Effect of ZrC Nanopowders on Enhancing the Hydro/Dehydrogenation Kinetics of MgH2 Powders
Abstract
:1. Introduction
2. Results and Discussions
2.1. Structural Analysis and Morphology
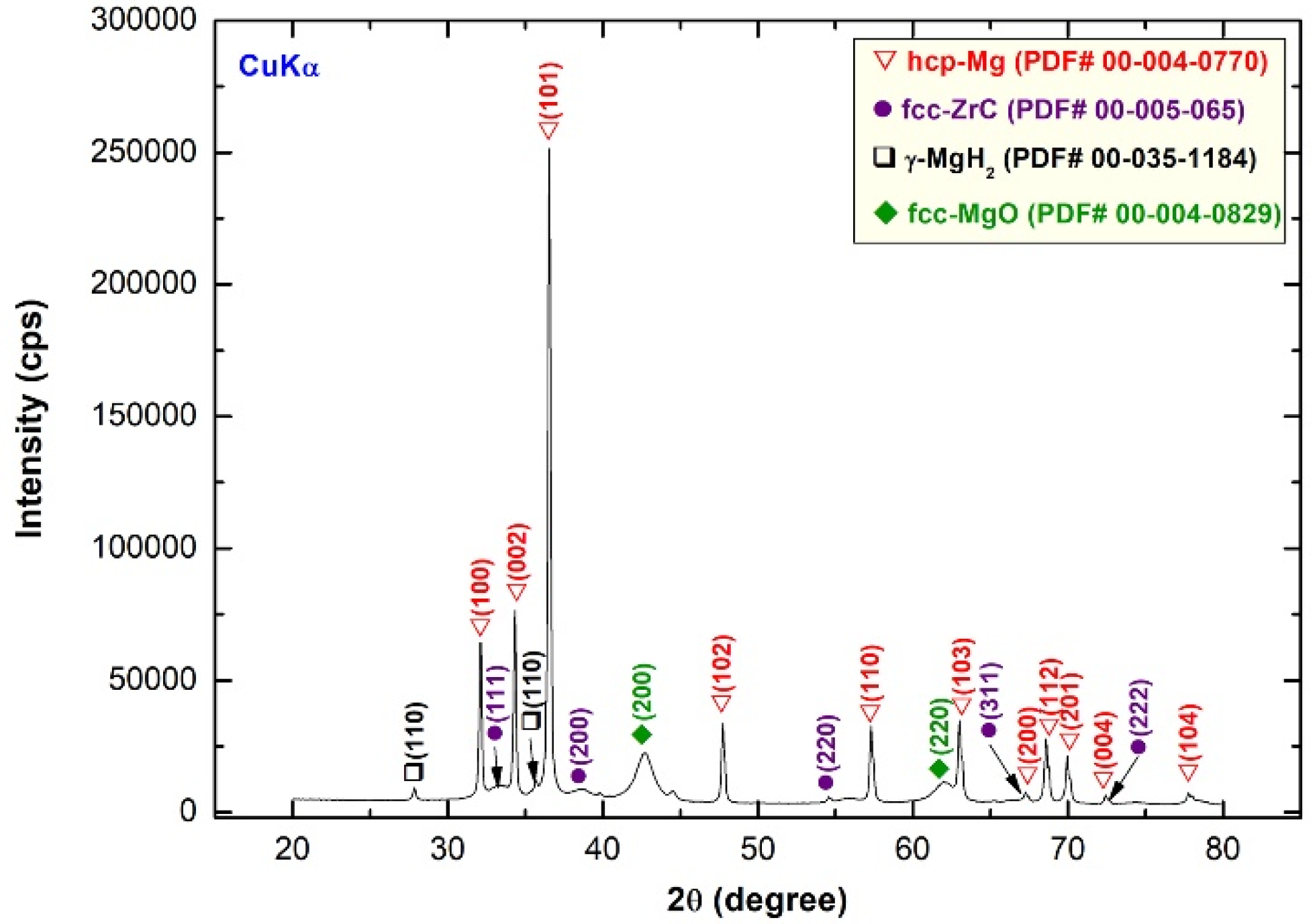
2.1.1. MgH2 Nanocrystalline Powders
2.1.2. ZrC Catalytic Agent Nanopowders
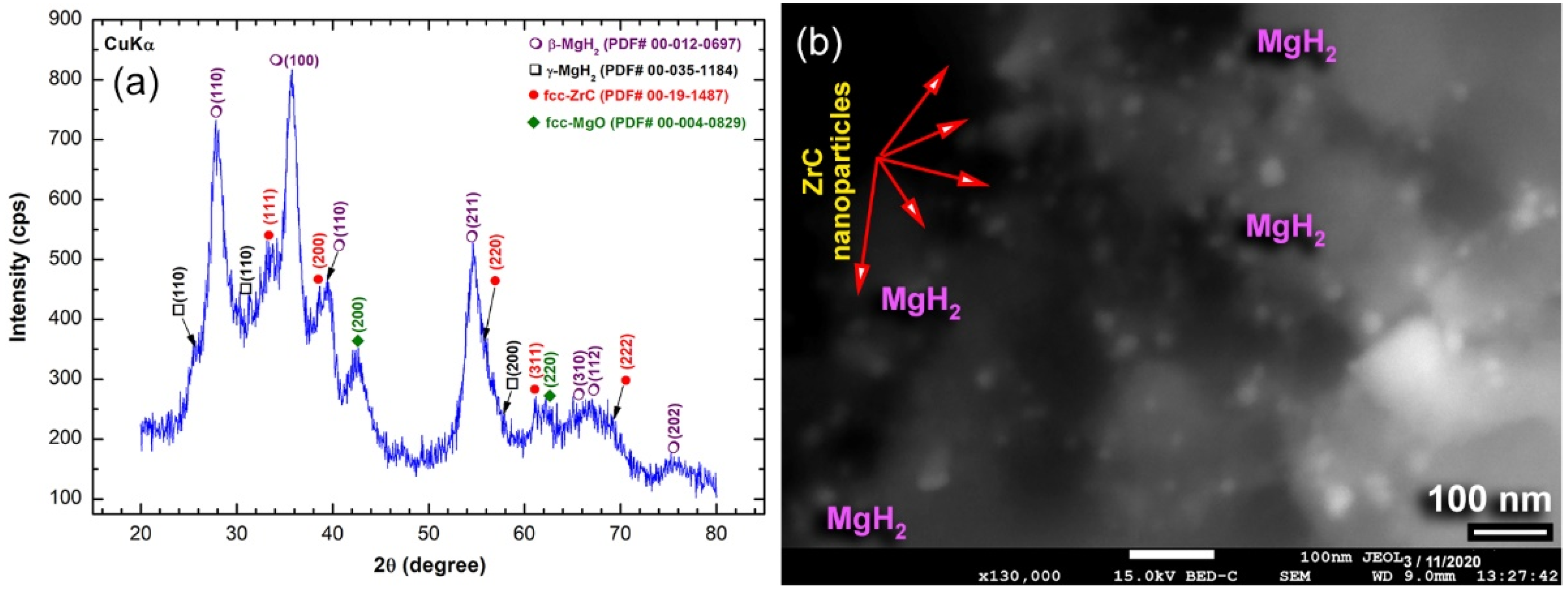
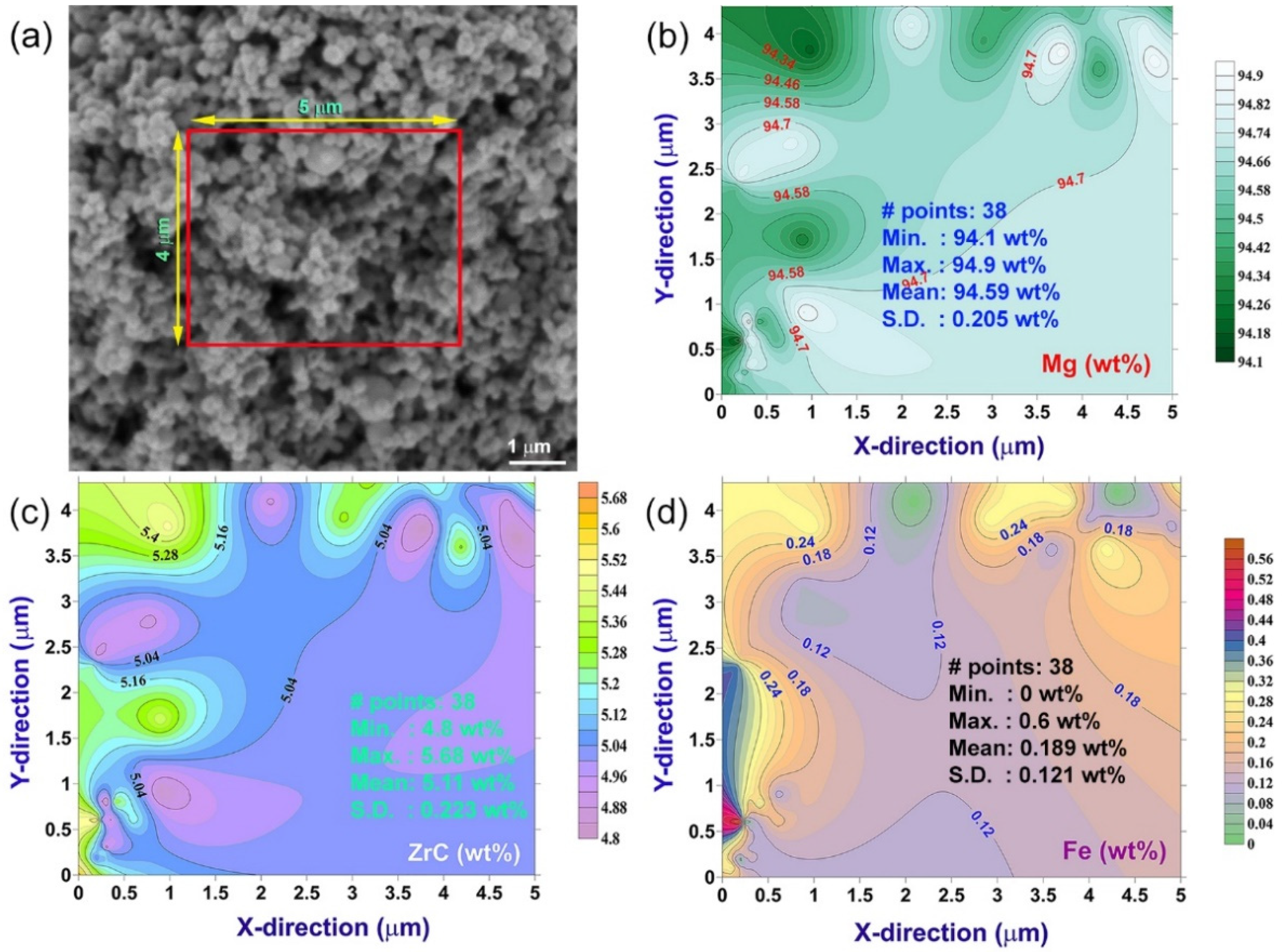
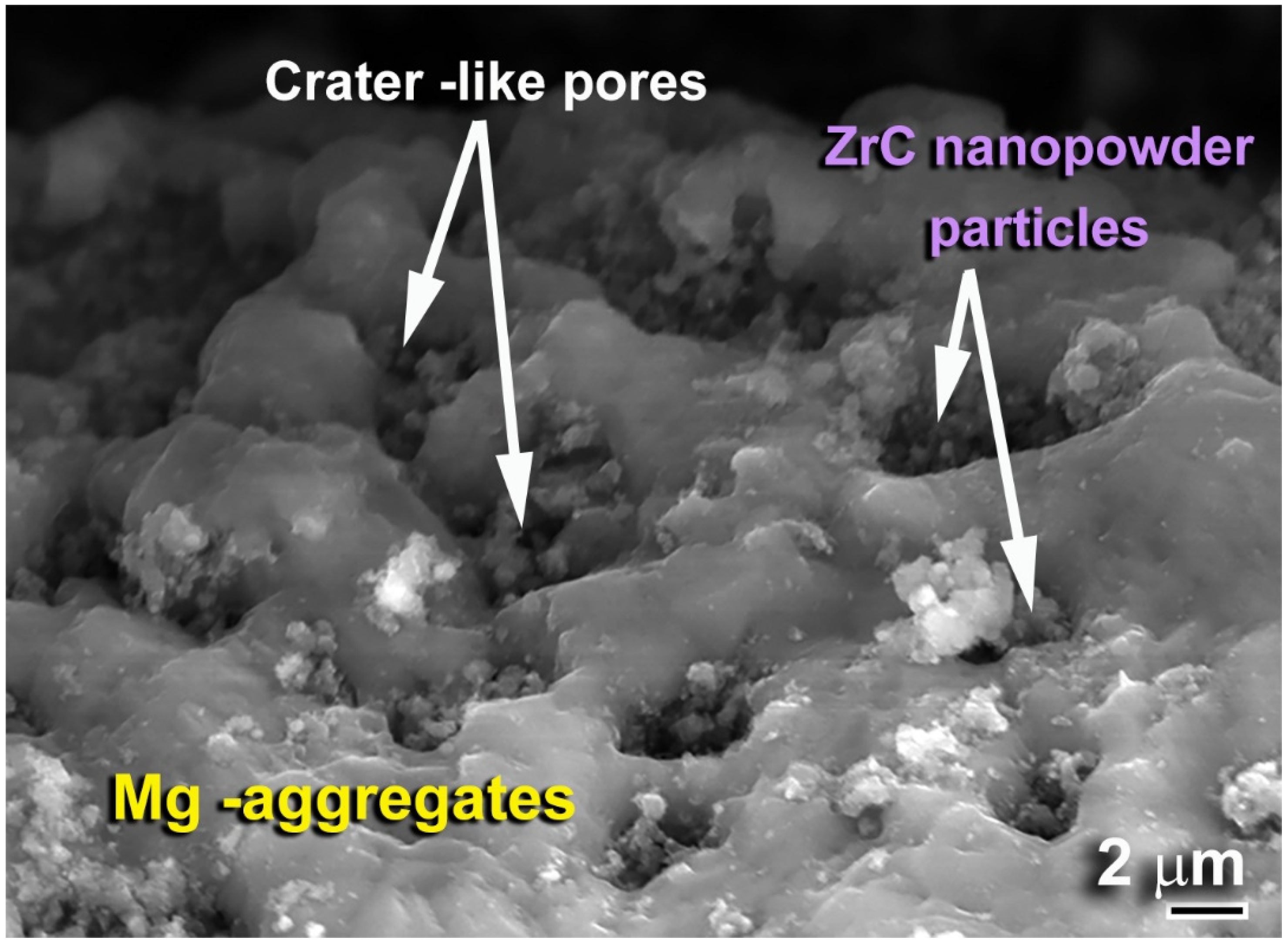
2.1.3. Nanocomposite MgH2/x-ZrC (x; 2, 5 and 7 wt.%) Powders
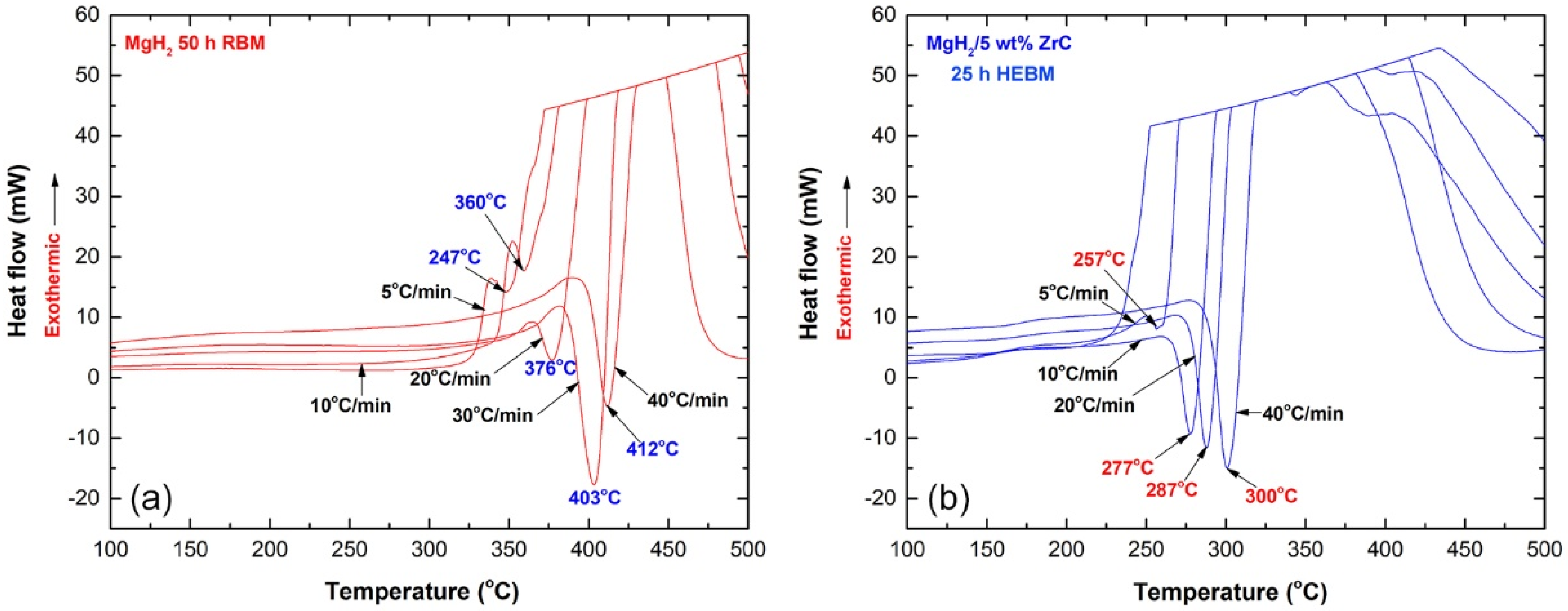
2.2. Thermal Stability
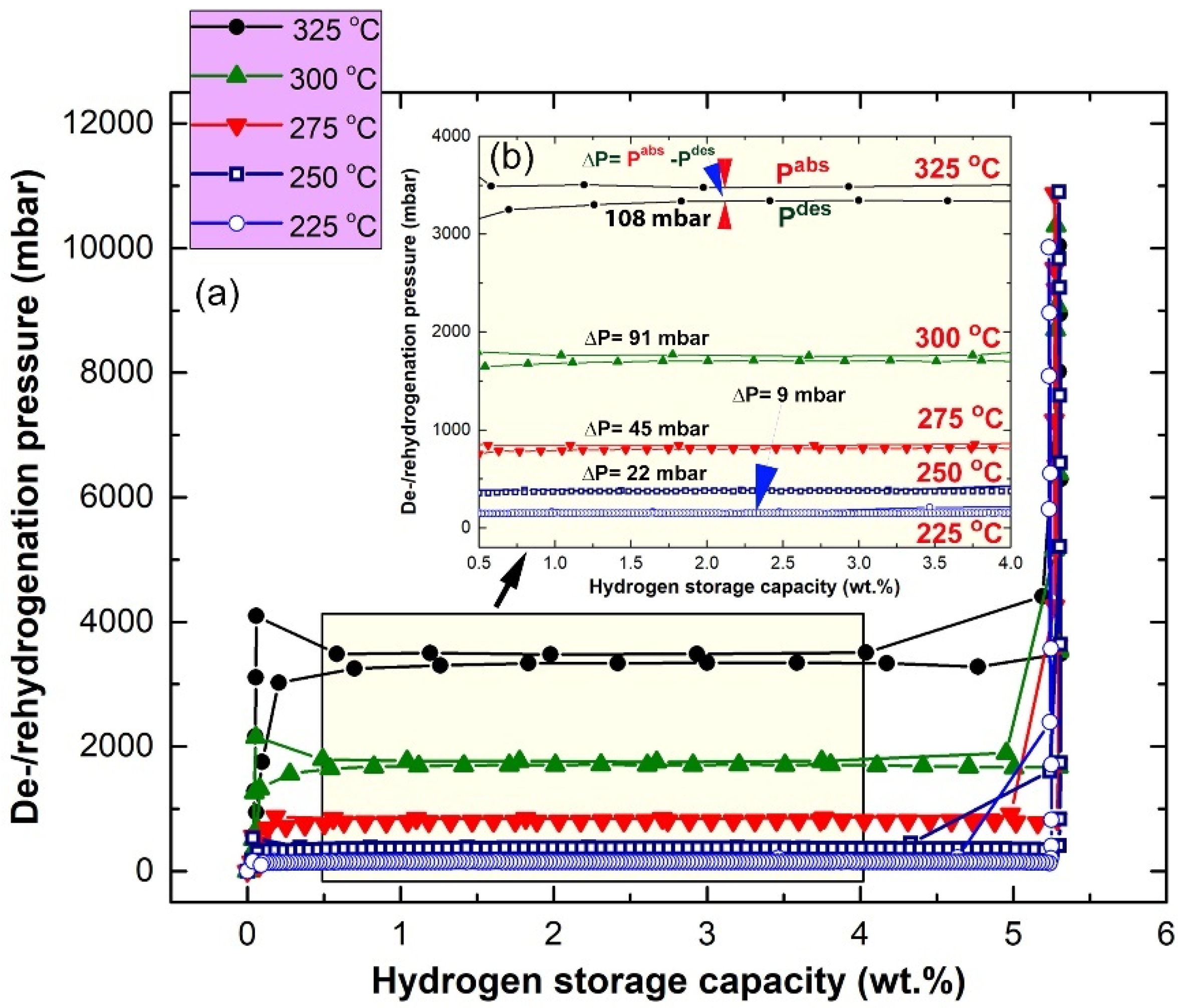
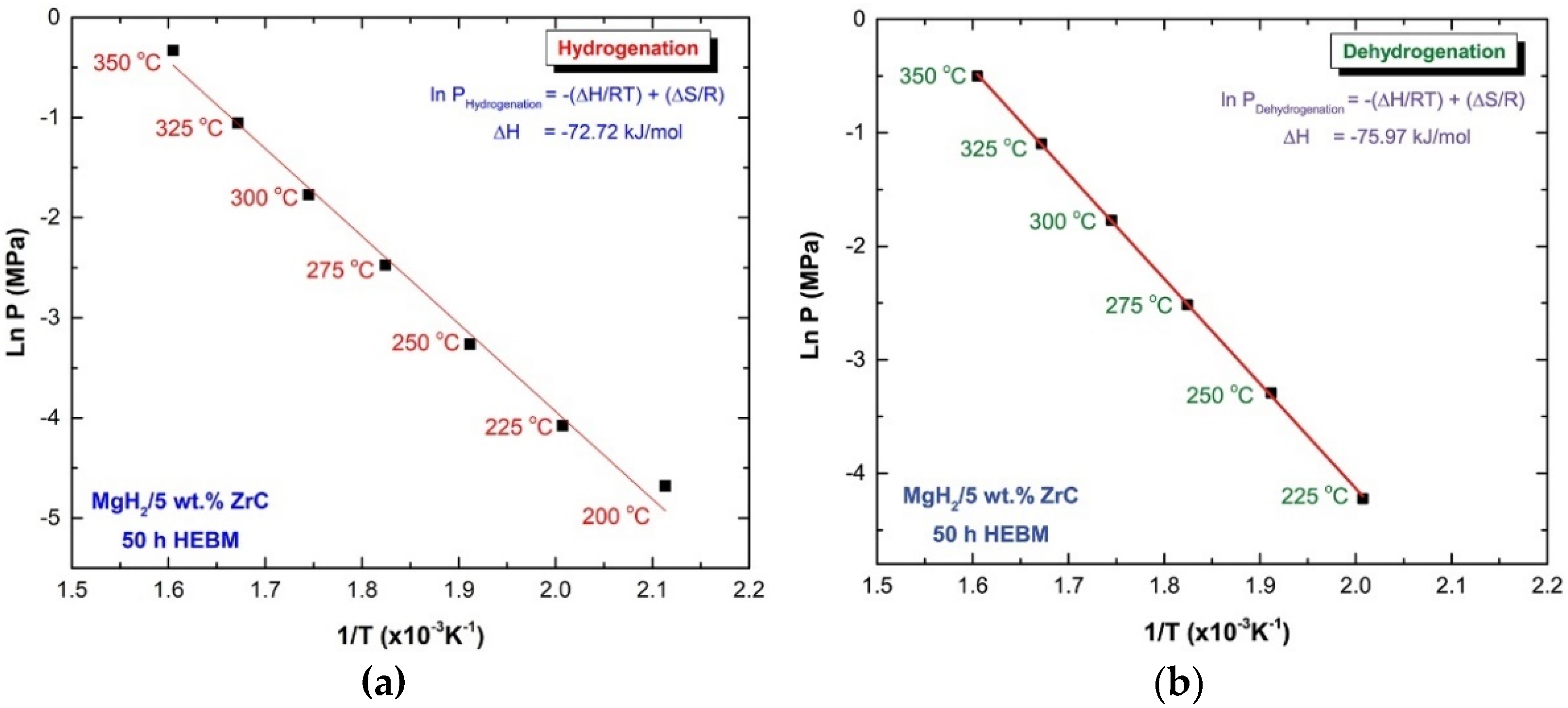
2.3. Pressure-Composition-Temperature
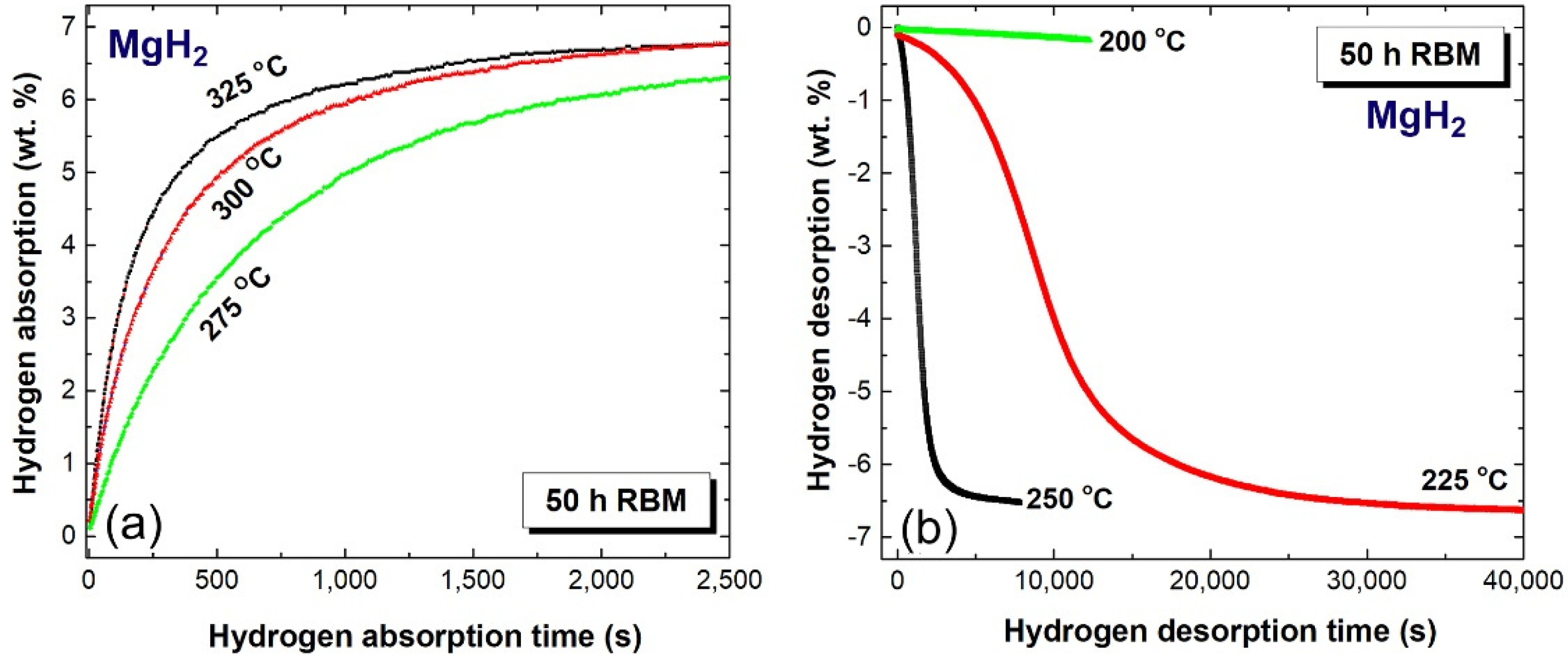
2.4. Hydrogenation/Dehydrogenation Kinetics
2.4.1. MgH2 Nanocrystalline Powders
2.4.2. Nanocomposite MgH2/x-ZrC (x; 2, 5, and 7 wt.%) Powders
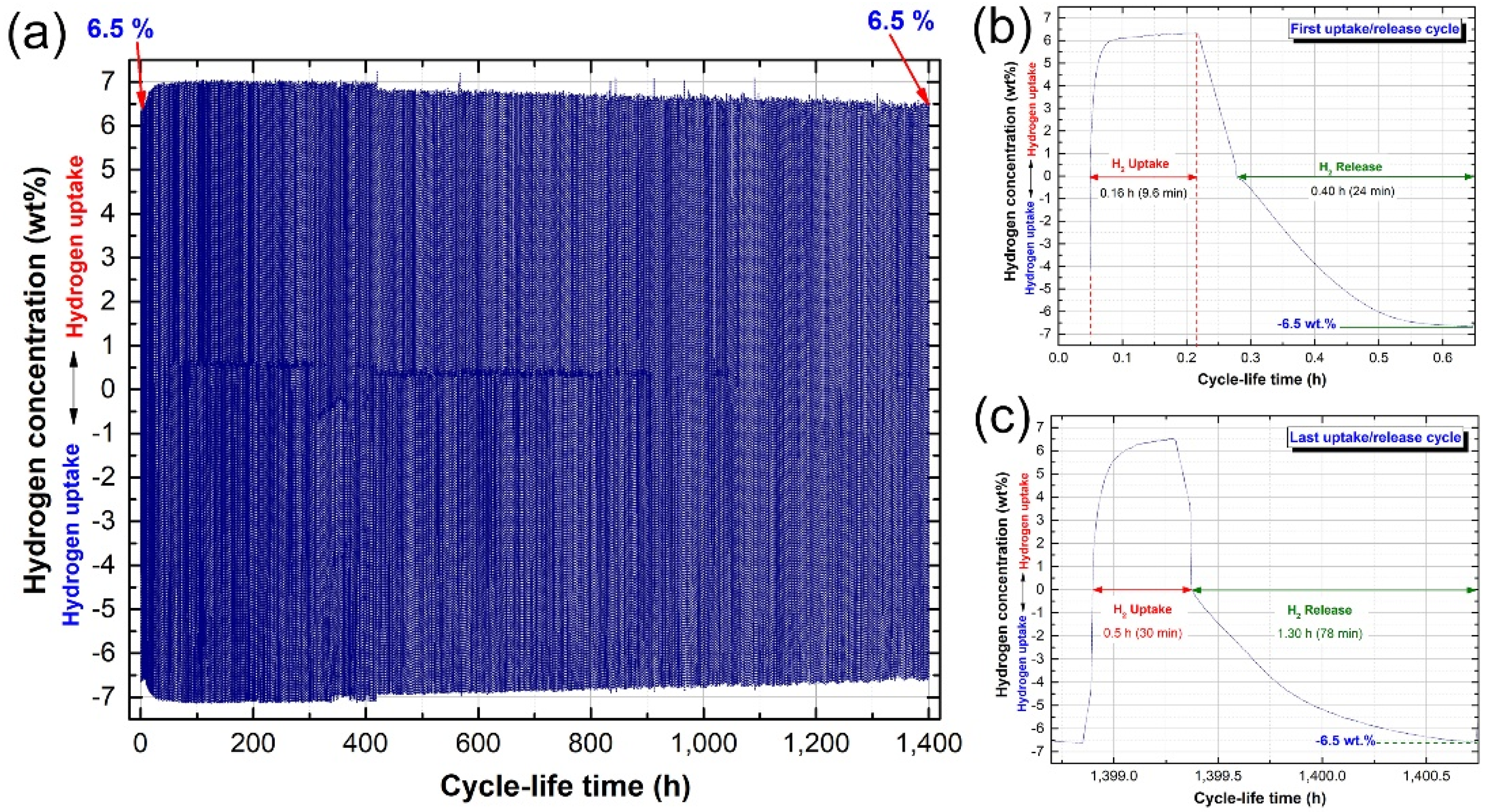
2.5. Cycle Lifetime
3. Materials and Methods
3.1. Materials Preparations
3.1.1. Preparations of Nanocrystalline MgH2 Powders
3.1.2. Preparations of ZrC Nanopowders
3.1.3. Preparations of Nanocomposite MgH2/ZrC Nanopowders
3.2. Sample Characterizations
3.2.1. Crystal Structure and Morphology
3.2.2. Thermal Stability
3.2.3. Hydrogenation/Dehydrogenation Kinetics
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Vohra, K.; Vodonos, A.; Schwartz, J.; Marais, E.; Sulprizio, M.; Mickley, L. Global mortality from outdoor fine particle pollution generated by fossil fuel combustion: Results from GEOS-Chem. Environ. Res. 2021, 195, 110754. [Google Scholar] [CrossRef]
