Low Energy Electron Attachment by Some Chlorosilanes
Abstract
:1. Introduction
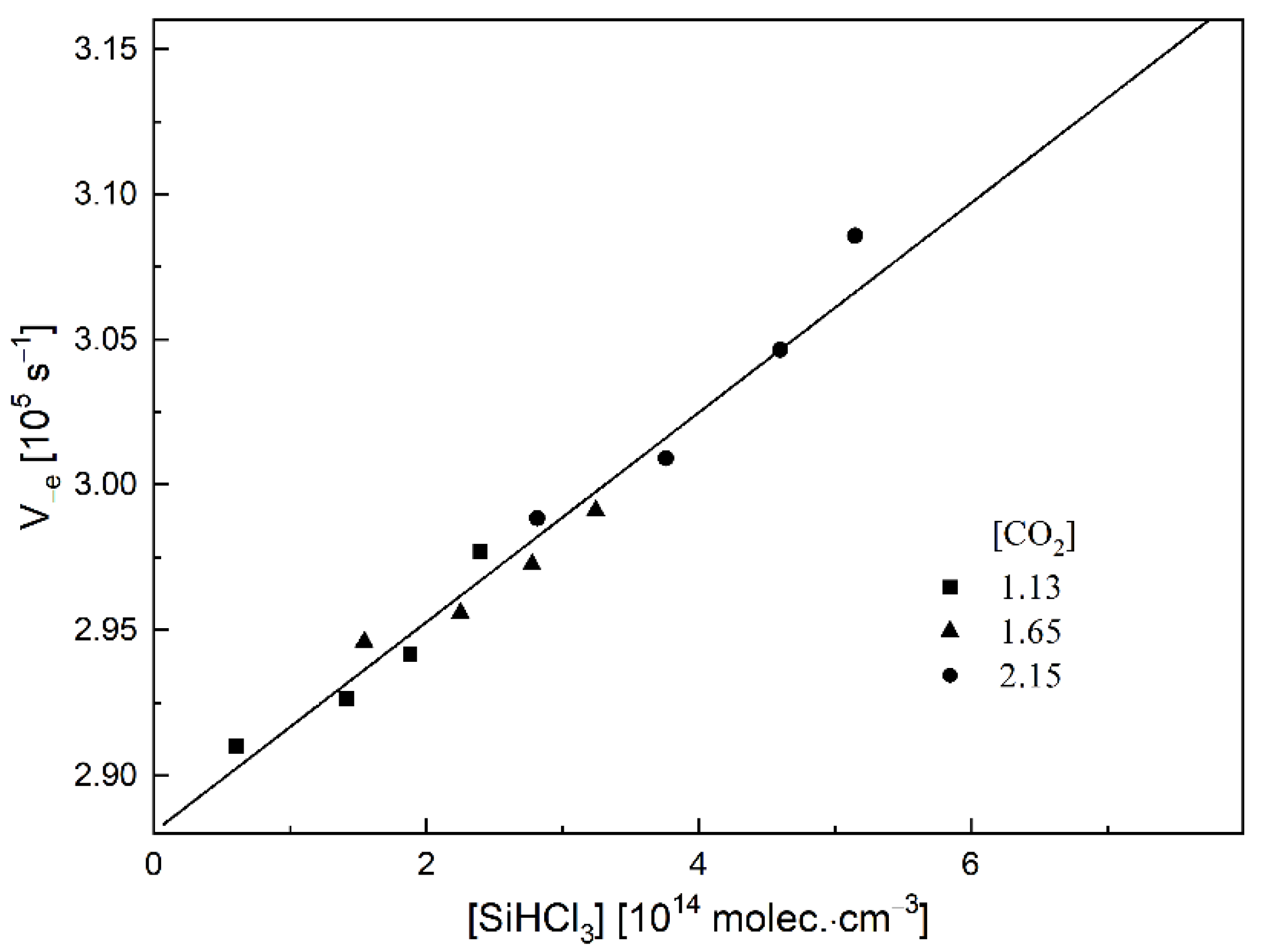
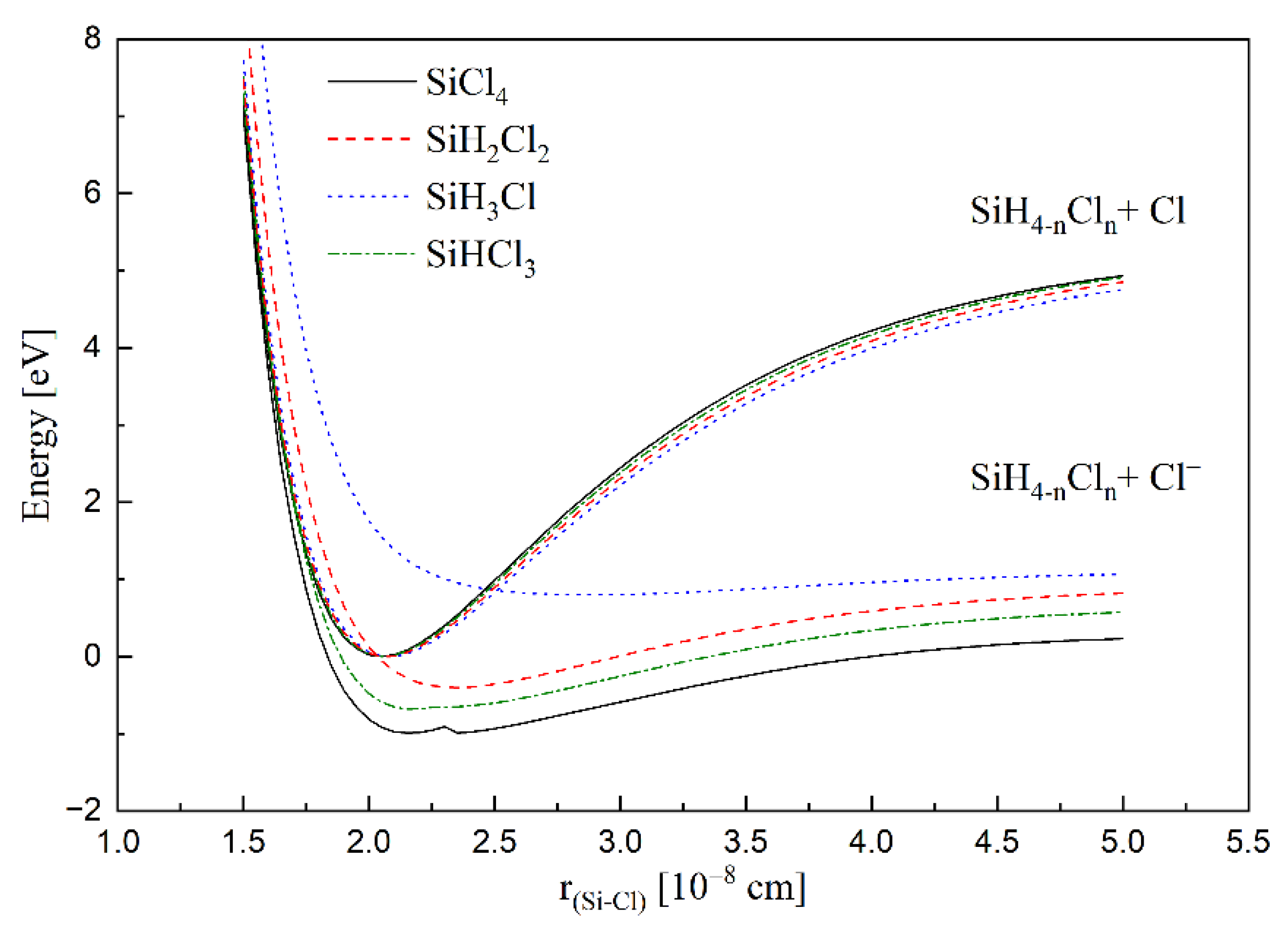
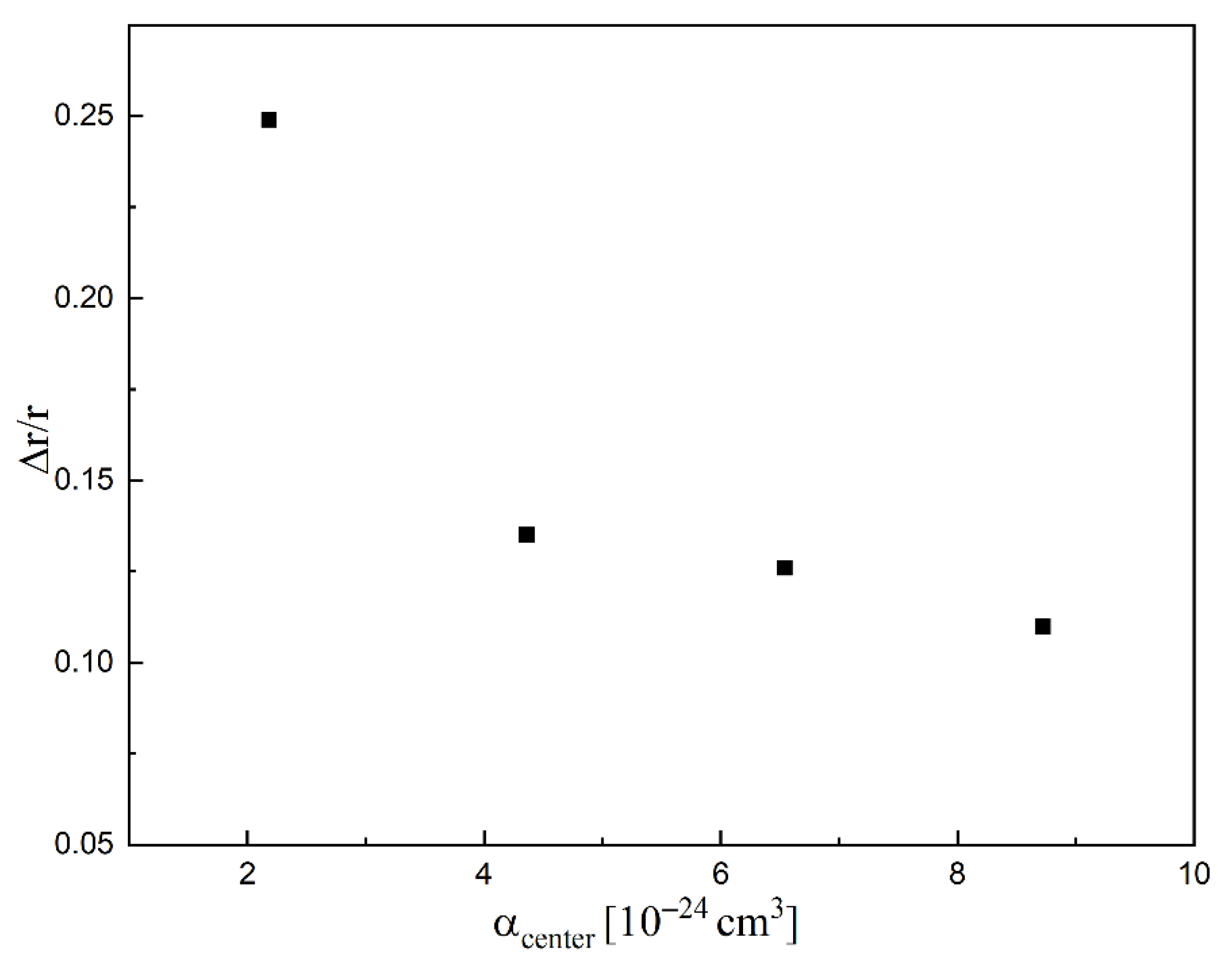
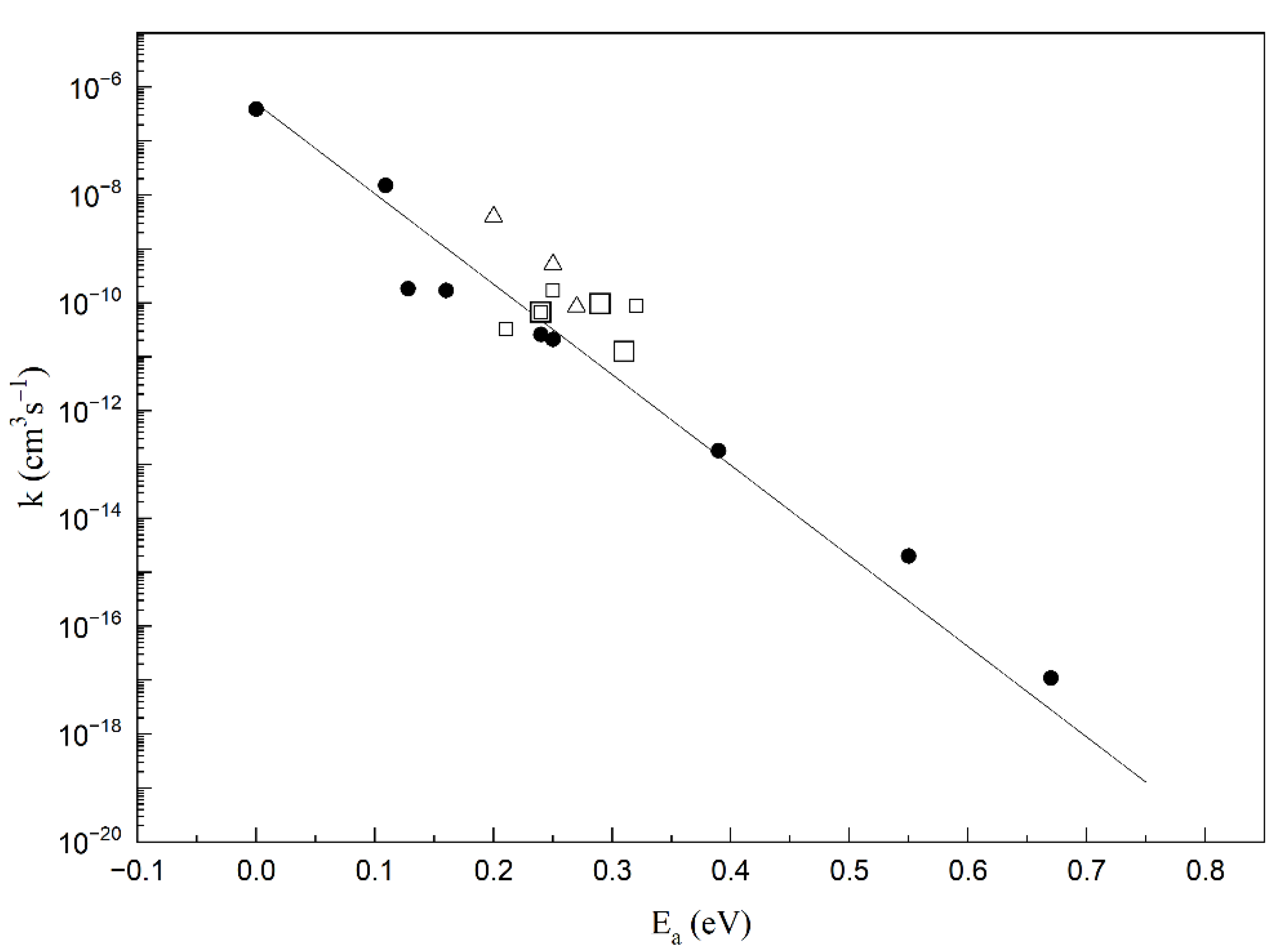
2. Results and Discussion
3. Materials and Methods
3.1. Chemicals
3.2. The Swarm Technique
3.3. Calculations
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Christophorou, L.G. (Ed.) Electron-Molecule Interactions and Their Applications; Academic Press: New York, NY, USA, 1984; ISBN 978-0-12-174401-4. [Google Scholar]
- Illenberger, E.; Momigny, J. Gaseous Molecular Ions; Topics in Physical Chemistry; Steinkopff: Heidelberg, Germany, 1992; Volume 2, ISBN 978-3-662-07385-8. [Google Scholar]
- National Research Council. Database Needs for Modeling and Simulation of Plasma Processing; The National Academies Press: Washington, DC, USA, 1996; ISBN 978-0-309-05591-8. [Google Scholar]
- Mason, N.J. Electron Driven Processes: Scientific Challenges and Technological Opportunities. In Electron Scattering; Whelan, C.T., Mason, N.J., Eds.; Springer: Boston, MA, USA, 2005; pp. 179–190. ISBN 978-0-306-48701-9. [Google Scholar]
- Abdoul-Carime, H.; Huels, M.A.; Illenberger, E.; Sanche, L. Formation of negative ions from gas phase halo-uracils by low-energy (0–18 eV) electron impact. Int. J. Mass Spectrom. 2003, 228, 703–716. [Google Scholar] [CrossRef]
- Boudaïffa, B.; Cloutier, P.; Hunting, D.; Huels, M.A.; Sanche, L. Resonant Formation of DNA Strand Breaks by Low-Energy (3 to 20 eV) Electrons. Science 2000, 287, 1658–1660. [Google Scholar] [CrossRef]
- Mandal, S.K.; Roesky, H.W. Interstellar molecules: Guides for new chemistry. Chem. Commun. 2010, 46, 6016–6041. [Google Scholar] [CrossRef]
- National Research Council. Plasma Science: Advancing Knowledge in the National Interest; The National Academies Press: Washington, DC, USA, 2007; ISBN 978-0-309-10943-7. [Google Scholar]
