Fluorescent Bis-Calix[4]arene-Carbazole Conjugates: Synthesis and Inclusion Complexation Studies with Fullerenes C60 and C70
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis and Structural Characterization
2.2. Photophysical Properties
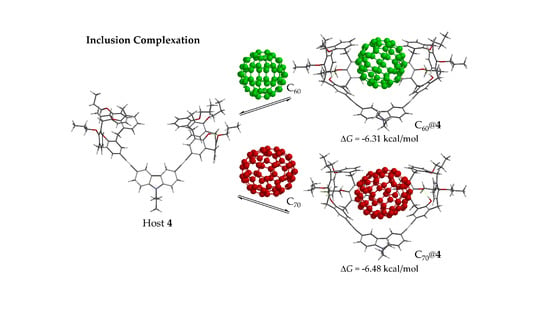
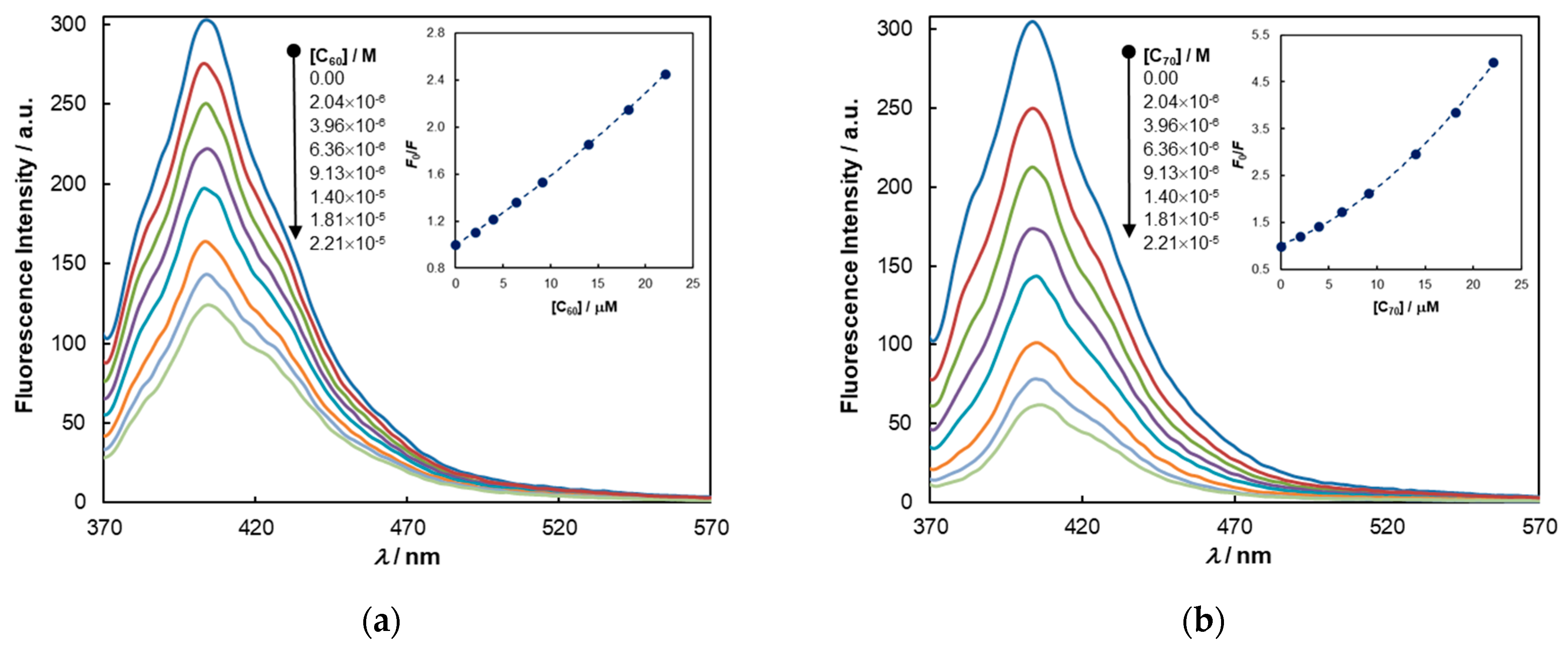
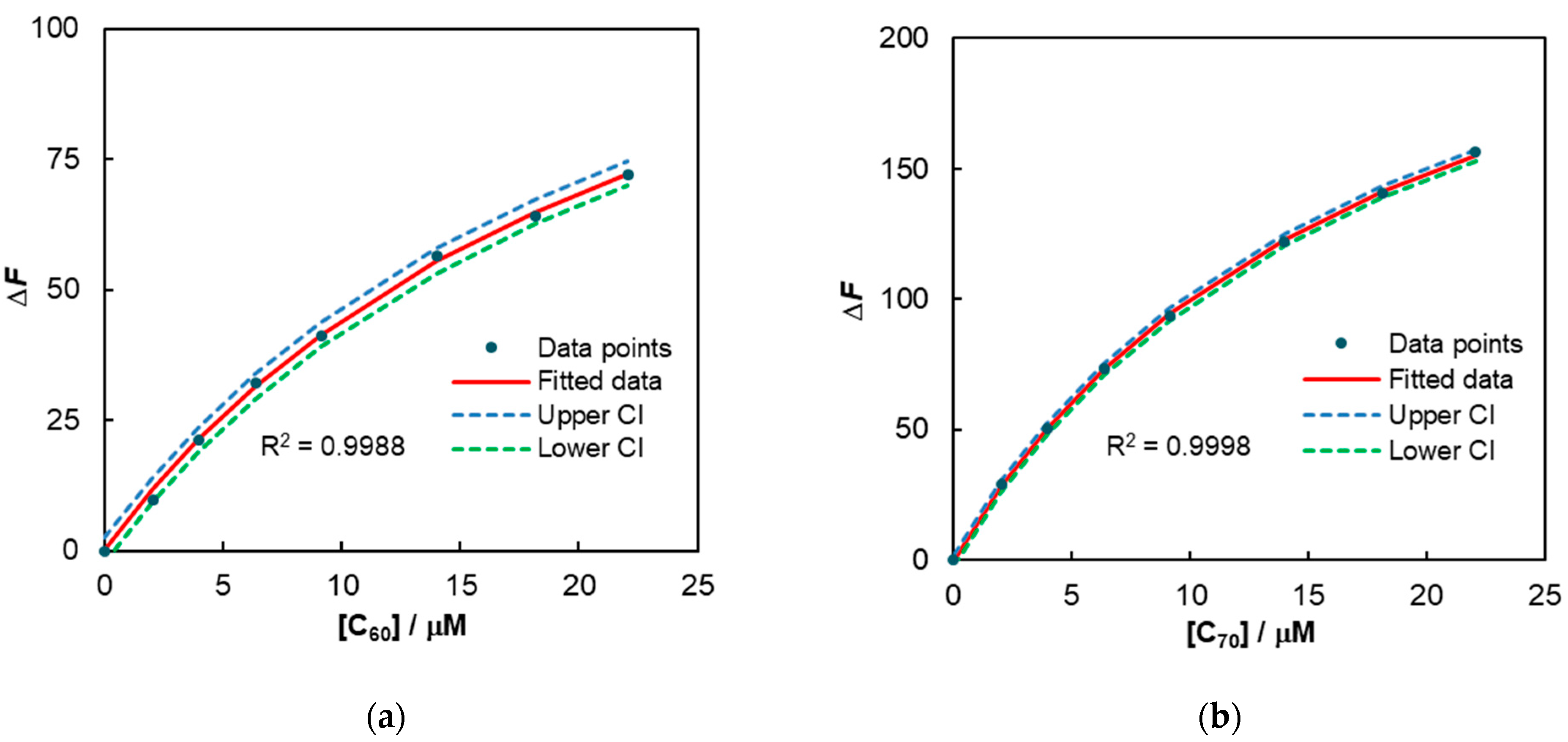
2.3. Complexation Studies with Fullerenes
3. Materials and Methods
3.1. Instruments and Methods
3.2. Materials
3.3. Synthesis
3.4. Computational Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Amabilino, D.B.; Gale, P.A. Supramolecular chemistry anniversary. Chem. Soc. Rev. 2017, 46, 2376–2377. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Zhao, Y. Biomedical applications of supramolecular systems based on Host−guest interactions. Chem. Rev. 2015, 115, 7794–7839. [Google Scholar] [CrossRef] [PubMed]
- Wagner, B.D. Hydrogen bonding of excited states in supramolecular host–guest inclusion complexes. Phys. Chem. Chem. Phys. 2012, 14, 8825–8835. [Google Scholar] [CrossRef]
- Steed, J.W.; Turner, D.R.; Wallace, K.J. Core Concepts in Supramolecular Chemistry and Nanochemistry; Wiley: Chichester, UK, 2007. [Google Scholar]
- Gutsche, C.D. Calixarenes-An Introduction. In Monographs in Supramolecular Chemistry; Stoddart, J.F., Ed.; The Royal Society of Chemistry: Cambridge, UK, 2008. [Google Scholar]
- Kumar, R.; Sharma, A.; Singh, H.; Suating, P.; Kim, H.S.; Sunwoo, K.; Shim, I.; Gibb, B.C.; Kim, J.S. Revisiting fluorescent calixarenes: From molecular sensors to smart materials. Chem. Rev. 2019, 119, 9657–9721. [Google Scholar] [CrossRef]
- Asfari, Z.; Böhmer, V.; Harrowfield, J.; Vicens, J. (Eds.) Synthesis of Calixarenes and Thiacalixarenes. In Calixarenes 2001; Kluwer Academic: Dordrecht, The Netherlands, 2001; pp. 1–25. [Google Scholar] [CrossRef]