- Jackson, R.B.; Quéré, C.L.; Andrew, R.M.; Canadell, J.G.; Peters, G.P.; Roy, J.; Wu, L. Warning signs for stabilizing global CO2 emissions. Environ. Res. Lett. 2017, 12, 110202–110206. [Google Scholar] [CrossRef] [Green Version]
- El-Eskandarany, M.S. Recent developments in the fabrication, characterization and implementation of MgH2-based solid-hydrogen materials in the Kuwait Institute for Scientific Research. RSC Adv. 2019, 9, 9907. [Google Scholar] [CrossRef] [Green Version]
- Rango, P.; Wen, J.; Skryabina, N.; Laversenne, L.; Fruchart, D.; Borges, M. Hydrogen Storage Properties of Mg-Ni Alloys Processed by Fast Forging. Energies 2020, 13, 3509. [Google Scholar] [CrossRef]
- El-Eskandarany, M.S.; Al-Salem, S.; Ali, N.; Banyan, M.; Al-Ajimi, F.; Al-Duweesh, A. From gangue to the fuel-cells application. Sci. Rep. 2020, 10, 20022. [Google Scholar] [CrossRef] [PubMed]
- El-Eskandarany, M.S. Mechanical Alloying: Energy, Surface Protective Coating and Medical Applications, 3rd ed.; Elsevier, Oxford University Press: New York, NY, USA, 2020. [Google Scholar]
- Sinigaglia, T.; Lewiski, F.; Martins, M.E.S.; Siluk, J.C.M. Production, storage, fuel stations of hydrogen and its utilization in automotive applications-a review. Int. J. Hydrogen Energy 2017, 42, 24597–24611. [Google Scholar] [CrossRef]
- El-Eskandarany, M.S.; Al-Nasrallah, E.; Banyan, M.; Al-Ajmi, F. Bulk nanocomposite MgH2/10 wt.% (8 Nb2O5/2 Ni) solid-hydrogen storage system for fuel cell applications. Int. J. Hydrogen Energy 2018, 27, 23382–23396. [Google Scholar] [CrossRef]