- Martin, F.; Burrow, P.D.; Cai, Z.; Cloutier, P.; Hunting, D.; Sanche, L. DNA Strand Breaks Induced by 0–4 eV Electrons: The Role of Shape Resonances. Phys. Rev. Lett. 2004, 93, 068101. [Google Scholar] [CrossRef] [Green Version]
- Lieberman, M.A. The Langmuir isotherm and the standard model of ion-assisted etching. Plasma Sources Sci. Technol. 2008, 18. [Google Scholar] [CrossRef]
- Lieberman, M.A.; Lichtenberg, A.J. Principles of Plasma Discharges and Materials Processing: Lieberman/Plasma, 2nd ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2005; ISBN 978-0-471-72425-4. [Google Scholar]
- Chen, F.F. Industrial applications of low-temperature plasma physics. Phys. Plasmas 1995, 2, 2164–2175. [Google Scholar] [CrossRef]
- Michalczuk, B.; Barszczewska, W. Kinetics of low energy electron attachment to some chlorosilanes in the gas phase. Chem. Phys. Lett. 2020, 740. [Google Scholar] [CrossRef]
- Michalczuk, B.; Barszczewska, W. Low-energy electron interactions with chlorotrimethylsilane (Si (CH3)3Cl), dichlorodimethylsilane (Si(CH3)2Cl2) and chloromethyldimethylsilane (SiH(CH3)2(CH2Cl)). Rapid Commun. Mass Spectrom. 2021, e9114. [Google Scholar] [CrossRef]
- Christophorou, L.G.; Olthoff, J.K. Fundamental Electron Interactions with Plasma Processing Gases; Physics of Atoms and Molecules; Springer US: Boston, MA, USA, 2004; ISBN 978-0-306-48037-9. [Google Scholar]
- Wilkerson, B.E.; Dillard, J.G. Resonant electron capture in silicon tetraisocyanate and silicon tetrachloride. J. Chem. Soc. D 1969, 212a. [Google Scholar] [CrossRef]
- Możejko, P.; Kasperski, G.; Szmytkowski, C.; Zecca, A.; Karwasz, G.; Del Longo, L.; Brusa, R. Absolute total cross-section measurements for electron scattering from silicon tetrachloride, SiCl. Eur. Phys. J. D 1999, 6, 481. [Google Scholar] [CrossRef]
- Wan, H.-X.; Moore, J.H.; Tossell, J.A. Electron attachment to the chlorosilanes and chloromethanes. J. Chem. Phys. 1991, 94, 1868–1874. [Google Scholar] [CrossRef]