- Loh, X. Supramolecular host–guest polymeric materials for biomedical applications. J. Mater. Horiz. 2014, 1, 185–195. [Google Scholar] [CrossRef]
- Ogoshi, T.; Yamagishi, T.-A.; Nakamoto, Y. Pillar-shaped macrocyclic hosts pillar[n]arenes: New key players for supramolecular chemistry. Chem. Rev. 2016, 116, 7937–8002. [Google Scholar] [CrossRef]
- Challa, R.; Ahuja, A.; Ali, J.; Khar, R.K. Cyclodextrins in drug delivery: An updated review. AAPS Pharm. Sci. Tech. 2005, 6, 43. [Google Scholar] [CrossRef]
- Zhang, W.; Gong, X.; Liu, C.; Piao, Y.; Sun, Y.; Diao, G. Water-soluble inclusion complex of fullerene with γ-cyclodextrin polymer for photodynamic therapy. J. Mater. Chem. B 2014, 2, 5107–5115. [Google Scholar] [CrossRef]
- Wang, L.; Li, L.-l.; Fan, Y.-S.; Wang, H. Host–guest supramolecular nanosystems for cancer diagnostics and therapeutics. Adv. Mater. 2013, 25, 3888–3898. [Google Scholar] [CrossRef]
- Uzunova, V.D.; Cullinane, C.; Brix, K.; Nau, W.M.; Day, A.I. Toxicity of cucurbit[7]uril and cucurbit[8]uril: An exploratory in vitro and in vivo study. Org. Biomol. Chem. 2010, 8, 2037–2042. [Google Scholar] [CrossRef]
- Barata, P.D.; Prata, J.V. Fluorescent Calix[4]arene-Carbazole-Containing Polymers as Sensors for Nitroaromatic Explosives. Chemosensors 2020, 8, 128. [Google Scholar] [CrossRef]
- Prata, J.V.; Costa, A.I.; Teixeira, C.M. A solid-state fluorescence sensor for nitroaromatics and nitroanilines based on a conjugated calix[4]arene polymer. J. Fluoresc. 2020, 30, 41–50. [Google Scholar] [CrossRef]
- Prata, J.V.; Barata, P.D. Fostering protein–calixarene interactions: From molecular recognition to sensing. RSC Adv. 2016, 6, 1659–1669. [Google Scholar] [CrossRef] [Green Version]
- Costa, A.I.; Barata, P.D.; Fialho, C.B.; Prata, J.V. Highly sensitive and selective fluorescent probes for Cu(II) detection based on calix[4]arene-oxacyclophane architectures. Molecules 2020, 25, 2456. [Google Scholar] [CrossRef] [PubMed]