- Walker, G. Hydrogen containment materials, In Solid-State Hydrogen Storage: Materials and Chemistry, 1st ed.; Woodhead Publishing Limited, Elsevier: New York, NY, USA, 2008. [Google Scholar]
- Jeon, S.; Roh, M.; Heshmati, A.; Kim, S. An assessment of corporate average fuel economy standards for passenger cars in South Korea. Energies 2020, 13, 4533. [Google Scholar] [CrossRef]
- Sakintuna, B.; Lamari-Darkrim, F.; Hirscher, M. Metal hydride materials for solid hydrogen storage: A review. Int. J. Hydrogen Energy 2007, 32, 1121–1140. [Google Scholar] [CrossRef]
- Shao, H. Heat modeling and material development of mg-based nanomaterials combined with solid oxide fuel cell for stationary energy storage. Energies 2017, 10, 1767. [Google Scholar] [CrossRef] [Green Version]
- Peska, M.; Crujko, T.; Polanski, M. Hydrogenation ability of Mg-Li alloys. Energies 2020, 13, 2080. [Google Scholar] [CrossRef] [Green Version]
- Shukla, V.; Bhatnagar, A.; Verma, S.; Pandey, A.; Vishwakarma, A.; Srivastava, P.; Yadav, T.; Srivastava, O. Simultaneous improvement of kinetics and thermodynamics based on SrF2 and SrF2@Gr additives on hydrogen sorption in MgH2. Mater. Adv 2021, 2, 4277–4290. [Google Scholar] [CrossRef]