- Ragesh Kumar, T.P.; Brynjarsson, B.; Ómarsson, B.; Hoshino, M.; Tanaka, H.; Limão-Vieira, P.; Jones, D.B.; Brunger, M.J.; Ingólfsson, O. Negative ion formation through dissociative electron attachment to the group IV tetrachlorides: Carbon tetrachloride, silicon tetrachloride and germanium tetrachloride. Int. J. Mass Spectrom. 2018, 426, 12–28. [Google Scholar] [CrossRef] [Green Version]
- Jäger, K.; Henglein, A. Die Bildung negativer Ionen aus SiCl4 und organischen Siliciumchloriden durch Elektronenstoß. Z. Nat. A 1968, 23, 1122–1127. [Google Scholar] [CrossRef]
- Wang, J.L.; Margrave, J.L.; Franklin, J.L. Interpretation of dissociative-electron attachment processes for silicon tetrachloride. J. Chem. Phys. 1974, 61, 1357. [Google Scholar] [CrossRef]
- Pabst, R.; Margrave, J.; Franklin, J. Electron impact studies of the tetrachlorides and tetrabromides of silicon and germanium. Int. J. Mass Spectrom. Ion Phys. 1977, 25, 361–374. [Google Scholar] [CrossRef]
- Moylan, C.R.; Green, S.B.; Brauman, J.I. Electron attachment chemistry of SiCl4. Relevance to plasma reactions. Int. J. Mass Spectrom. Ion Process. 1990, 96, 299–307. [Google Scholar] [CrossRef]
- Böhler, E.; Postler, J.; Gschliesser, D.; Borrmann, T.; Scheier, P.; Denifl, S.; Beckmann, J.; Swiderek, P. Electron-induced dissociation of chlorosilanes: Role of aromatic side groups in gas phase and solution chemistry. Int. J. Mass Spectrom. 2014, 365-366, 169–176. [Google Scholar] [CrossRef]
- Kočišek, J.; Illenberger, E.; Matejčík, S. Dissociative electron attachment to the silane derivatives trichlorovinylsilane (SiCl3C2H3), tetravinylsilane (Si(C2H3)4) and trimethylvinylsilane (Si(CH3)3C2H3). Int. J. Mass Spectrom. 2012, 315, 40–45. [Google Scholar] [CrossRef]
- Bjarnason, E.H.; Ómarsson, B.; Jónsdóttir, N.R.; Árnason, I.; Ingólfsson, O. Dissociative electron attachment and dissociative ionization of 1,1-dichloro-1-silacyclohexane and silacyclohexane. Int. J. Mass Spectrom. 2014, 370, 39–43. [Google Scholar] [CrossRef]