- Kroto, H.; Heath, J.; O’Brien, S.; Curl, R.F.; Smalley, R.E. C60: Buckminsterfullerene. Nature 1985, 318, 162–163. [Google Scholar] [CrossRef]
- Goodarzi, S.; Ros, T.D.; Conde, J.; Sefat, F.; Mozafari, M. Fullerene: Biomedical engineers get to revisit an old friend. Mat. Today 2017, 20, 460–480. [Google Scholar] [CrossRef] [Green Version]
- Patel, K.D.; Singh, R.K.; Kim, H.-W. Carbon-based nanomaterials as an emerging platform for theranostics. Mater. Horiz. 2019, 6, 434–469. [Google Scholar] [CrossRef]
- Zhong, Z.-L.; Ikeda, A.; Shinkai, S. Complexation of Fullerenes. In Calixarenes 2001; Asfari, Z., Bohmer, V., Harrowfield, J., Vicens, J., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2001; pp. 476–495. [Google Scholar]
- Cruz, J.L.D.; Nierengarten, J.F. Fullerenes and Calixarenes. In Calixarenes in the Nanoworld; Vicens, J., Harrowfield, J., Baklouti, L., Eds.; Springer: Dordrecht, The Netherlands, 2007; pp. 173–196. [Google Scholar] [CrossRef]
- Georghiou, P.E. Calixarenes and Fullerenes. In Calixarenes and Beyond; Neri, P., Sessler, J.L., Wang, M.-X., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 879–919. ISBN 978-3-319-31867-7. [Google Scholar]
- Kawase, T.; Kurata, H. Ball-, Bowl, and belt-shaped conjugated systems and their complexing abilities: exploration of the concave−convex π-π interaction. Chem. Rev. 2006, 106, 5250–5273. [Google Scholar] [CrossRef]
- Boyd, P.D.W.; Reed, C.A. Fullerene—Porphyrin Constructs. Acc. Chem. Res. 2005, 38, 235–242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, G.; Li, G. Preparation and biological activity of novel cucurbit[8]uril–fullerene complex. J. Photochem. Photobiol. B Biol. 2006, 85, 223–227. [Google Scholar] [CrossRef]
