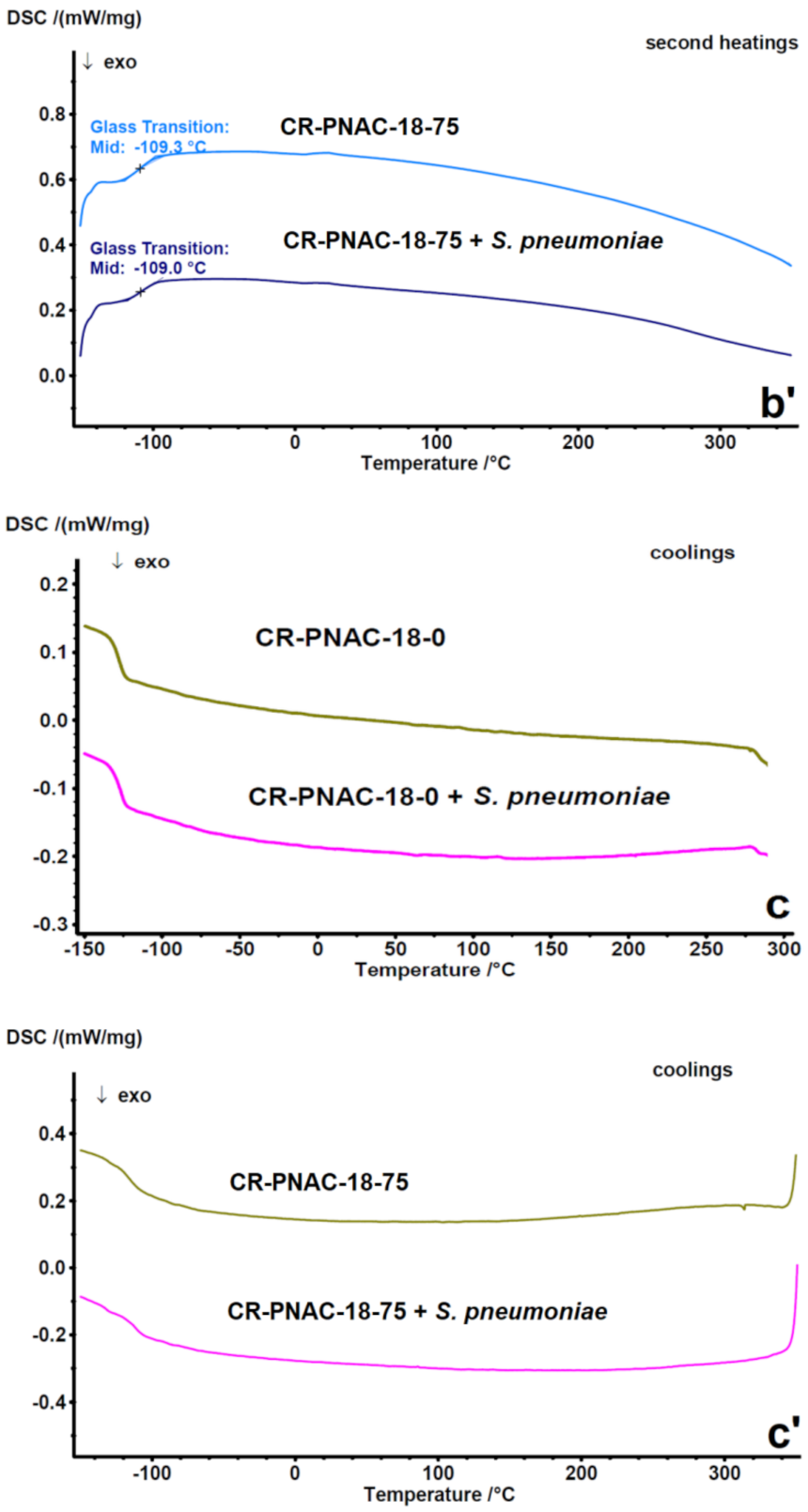
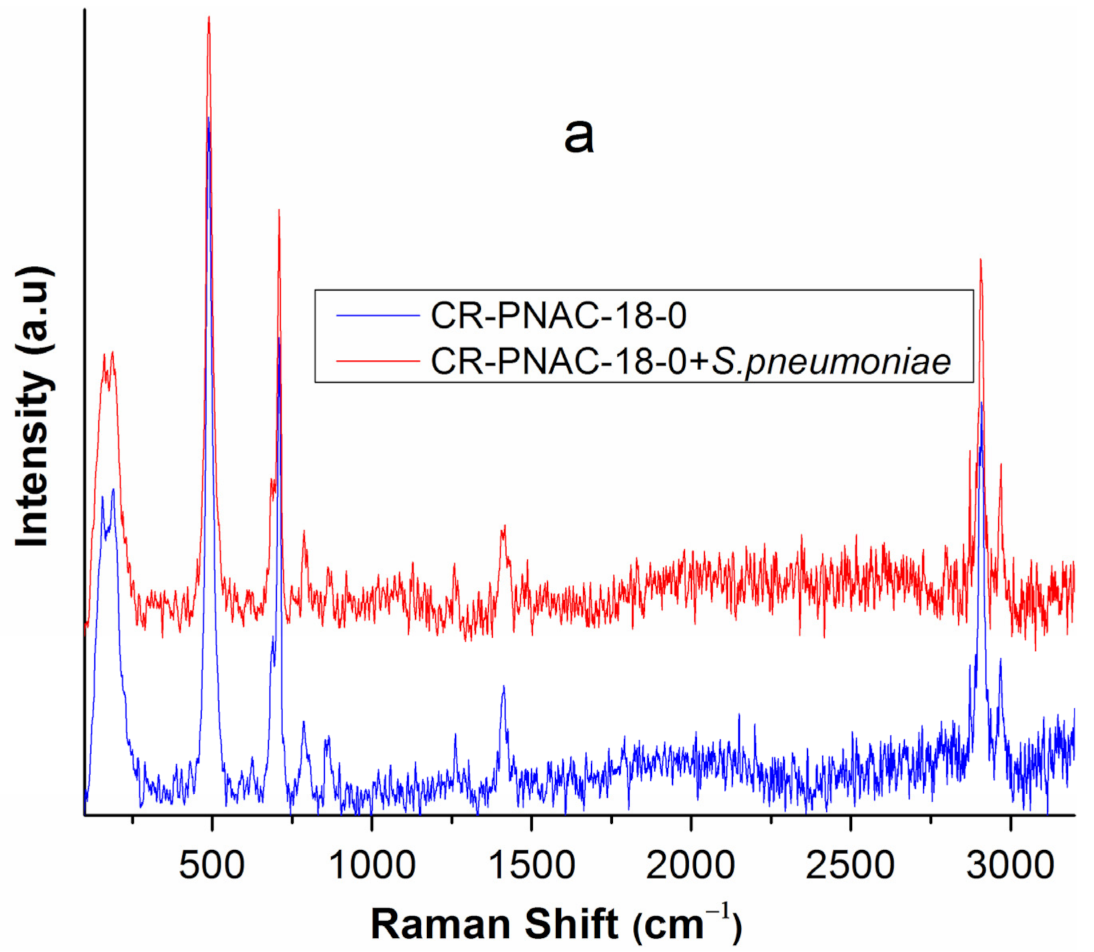
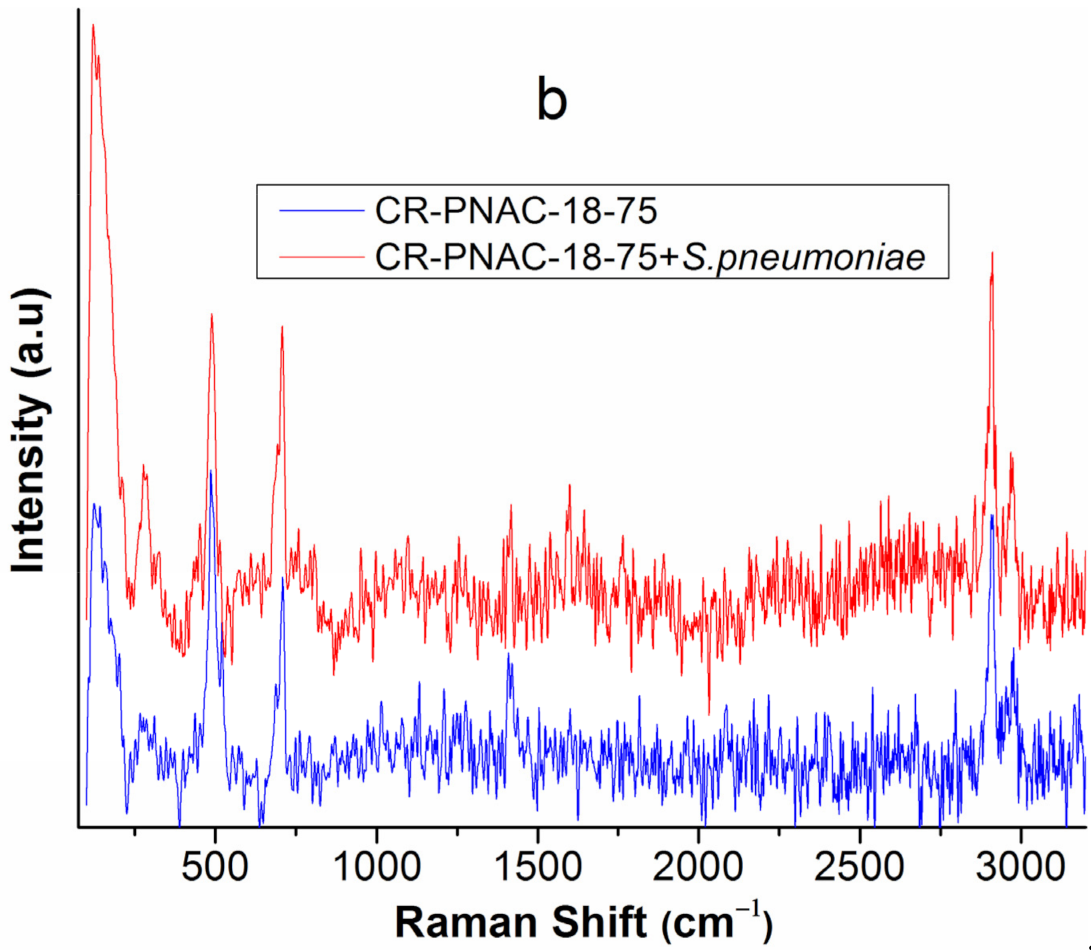
2.3. Compositional Stability of Samples before and after In Vitro Tests
2.4. In Vivo Tests
2.5. Surface Characterizations of the Samples after In Vitro and In Vivo Tests
3. Materials and Methods
3.1. Materials
3.2. Synthesis of CoSH Crosslinker Copolymer
3.3. Synthesis of PNAC-X-Y
3.3.1. Synthesis of PNAC-10-100
3.3.2. Synthesis of PNAC-18-75
3.3.3. Synthesis of PNAC-18-30
3.4. Preparation of Crosslinked Film
3.5. In Vitro Antimicrobial Tests
3.6. In Vivo Antimicrobial Tests
3.7. Morphological Characterization
3.8. X-ray Diffraction (XRD)
3.9. Differential Scanning Calorimetry (DSC)
3.10. Raman Spectroscopy
3.11. Surface Properties
3.12. Nuclear Magnetic Resonance (NMR) Spectroscopy