- Fabrikant, I.I.; Hotop, H. On the validity of the Arrhenius equation for electron attachment rate coefficients. J. Chem. Phys. 2008, 128, 124308. [Google Scholar] [CrossRef] [Green Version]
- Miller, T.M.; Friedman, J.F.; Schaffer, L.C.; Viggiano, A.A. Electron Attachment to Halomethanes at High Temperature: CH2Cl2, CF2Cl2, CH3Cl, and CF3Cl Attachment Rate Constants up to 1100 K. J. Chem. Phys. 2009, 131, 84302. [Google Scholar] [CrossRef]
- Kopyra, J.; Szamrej, I.; Graupner, K.; Graham, L.; Field, T.; Sulzer, P.; Denifl, S.; Märk, T.; Scheier, P.; Fabrikant, I.; et al. Low-energy electron attachment to chloroform (CHCl3) molecules: A joint experimental and theoretical study. Int. J. Mass Spectrom. 2008, 277, 130–141. [Google Scholar] [CrossRef]
- Smith, D.; Adams, N.G.; Alge, E. Attachment Coefficients for the Reactions of Electrons with CCl4, CCl3F, CCl2F2, CHCl3, Cl2 and SF6 Determined between 200 and 600 K Using the FALP Technique. J. Phys. B At. Mol. Phys. 1984, 17, 461–472. [Google Scholar] [CrossRef]
- Rosa, A.; Barszczewska, W.; Foryś, M.; Szamrej, I. Electron capture by haloethanes in a carbon dioxide buffer gas. Int. J. Mass Spectrom. 2001, 205, 85–92. [Google Scholar] [CrossRef]
- Chen, E.C.M.; Chen, E.S.D. The Electron Capture Detector and the Study of Reactions with Thermal Electrons; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2004; ISBN 978-0-471-65989-1. [Google Scholar]
- Christophorou, L.G. The Dependence of the Thermal Electron Attachment Rate Constant in Gases and Liquids on the Energy Position of the Electron Attaching State. Zeitschrift für Physikalische Chemie 1996, 195, 195–215. [Google Scholar] [CrossRef]
- Smith, D.; Herd, C.; Adams, N. Studies of dissociative electron attachment to some haloethanes using the falp apparatus: Comparisons with data obtained using non-thermal techniques. Int. J. Mass Spectrom. Ion Process. 1989, 93, 15–22. [Google Scholar] [CrossRef]