- Haino, T.; Yanase, M.; Fukunaga, C.; Fukazawa, Y. Fullerene encapsulation with calix[5]arenes. Tetrahedron 2006, 62, 2025–2035. [Google Scholar] [CrossRef]
- Kás, M.; Lang, K.; Stibor, I.; Lhoták, P. Novel fullerene receptors based on calixarene–porphyrin conjugates. Tetrahedron Lett. 2007, 48, 477–481. [Google Scholar] [CrossRef]
- Le, V.H.; Yanney, M.; McGuire, M.; Sygula, A.; Lewis, E.A. Thermodynamics of host—guest interactions between fullerenes and a buckycatcher. J. Phys. Chem. B 2014, 118, 11956–11964. [Google Scholar] [CrossRef] [PubMed]
- Haino, T.; Yanase, M.; Fukazawa, Y. Fullerenes enclosed in bridged calix[5]arenes. Angew. Chem. Int. Ed. 1998, 37, 997–998. [Google Scholar] [CrossRef]
- Haino, T.; Araki, H.; Fujiwara, Y.; Tanimoto, Y.; Fukazawa, Y. Fullerene sensors based on calix[5]arene. Chem Commun. 2002, 2148–2149. [Google Scholar] [CrossRef]
- Kawase, T.; Tanaka, K.; Seirai, Y.; Shiono, N.; Oda, M. Complexation of carbon nanorings with fullerenes: Supramolecular dynamics and structural tuning for a fullerene sensor. Angew. Chem. Int. Ed. 2003, 42, 5597–5600. [Google Scholar] [CrossRef]
- Campbell, K.; Zappas, A.; Bunz, U.; Thio, Y.; Bucknall, D.G. Fluorescence quenching of a poly(para-phenylene ethynylenes) by C60 fullerenes. J. Photochem. Photobiol. A Chem. 2012, 249, 41–46. [Google Scholar] [CrossRef]
- Wu, H.; Zhang, C.; Li, L.; Chao, J.; Han, Y.; Dong, C.; Guo, Y.; Shuang, S. Cyclotriveratrylene-carbazole cage for self-assembly of C₆₀. Talanta 2013, 106, 454–458. [Google Scholar] [CrossRef]
- Li, H.; Chen, Q.; Schönbeck, C.; Han, B.-H. Sugar-functionalized water-soluble pillar[5]arene and its host–guest interaction with fullerene. RSC Adv. 2015, 5, 19041–19047. [Google Scholar] [CrossRef]
- Iglesias-Sánchez, J.C.; Fragoso, A.; Mendoza, J.; Prados, P. Aryl—Aryl Linked Bi-5,5′-p-tert-butylcalix[4]arene Tweezer for Fullerene Complexation. Org. Lett. 2006, 8, 2571–2574. [Google Scholar] [CrossRef]