3.13. Fourier Transform Infrared (FT-IR) Spectroscopy
3.14. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Niparko, J.K. Spoken Language Development in Children Following Cochlear Implantation. JAMA 2010, 303, 1498–1506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szyfter, W.; Karlik, M.; Sekula, A.; Harris, S.; Gawęcki, W. Current indications for cochlear implantation in adults and children. Otolaryngol. Pol. 2019, 73, 1–5. [Google Scholar] [CrossRef]
- Ciorba, A.; Bovo, R.; Trevisi, P.; Rosignoli, M.; Aimoni, C.; Castiglione, A.; Martini, A. Postoperative Complications in Cochlear Implants: A Retrospective Analysis of 438 Consecutive Cases. Eur. Arch. Otorhinolaryngol. 2012, 269, 1599–1603. [Google Scholar] [CrossRef]
- Infectious Complications in Pediatric Cochlear Implants-Hopfenspirger-2007-The Laryngoscope-Wiley Online Library. Available online: https://onlinelibrary.wiley.com/doi/abs/10.1097/MLG.0b013e3180de4d35 (accessed on 21 July 2021).
- Vila, P.M.; Ghogomu, N.T.; Odom-John, A.R.; Hullar, T.E.; Hirose, K. Infectious Complications of Pediatric Cochlear Implants Are Highly Influenced by Otitis Media. Int. J. Pediatr. Otorhinolaryngol. 2017, 97, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Ray, J.; Proops, D.; Donaldson, I.; Fielden, C.; Cooper, H. Explantation and Reimplantation of Cochlear Implants. Cochlear Implant. Int. 2004, 5, 160–167. [Google Scholar] [CrossRef]
- Evolving Strategies to Prevent Implant-Associated Infections: JAAOS-Journal of the American Academy of Orthopaedic Surgeons. Available online: https://journals.lww.com/jaaos/fulltext/2012/07000/evolving_strategies_to_prevent_implant_associated.9.aspx (accessed on 21 July 2021).