- El-Eskandarany, M.S.; Saeed, M.; Al-Nasrallah, E.; Al-Ajmi, F.; Banyan, M. Effect of LaNi3 amorphous alloy nanopowders on the performance and hydrogen storage properties of MgH2. Energies 2019, 12, 1005. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Liu, Y.; Zhang, X.; Hu, J.; Gao, M.; Pan, H. Empowering hydrogen storage performance of MgH2 by nanoengineering and nanocatalysis. Mater. Today Nano 2020, 9, 100064. [Google Scholar] [CrossRef]
- El-Eskandarany, M.S.; Shaban, E.; Al-Matrouk, H.; Behbehani, M.; Alkandary, A.; Aldakheel, F.; Ali, N.; Ahmed, S.A. Structure, morphology and hydrogen storage kinetics of nanocomposite MgH2/10 wt% ZrNi5 powders. Mater. Today Energy 2017, 3, 60–71. [Google Scholar] [CrossRef]
- El-Eskandarany, M.S. Metallic glassy Zr70Ni20 Pd10 powders for improving the hydrogenation/dehydrogenation behavior of MgH2. Sci. Rep. 2016, 6, 26936. [Google Scholar] [CrossRef] [PubMed]
- Luo, Q.; Li, J.; Li, B.; Liu, B.; Shao, H.; Li, Q. Kinetics in Mg-based hydrogen storage materials: Enhancement and mechanism. J. Magnes. Alloy 2019, 7, 58–71. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Y.J. Recent advances in additive-enhanced magnesium hydride for hydrogen storage. Prog. Nat. Sci. 2017, 27, 41–49. [Google Scholar] [CrossRef]
- El-Eskandarany, M.S.; Shaban, E.; Al-Halaili, B. Nanocrystalline β-γ-β cyclic phase transformation in reacted ball milled MgH2 powders. Int. J. Hydrogen Energy 2014, 39, 12727–12740. [Google Scholar] [CrossRef]
- Amira, S.; Huot, J. Effect of cold rolling on hydrogen sorption properties of die-cast and as-cast magnesium alloys. J. Alloys Compd. 2012, 520, 287–294. [Google Scholar] [CrossRef]