- Golan, A.; Goldberg, I.; Vigalok, A. Synthesis and C70 complexation studies of a fluorescent 5,5′-bi-p-tert-butylcalix[4]arene scaffold. Supramol. Chem. 2016, 28, 526–535. [Google Scholar] [CrossRef]
- Bovonsombat, P.; Leykajarakul, J.; Khan, C.; Pla-on, K.; Krause, M.M.; Khanthapura, P.; Ali, R.; Doowa, N. Regioselective iodination of phenol and analogues using N-iodosuccinimide and p-toluenesulfonic acid. Tetrahedron Lett. 2009, 50, 2664–2667. [Google Scholar] [CrossRef]
- Barata, P.D.; Costa, A.I.; Prata, J.V. Calix[4]arene-carbazole-containing polymers: Synthesis and properties. React. Funct. Polym. 2012, 72, 627–634. [Google Scholar] [CrossRef] [Green Version]
- Valeur, B.; Berberan-Santos, M.N. Molecular Fluorescence: Principles and Applications, 2nd ed.; Wiley-VCH: Weinheim, Germany, 2012; pp. 146–158. [Google Scholar]
- Stella, L.; Capodilupo, A.L.; Bietti, M. A reassessment of the association between azulene and [60]fullerene. Possible pitfalls in the determination of binding constants through fluorescence spectroscopy. Chem. Commun. 2008, 4744–4746. [Google Scholar] [CrossRef]
- Borissevitch, I.E. More about the inner filter effect: Corrections of Stern–Volmer fluorescence quenching constants are necessary at very low optical absorption of the quencher. J. Lumin. 1999, 81, 219–224. [Google Scholar] [CrossRef]
- Thordarson, P. Determining association constants from titration experiments in supramolecular chemistry. Chem. Soc. Rev. 2011, 40, 1305. [Google Scholar] [CrossRef]
- van de Weert, M.; Stella, L. Fluorescence quenching and ligand binding: A critical discussion of a popular methodology. J. Mol. Struct. 2011, 998, 144–150. [Google Scholar] [CrossRef]
- Spartan’18 Molecular Modeling Program-Parallel Suite; Version 1.4.5; Wavefunction, Inc.: Irvine, CA, USA, 2020.