- Ketonis, C.; Barr, S.; Adams, C.S.; Shapiro, I.M.; Parvizi, J.; Hickok, N.J. Vancomycin Bonded to Bone Grafts Prevents Bacterial Colonization. Antimicrob. Agents Chemother. 2011, 55, 487–494. [Google Scholar] [CrossRef] [Green Version]
- Xue, T.; Attarilar, S.; Liu, S.; Liu, J.; Song, X.; Li, L.; Zhao, B.; Tang, Y. Surface Modification Techniques of Titanium and Its Alloys to Functionally Optimize Their Biomedical Properties: Thematic Review. Front. Bioeng. Biotechnol. 2020, 8, 1261. [Google Scholar] [CrossRef]
- Klibanov, A.M. Permanently Microbicidal Materials Coatings. J. Mater. Chem. 2007, 17, 2479–2482. [Google Scholar] [CrossRef]
- Majumdar, P.; He, J.; Lee, E.; Kallam, A.; Gubbins, N.; Stafslien, S.J.; Daniels, J.; Chisholm, B.J. Antimicrobial Activity of Polysiloxane Coatings Containing Quaternary Ammonium-Functionalized Polyhedral Oligomeric. Silsesquioxane. J. Coat. Technol. Res. 2010, 7, 455–467. [Google Scholar] [CrossRef]
- Stöver, T.; Lenarz, T. Biomaterials in Cochlear Implants. GMS Curr. Top. Otorhinolaryngol. Head Neck Surg. 2011, 8, Doc10. [Google Scholar] [CrossRef]
- Lehnhardt, E. Biokompatibilität der Cochlear-Implants. In Proceedings of the Teil I: Referate; Herberhold, C., Ed.; Springer: Berlin/Heidelberg, Germany, 1992; pp. 223–233. [Google Scholar]
- Leslie, L.J.; Jenkins, M.J.; Shepherd, D.E.T.; Kukureka, S.N. The Effect of the Environment on the Mechanical Properties of Medical Grade Silicones. J. Biomed. Mater. Res. Part B Appl. Biomater. 2008, 86B, 460–465. [Google Scholar] [CrossRef]
- Nowacka, M.; Rygała, A.; Kręgiel, D.; Kowalewska, A. New Antiadhesive Hydrophobic Polysiloxanes. Molecules 2021, 26, 814. [Google Scholar] [CrossRef]
- Nowacka, M.; Rygała, A.; Kręgiel, D.; Kowalewska, A. Poly(Silsesquioxanes) and Poly(Siloxanes) Grafted with N-Acetylcysteine for Eradicating Mature Bacterial Biofilms in Water Environment. Colloids Surf. B Biointerfaces 2018, 172, 627–634. [Google Scholar] [CrossRef]
- Kregiel, D.; Rygala, A.; Kolesinska, B.; Nowacka, M.; Herc, A.S.; Kowalewska, A. Antimicrobial and Antibiofilm N-Acetyl-L-Cysteine Grafted Siloxane Polymers with Potential for Use in Water Systems. Int. J. Mol. Sci. 2019, 20, E2011. [Google Scholar] [CrossRef] [Green Version]
- Olofsson, A.-C.; Hermansson, M.; Elwing, H. N-Acetyl-l-Cysteine Affects Growth, Extracellular Polysaccharide Production, and Bacterial Biofilm Formation on Solid Surfaces. Appl. Environ. Microbiol. 2003, 69, 4814–4822. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamed, S.; Emara, M.; Shawky, R.M.; El-Domany, R.A.; Youssef, T. Silver Nanoparticles: Antimicrobial Activity, Cytotoxicity, and Synergism with N-Acetyl Cysteine. J. Basic Microbiol. 2017, 57, 659–668. [Google Scholar] [CrossRef] [PubMed]
- Opris, D.M.; Dünki, S.; Racles, C.; Bele, A.; Cazacu, M. High Permittivity Polymers Based on Functionalized Silicones. Patent No. WO2015135086, 17 September 2015. [Google Scholar]