- Huot, J.; Tousignant, M. Effect of cold rolling on metal hydrides. Mater. Trans. 2019, 60, 1571–1576. [Google Scholar] [CrossRef] [Green Version]
- Jorge, A.M., Jr.; de Lima, G.F.; Triques, M.R.M.; Botta, W.J.; Kiminami, C.S.; Nogueira, R.P.; Yavari, A.R.; Langdon, T.G. Correlation between hydrogen storage properties and textures induced in magnesium through ecap and cold rolling. Int. J. Hydrogen Energy 2014, 39, 3810–3821. [Google Scholar] [CrossRef]
- Valiev, R.Z.; Islamgaliev, R.K.; Alexandrov, I.V. Bulk nanostructured materials from severe plastic deformation. Prog. Mater. Sci. 2000, 45, 103–189. [Google Scholar] [CrossRef]
- Vajeeston, P.; Ravindran, P.; Kjekshus, A.; Fjellvåg, H. Pressure-induced structural transitions in MgH2. Phys. Rev. Lett. 2002, 89, 175506. [Google Scholar] [CrossRef] [Green Version]
- Xie, L.S.; Li, J.S.; Zhang, T.B.; Kou, H.C. Role of milling time and ni content on dehydrogenation behavior of MgH2/Ni composite. T. Nonferr. Metal SOC 2017, 27, 569–577. [Google Scholar] [CrossRef]
- Liang, G.; Huot, J.; Boily, S.; Van Neste, A.; Schulz, R. Catalytic effect of transition metals on hydrogen sorption in nanocrystalline ball milled MgH2–TM (TM= Ti, V, Mn, Fe and Ni) systems. J. Alloys Compd. 1999, 292, 247–252. [Google Scholar] [CrossRef]
- House, S.D.; Vajo, J.J.; Ren, C.; Rockett, A.A.; Robertson, I.M. Effect of ball-milling duration and dehydrogenation on the morphology, microstructure and catalyst dispersion in Ni-catalyzed MgH2 hydrogen storage materials. Acta Mater. 2015, 86, 55–68. [Google Scholar] [CrossRef] [Green Version]