- Barszczewska, W.; Kopyra, J.; Wnorowska, J.; Szamrej, I. Low-Energy Electron Attachment by Chloroalkanes. J. Phys. Chem. A 2003, 107, 11427–11432. [Google Scholar] [CrossRef]
- O’Malley, T.F. Theory of Dissociative Attachment. Phys. Rev. 1966, 150, 14–29. [Google Scholar] [CrossRef]
- Illenberger, E.; Baumgärtel, H.; Süzer, S. Electron-attachment spectroscopy: Formation and dissociation of negative ions in the fluorochloroethylenes. J. Electron Spectrosc. Relat. Phenom. 1984, 33, 123–139. [Google Scholar] [CrossRef]
- Olthoff, J.K.; Tossell, J.A.; Moore, J.H. Electron attachment by haloalkenes and halobenzenes. J. Chem. Phys. 1985, 83, 5627–5634. [Google Scholar] [CrossRef]
- Johnson, J.P.; Christophorou, L.G.; Carter, J.G. Fragmentation of aliphatic chlorocarbons under low-energy (≲10 eV) electron impact. J. Chem. Phys. 1977, 67, 2196. [Google Scholar] [CrossRef]
- Dispert, H.; Lacmann, K. Negative ion formation in collisions between potassium and fluoro- and chloromethanes: Electron affinities and bond dissociation energies. Int. J. Mass Spectrom. Ion Phys. 1978, 28, 49–67. [Google Scholar] [CrossRef]
- Gaines, A.F.; Kay, J.; Page, F.M. Determination of electron affinities. Part 8.—Carbon tetrachloride, chloroform and hexachloroethane. Trans. Faraday Soc. 1966, 62, 874–880. [Google Scholar] [CrossRef]
- Luo, Y.-R. Comprehensive Handbook of Chemical Bond Energies; CRC Press: New York, NY, USA, 2007. [Google Scholar]
- Foryś, M.; Szamrej, I. The dependence of electron capture rate constants on some molecular parameters. J. Radioanal. Nucl. Chem. 1998, 232, 67–69. [Google Scholar] [CrossRef]
- Pezler, B.; Szamrej, I. Geometry and energy changes in halomethanes due to electron capture. Res. Chem. Intermed. 2001, 27, 787–794. [Google Scholar] [CrossRef]
- Barszczewska, W.; Rosa, A.; Kopyra, J.; Szamrej, I. Electron attachment processes in gas mixtures containing haloethanes. Res. Chem. Intermed. 2001, 27, 699–707. [Google Scholar] [CrossRef]
- Kopyra, J.; Barszczewska, W.; Wnorowska, J.; Foryś, M.; Szamrej, I. Low Energy electron interaction with haloethanes. Acta Physica Slovaca 2005, 55, 447–451. [Google Scholar]