- Zuo, Y.; Lu, H.; Xue, L.; Wang, X.; Ning, L.; Feng, S. Preparation and Characterization of Luminescent Silicone Elastomer by Thiol–Ene “Click” Chemistry. J. Mater. Chem. C 2014, 2, 2724–2734. [Google Scholar] [CrossRef]
- Balan, G.G.; Rosca, I.; Ursu, E.-L.; Fifere, A.; Varganici, C.-D.; Doroftei, F.; Turin-Moleavin, I.-A.; Sandru, V.; Constantinescu, G.; Timofte, D.; et al. Duodenoscope-Associated Infections beyond the Elevator Channel: Alternative Causes for Difficult Reprocessing. Molecules 2019, 24, 2343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vlad, A.; Zaltariov, M.-F.; Shova, S.; Novitchi, G.; Varganici, C.-D.; Train, C.; Cazacu, M. Flexible Linkers and Dinuclear Metallic Nodes Build up an Original Metal–Organic Framework. CrystEngComm 2013, 15, 5368–5375. [Google Scholar] [CrossRef]
- Rosu, L.; Varganici, C.; Crudu, A.; Rosu, D.; Bele, A. Ecofriendly Wet–White Leather vs. Conventional Tanned Wet–Blue Leather. A Photochemical Approach. J. Clean. Prod. 2018, 177, 708–720. [Google Scholar] [CrossRef]
- Varganici, C.-D.; Ursache, O.; Gaina, C.; Gaina, V.; Simionescu, B.C. Studies on New Hybrid Materials Prepared by Both Diels–Alder and Michael Addition Reactions. J. Anal. Calorim. 2013, 111, 1561–1570. [Google Scholar] [CrossRef]
- Deshpande, G.; Rezac, M.E. Kinetic Aspects of the Thermal Degradation of Poly(Dimethyl Siloxane) and Poly(Dimethyl Diphenyl Siloxane). Polym. Degrad. Stab. 2002, 76, 17–24. [Google Scholar] [CrossRef]
- Brandon, H.J.; Jerina, K.L.; Wolf, C.J.; Young, V.L. In Vivo Aging Characteristics of Silicone Gel Breast Implants Compared to Lot-Matched Controls. Plast. Reconstr. Surg. 2002, 109, 1927–1933. [Google Scholar] [CrossRef]
- Ramião, N.G.; Martins, P.S.; Barroso, M.L.; Santos, D.C.; Fernandes, A.A. In Vitro Degradation of Polydimethylsiloxanes in Breast Implant Applications. J. Appl. Biomater. Funct. Mater. 2017, 15, e369–e375. [Google Scholar] [CrossRef]
- Comparison of Contact Angle Hysteresis of Different Probe Liquids on the Same Solid Surface | SpringerLink. Available online: https://link.springer.com/article/10.1007/s00396-012-2777-9 (accessed on 22 July 2021).
- Racles, C.; Asandulesa, M.; Tiron, V.; Tugui, C.; Vornicu, N.; Ciubotaru, B.-I.; Mičušík, M.; Omastová, M.; Vasiliu, A.-L.; Ciomaga, C. Elastic Composites with PDMS Matrix and Polysulfone-Supported Silver Nanoparticles as Filler. Polymer 2021, 217, 123480. [Google Scholar] [CrossRef]
- Schrader, M.E. On Adhesion of Biological Substances to Low Energy Solid Surfaces. J. Colloid Interface Sci. 1982, 88, 296–297. [Google Scholar] [CrossRef]
- Kanematsu, H.; Oizumi, A.; Sato, T.; Kamijo, T.; Honma, S.; Barry, D.M.; Hirai, N.; Ogawa, A.; Kogo, T.; Kuroda, D.; et al. Biofilm Formation of a Polymer Brush Coating with Ionic Liquids Compared to a Polymer Brush Coating with a Non-Ionic Liquid. Coatings 2018, 8, 398. [Google Scholar] [CrossRef] [Green Version]
- Sanchez, C.J.; Kumar, N.; Lizcano, A.; Shivshankar, P.; Dunning Hotopp, J.C.; Jorgensen, J.H.; Tettelin, H.; Orihuela, C.J. Streptococcus Pneumoniae in Biofilms Are Unable to Cause Invasive Disease Due to Altered Virulence Determinant Production. PLoS ONE 2011, 6, e28738. [Google Scholar] [CrossRef]