- Xu, C.C.; Xiao, X.Z.; Jie, S.; Liu, L.X.; Teng, Q.; Chen, L.X. Effects of Ti-based additives on Mg2FeH6 dehydrogenation properties. T. Nonferr. Metal SOC 2016, 26, 791–798. [Google Scholar] [CrossRef]
- Yu, X.; Yang, Z.; Liu, H.-K.; Grant, D.; Walker, G.S. The effect of a Ti-V-based bcc alloy as a catalyst on the hydrogen storage properties of MgH2. Int. J. Hydrogen Energy 2010, 35, 6338–6344. [Google Scholar] [CrossRef]
- Zhou, C.; Fang, Z.Z.; Ren, C.; Li, J.; Lu, J. Effect of Ti intermetallic catalysts on hydrogen storage properties of magnesium hydride. J. Phys. Chem. C 2013, 117, 12973–12980. [Google Scholar] [CrossRef]
- El-Eskandarany, M.S.; Al-Matrouk, H.; Shaban, E.; Al-Duweesh, A. Effect of mechanically-induced solid-state doping time on the morphology and hydrogenation cyclability of MgH2/7 Mn3.6Ti2.4 nanocomposite powders. Int. J. Hydrogen Energy 2015, 40, 10139–10149. [Google Scholar] [CrossRef]
- Ren, C.; Fang, Z.Z.; Zhou, C.; Lu, J.; Ren, Y.; Zhang, X. Hydrogen storage properties of magnesium hydride with V-based additives. J. Phys. Chem. C 2014, 118, 21778–21784. [Google Scholar] [CrossRef]
- El-Eskandarany, M.S.; Al-Matrouk, H.; Shaban, E.; Al-Duweesh, A. Superior catalytic effect of nanocrystalline big-cube Zr2 Ni metastable phase for improving the hydrogen sorption/desorption kinetics and cyclability of MgH2 powders. Energy 2015, 91, 274–282. [Google Scholar] [CrossRef]