- Dashevskaya, E.I.; Litvin, I.; Nikitin, E.E.; Troe, J. Modelling low-energy electron–molecule capture processes. Phys. Chem. Chem. Phys. 2007, 10, 1270–1276. [Google Scholar] [CrossRef] [Green Version]
- Vogt, E.; Wannier, G.H. Scattering of Ions by Polarization Forces. Phys. Rev. 1954, 95, 1190–1198. [Google Scholar] [CrossRef]
- Fabrikant, I.I.; Hotop, H. Low-energy behavior of exothermic dissociative electron attachment. Phys. Rev. A 2001, 63, 022706. [Google Scholar] [CrossRef]
- Klar, D.; Ruf, M.-W.; Fabrikant, I.I.; Hotop, H. Dissociative electron attachment to dipolar molecules at low energies with meV resolution: CFCl3, 1,1,1-C2Cl3F3, and HI. J. Phys. B: At. Mol. Opt. Phys. 2001, 34, 3855–3878. [Google Scholar] [CrossRef]
- O’Reilly, W. Numerical Data and Functional Relationships in Science and Technology. Phys. Earth Planet. Inter. 1987, 45, 304–305. [Google Scholar] [CrossRef]
- Barszczewska, W.; Kopyra, J.; Wnorowska, J.; Foryś, M.; Szamrej, I.; Asfandiarov, N.; Pshenichnyuk, S.; Fal’Ko, V. Thermal electron capture by some halopropanes. Radiat. Phys. Chem. 2007, 76, 1017–1025. [Google Scholar] [CrossRef]
- Aflatooni, K.; Gallup, G.; Burrow, P. Dissociative electron attachment in chloroalkanes and the correlation with vertical attachment energies. Chem. Phys. Lett. 1998, 282, 398–402. [Google Scholar] [CrossRef]
- Modelli, A.; Guerra, M.; Jones, D.; Distefano, G.; Tronc, M. Low-energy electron capture in group 14 methyl chlorides and tetrachlorides: Electron transmission and dissociative electron attachment spectra and MS-Xα calculations. J. Chem. Phys. 1998, 108, 9004–9015. [Google Scholar] [CrossRef]
- Kopyra, J.; Wnorowska, J.; Foryš, M.; Szamrej, I. A new apparatus for measuring rate constants and activation energies of thermal electron capture processes in the gas phase. Int. J. Mass Spectrom. 2007, 268, 60–65. [Google Scholar] [CrossRef]