- Lawford, H.G.; Rousseau, J.D. Studies on Nutrient Requirements and Cost-Effective Supplements for Ethanol Production by RecombinantE. Coli. Appl. Biochem. Biotechnol. 1996, 57, 307. [Google Scholar] [CrossRef]
- Cazacu, M.; Marcu, M.; Holercă, M.N.; Petrovan, S.; Lăzărescu, S. Heterogeneous Catalyzed Copolymerization of Octamethylcyclotetrasiloxane with 1,3,5,7-Tetravinyl-1,3,5,7-Tetramethylcyclo-Tetrasiloxane. J. Macromol. Sci. Part A 1996, 33, 65–76. [Google Scholar] [CrossRef]
- Cazacu, M.; Marcu, M.; Ibanescu, C.; Petrovan, S.; Holerca, M.; Simionescu, M. Cationic Heterogeneous Copolymerization of Octamethylcyclotetrasiloxane with 1,3,5,7-Tetramethyl-1,3,5,7-Tetravinylcyclotetra-Siloxane: Optimization of Reaction Conditions. Polym. Plast. Technol. Eng. 1996, 35, 327–347. [Google Scholar] [CrossRef]
- Harabagiu, V.; Pinteala, M.; Cotzur, C.; Holerca, M.N.; Ropot, M. Functional Polysiloxanes. 3. Reaction of 1,3-Bis(3-Glycidoxypropyl)-1,1,3,3-Tetramethyldisiloxane with Amino Compounds. J. Macromol. Sci. Part A 1995, 32, 1641–1648. [Google Scholar] [CrossRef]
- XLSTAT | Statistical Software for Excel. Available online: https://www.xlstat.com (accessed on 22 July 2021).






| Element | CR-PNAC-18-0 | CR-PNAC-18-75 | ||||
|---|---|---|---|---|---|---|
| Pristine | In Vitro | In Vivo | Pristine | In Vitro | In Vivo | |
| C | 29.06 ± 0.16 | 26.96 ± 0.26 | 35.36 ± 0.98 | 31.42 ± 0.01 | 30.36 ± 0.17 | 26.20 ± 0.91 |
| N | 0.67± 0.30 | 9.78 ± 0.03 | 2.82 ± 0.79 | 11.18 ± 0.15 | 10.76 ± 0.36 | 4.23 ± 0.43 |
| O | 27.72 ± 0.41 | 30.20 ± 0.88 | 26.11 ± 1.13 | 25.13 ± 0.47 | 28.35 ± 0.28 | 27.27 ± 0.43 |
| Na | - | 2.39 ± 0.07 | 0.395 ± 0.07 | - | 2.39 ± 0.88 | 1.13 ± 0.01 |
| Si | 47.60 ± 0.28 | 46.86 ± 0.79 | 33.93 ± 0.21 | 33.17± 0.12 | 27.10 ± 0.73 | 29.51 ± 0.12 |
| P | - | - | 0.12 ± 0.01 | - | - | - |
| S | - | 0.12 ± 0.25 | 0.07 ± 0.05 | - | 2.92 ± 0.14 | 3.31 ± 0.04 |
| Cl | - | - | 0.58 ± 0.70 | - | 0.83 ± 0.01 | - |
| K | - | 0.19 ± 0.02 | 0.09 ± 0.13 | - | 0.18 ± 0.01 | 0.19 ± 0.11 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cozma, V.; Rosca, I.; Radulescu, L.; Martu, C.; Nastasa, V.; Varganici, C.-D.; Ursu, E.-L.; Doroftei, F.; Pinteala, M.; Racles, C. Antibacterial Polysiloxane Polymers and Coatings for Cochlear Implants. Molecules 2021, 26, 4892. https://doi.org/10.3390/molecules26164892
Cozma V, Rosca I, Radulescu L, Martu C, Nastasa V, Varganici C-D, Ursu E-L, Doroftei F, Pinteala M, Racles C. Antibacterial Polysiloxane Polymers and Coatings for Cochlear Implants. Molecules. 2021; 26(16):4892. https://doi.org/10.3390/molecules26164892
Chicago/Turabian StyleCozma, Vlad, Irina Rosca, Luminita Radulescu, Cristian Martu, Valentin Nastasa, Cristian-Dragos Varganici, Elena-Laura Ursu, Florica Doroftei, Mariana Pinteala, and Carmen Racles. 2021. "Antibacterial Polysiloxane Polymers and Coatings for Cochlear Implants" Molecules 26, no. 16: 4892. https://doi.org/10.3390/molecules26164892