- Polanski, M.; Bystrzycki, J.; Varin, R.A.; Plocinski, T.; Pisarek, M. The effect of chromium (iii) oxide (Cr2O3) nanopowder on the microstructure and cyclic hydrogen storage behavior of magnesium hydride (MgH2). J. Alloys Compd. 2011, 509, 2386–2391. [Google Scholar] [CrossRef]
- Ma, Z.; Liu, J.; Zhu, Y.; Zhao, Y.; Lin, H.; Zhang, Y.; Li, H.; Zhang, J.; Liu, Y.; Gao, W. Crystal-facet-dependent catalysis of anatase TiO2 on hydrogen storage of MgH2. J. Alloys Compd. 2020, 822, 153553. [Google Scholar] [CrossRef]
- Gupta, R.; Agresti, F.; Russo, S.L.; Maddalena, A.; Palade, P.; Principi, G. Structure and hydrogen storage properties of MgH2 catalysed with La2O3. J. Alloys Compd. 2008, 450, 310–313. [Google Scholar] [CrossRef]
- El-Eskandarany, M.S.; Alkandary, A.; Aldakheel, F.; Al-Saidi, M.; Al-Ajmi, F.; Banyan, M. Performance and fuel cell applications of reacted ball-milled MgH2/5.3 wt.% TiH2 nanocomposite powders. RSC Adv. 2018, 8, 38175–38185. [Google Scholar] [CrossRef] [Green Version]
- Song, J.Z.; Zhao, Z.Y.; Zhao, X.; Fu, R.D.; Han, S.M. Hydrogen storage properties of MgH2 Co-catalyzed by LaH3 and NbH. Int. J. Miner. Metall. Mater. 2017, 24, 1183–1191. [Google Scholar] [CrossRef] [Green Version]
- Ranjbar, A.; Guo, Z.; Yu, X.; Wexler, D.; Calka, A.; Kim, C.; Liu, H.-K. Hydrogen storage properties of MgH2–SiC composites. Mater. Chem. Phys. 2009, 114, 168–172. [Google Scholar] [CrossRef]
- El-Eskandarany, M.S.; Shaban, E.; Alsairafi, A.A. Synergistic dosing effect of TiC/FeCr nanocatalysts on the hydrogenation/dehydrogenation kinetics of nanocrystalline MgH2 powders. Energy 2016, 104, 158–170. [Google Scholar] [CrossRef]