- Wnorowski, K.; Wnorowska, J.; Michalczuk, B.; Jówko, A.; Barszczewska, W. The influence of the temperature on electron attachment to some Br-substituted alkanes. Chem. Phys. Lett. 2013, 586, 29–33. [Google Scholar] [CrossRef]
- Orient, O.J.; Chutjian, A.; Crompton, R.W.; Cheung, B. Comparison of experimental and calculated attachment rate constants forCFCl3andCCl4in the temperature range 294–500 K. Phys. Rev. A 1989, 39, 4494–4501. [Google Scholar] [CrossRef] [PubMed]
- Wnorowski, K.; Wnorowska, J.; Michalczuk, B.; Pshenichnyuk, S.; Nafikova, E.; Asfandiarov, N.; Barszczewska, W. Electron attachment to chlorinated alcohols. Chem. Phys. Lett. 2015, 634, 203–209. [Google Scholar] [CrossRef]
- Wnorowski, K.; Wnorowska, J.; Kopyra, J.; Michalczuk, B.; Szamrej, I.; Barszczewska, W. Kinetics of low energy electron attachment to some fluorinated alcohols in the gas phase. Chem. Phys. Lett. 2014, 591, 282–286. [Google Scholar] [CrossRef]
- Kopyra, J.; Wnorowska, J.; Barszczewska, W.; Karolczak, S.; Szamrej, I. On the kinetics of thermal electron attachment to perfluoroethers. Chem. Phys. Lett. 2012, 519-520, 25–28. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision C.01; Gaussian, Inc.: Wallingford, CT, USA, 2019. [Google Scholar]
- Becke, A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef] [Green Version]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef] [Green Version]




| Molecules | k298 (cm3·s−1) | Ea (eV) | Trange (K) | |
|---|---|---|---|---|
| Chlorinated silanes (present data) | SiHCl3 | (5.18 ± 0.22) × 10−10 | 0.25 ± 0.01 | 298–368 |
| SiCl4 | (3.98 ± 1.8) × 10−9 | 0.20 ± 0.01 | 298–368 | |
| Si(CH3)2(CH2Cl)Cl | (8.46 ± 0.23) × 10−11 | 0.27 ± 0.01 | 298–378 | |
| Chlorinated silanes | SiH(CH3)2Cl | (6.72 ± 0.08) × 10−11 [13] | 0.24 ± 0.01 [13] | 298–368 |
| SiHCH3Cl2 | (16.80 ± 0.06) × 10−11 [13] | 0.25 ± 0.10 [13] | 298–378 | |
| SiCH3Cl3 | (8.72 ± 0.07) × 10−11 [13] | 0.32 ± 0.02 [13] | 298–378 | |
| Si(C2H5)3Cl | (3.27 ± 0.02) × 10−11 [13] | 0.21 ± 0.01 [13] | 298–378 | |