- Pandyan, R.K.; Seenithurai, S.; Mahendran, M. Hydrogen storage in MgH2 coated single walled carbon nanotubes. Int. J. Hydrogen Energy 2011, 36, 3007–3015. [Google Scholar] [CrossRef]
- Singh, M.K.; Bhatnagar, A.; Pandey, S.K.; Mishra, P.; Srivastava, O. Experimental and first principle studies on hydrogen desorption behavior of graphene nanofiber catalyzed MgH2. Int. J. Hydrogen Energy 2017, 42, 960–968. [Google Scholar] [CrossRef]
- El-Eskandarany, M.S.; Ali, N.; Banyan, M.; Al-Ajmi, F. Cold gas-dynamic spray for catalyzation of plastically deformed Mg-strips with Ni powder. Nanomaterials 2021, 11, 1169. [Google Scholar] [CrossRef] [PubMed]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Eskandarany, M.S.; Ali, N.; Al-Ajmi, F.; Banyan, M. Effect of ZrC Nanopowders on Enhancing the Hydro/Dehydrogenation Kinetics of MgH2 Powders. Molecules 2021, 26, 4962. https://doi.org/10.3390/molecules26164962
El-Eskandarany MS, Ali N, Al-Ajmi F, Banyan M. Effect of ZrC Nanopowders on Enhancing the Hydro/Dehydrogenation Kinetics of MgH2 Powders. Molecules. 2021; 26(16):4962. https://doi.org/10.3390/molecules26164962
Chicago/Turabian StyleEl-Eskandarany, Mohamed Sherif, Naser Ali, Fahad Al-Ajmi, and Mohammad Banyan. 2021. "Effect of ZrC Nanopowders on Enhancing the Hydro/Dehydrogenation Kinetics of MgH2 Powders" Molecules 26, no. 16: 4962. https://doi.org/10.3390/molecules26164962