| Si(CH3)3Cl | (9.56 ± 0.02) × 10−11 [14] | 0.29 ± 0.01 [14] | 298–368 | |
| Si(CH3)2Cl2 | (6.62 ± 0.02) × 10−11 [14] | 0.24 ± 0.01 [14] | 298–368 | |
| SiH(CH3)2(CH2Cl) | (1.24 ± 0.05) × 10−11 [14] | 0.31 ± 0.01 [14] | 298–378 | |
| Chlorinated alkanes | CH3Cl | 1.1 × 10−17 (300 K) [28] | 0.67 [28] | 700–1100 |
| CH2Cl2 | 1.8×10−13 (300 K) [28] | 0.39 [28] | 495–973 | |
| CHCl3 | 3.7 × 10−9 [29] | 0.11 [29] | 295–373 | |
| CCl4 | 3.9 × 10−7 (300 K) [30] | 0.00 [30] | 205–590 | |
| CH3CH2Cl | 3.4 × 10−14 [31] | - | 298 | |
| CH2ClCH2Cl | 2.6 × 10−11 [31] | 0.24 [32] | 298–463 | |
| CHCl2CH3 | 2.1 × 10−11 [33] | 0.25 [32] | 298–463 | |
| CH3CCl3 | 1.5 × 10−8 [34] | 0.11 [34] | 298–470 | |
| CHCl2CH2Cl | 1.8 × 10−10 [34] | 0.13 [34] | 298–470 | |
| CHCl2CHCl2 | 3.5 × 10−8 [35] | - | 298 | |
| CH2ClCCl3 | 3.1 × 10−7 [35] | - | 298 | |
| Compound | r(Si-Cl) [10−8 cm] | rn(Si-Cl) [10−8 cm] | Δr/r 1 | AEA [eV] | αcenter [10−24 cm3] | ∠HSiCl [°] | ∠nHSiCl [°] | Δ∠/∠ 2 |
|---|---|---|---|---|---|---|---|---|
| SiH3Cl | 2.079 | 2.596 | 0.249 | −0.80 | 2.18 | 108.63 | 157.36 | 0.45 |
| SiH2Cl2 | 2.066 | 2.344 | 0.135 | 0.40 | 4.36 | 108.28 | 95.22 | 0.12 |
| SiHCl3 | 2.055 | 2.314 | 0.126 | 0.68 | 6.54 | 109.71 | 114.32 | 0.04 |
| SiCl4 | 2.047 | 2.273 | 0.110 | 0.99 | 8.72 | 109.47 | 109.47 | 0.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Michalczuk, B.; Barszczewska, W.; Wysocki, W.; Matejčík, Š. Low Energy Electron Attachment by Some Chlorosilanes. Molecules 2021, 26, 4973. https://doi.org/10.3390/molecules26164973
Michalczuk B, Barszczewska W, Wysocki W, Matejčík Š. Low Energy Electron Attachment by Some Chlorosilanes. Molecules. 2021; 26(16):4973. https://doi.org/10.3390/molecules26164973
Chicago/Turabian StyleMichalczuk, Bartosz, Wiesława Barszczewska, Waldemar Wysocki, and Štefan Matejčík. 2021. "Low Energy Electron Attachment by Some Chlorosilanes" Molecules 26, no. 16: 4973. https://doi.org/10.3390/molecules26164973