- Wang, J.; Wang, D.; Miller, E.K.; Moses, D.; Bazan, G.C.; Heeger, A.J. Photoluminescence of water-soluble conjugated polymers: Origin of enhanced quenching by charge transfer. Molecules 2020, 33, 5153–5158. [Google Scholar] [CrossRef]
- Shao, Y.; Gan, Z.; Epifanovsky, E.; Gilbert, A.T.B.; Wormit, M.; Kussmann, J.; Lange, A.W.; Behn, A.; Deng, J.; Feng, X.; et al. Advances in molecular quantum chemistry contained in the Q-Chem 4 program package. Mol. Phys. 2015, 113, 184–215. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Truhlar, D.G. Computational characterization and modeling of buckyball tweezers: Density functional study of concave–convex π⋯π interactions. Phys. Chem. Chem. Phys. 2008, 10, 2813–2818. [Google Scholar] [CrossRef]
- Sure, R.; Grimme, S. Comprehensive benchmark of association (free) energies of realistic host−guest complexes. J. Chem. Theory Comput. 2015, 11, 3785–3801. [Google Scholar] [CrossRef]
- Mardirossian, N.; Head-Gordon, M. Thirty years of density functional theory in computational chemistry: An overview and extensive assessment of 200 density functionals. Mol. Phys. 2017, 115, 2315–2372. [Google Scholar] [CrossRef]
- Řezáč, J.; Hobza, P. Advanced corrections of hydrogen bonding and dispersion for semiempirical quantum mechanical methods. J. Chem. Theory Comput. 2012, 8, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Stewart, J.J.P. Stewart Computational Chemistry; Colorado Springs, CO, USA; http://OpenMOPAC.net/.
- Grimme, S. Supramolecular binding thermodynamics by dispersion-corrected density functional. Chem. Eur. J. 2012, 18, 9955–9964. [Google Scholar] [CrossRef] [PubMed]
- Graton, J.; Questel, J.-Y.L.; Legouin, B.; Uriac, P.; van de Weghe, P.; Jacquemin, D. A DFT-D evaluation of the complexation of a molecular tweezer with small aromatic molecules. Chem. Phys. Lett. 2012, 522, 11–16. [Google Scholar] [CrossRef]
- Camaioni, D.M.; Schwerdtfeger, C.A. Comment on “Accurate experimental values for the free energies of hydration of H+, OH-, and H3O+”. J. Phys. Chem. A 2005, 109, 10795–10797. [Google Scholar] [CrossRef]
- All NMR Spectra Were Post-Processed by MestReNova, Version: 14.1.2-25024; Mestrelab Research: Santiago de Compostela, Spain, 2020.
- Eaton, D.F. Reference materials for fluorescence measurement. Pure Appl. Chem. 1988, 60, 1107–1114. [Google Scholar] [CrossRef]
- A Guide to Recording Fluorescence Quantum Yields, Horiba Scientific. Available online: http://www.horiba.com/fileadmin/uploads/Scientific/Documents/Fluorescence/quantumyieldstrad.pdf (accessed on 2 March 2020).
- Liu, Y.; Han, B.-H.; Chen, Y.-T. Molecular recognition and complexation thermodynamics of dye guest molecules by modified cyclodextrins and calixarenesulfonates. J. Phys. Chem. B 2002, 106, 4678–4687. [Google Scholar] [CrossRef]
- Brown, A.M. A step-by-step guide to non-linear regression analysis of experimental data using a Microsoft Excel spreadsheet. Comput. Meth. Programs Biomed. 2001, 65, 191–200. [Google Scholar] [CrossRef]
- Dondoni, A.; Ghiglione, C.; Marra, A.; Scoponi, M. Synthesis of calix[4]arenylvinylene and calix[4]arenylphenylene oligomers by Stille and Suzuki cross-coupling reactions. J. Org. Chem. 1998, 63, 9535–9539. [Google Scholar] [CrossRef]
- Gutsche, C.D.; Iqbal, M. para-tert-Butylcalix[4]arene. Org. Synth. 1990, 68, 234–237. [Google Scholar] [CrossRef]
- Gutsche, C.D.; Lin, L.G. The Synthesis of Functionalized Calixarenes. Tetrahedron 1986, 42, 1633–1640. [Google Scholar] [CrossRef]
- Gunji, A.; Takahashi, K. Selective and Efficient Iodination of the p-Positions of Calix[4]arene Derivatives. Synth. Commun. 1998, 28, 3933–3941. [Google Scholar] [CrossRef]
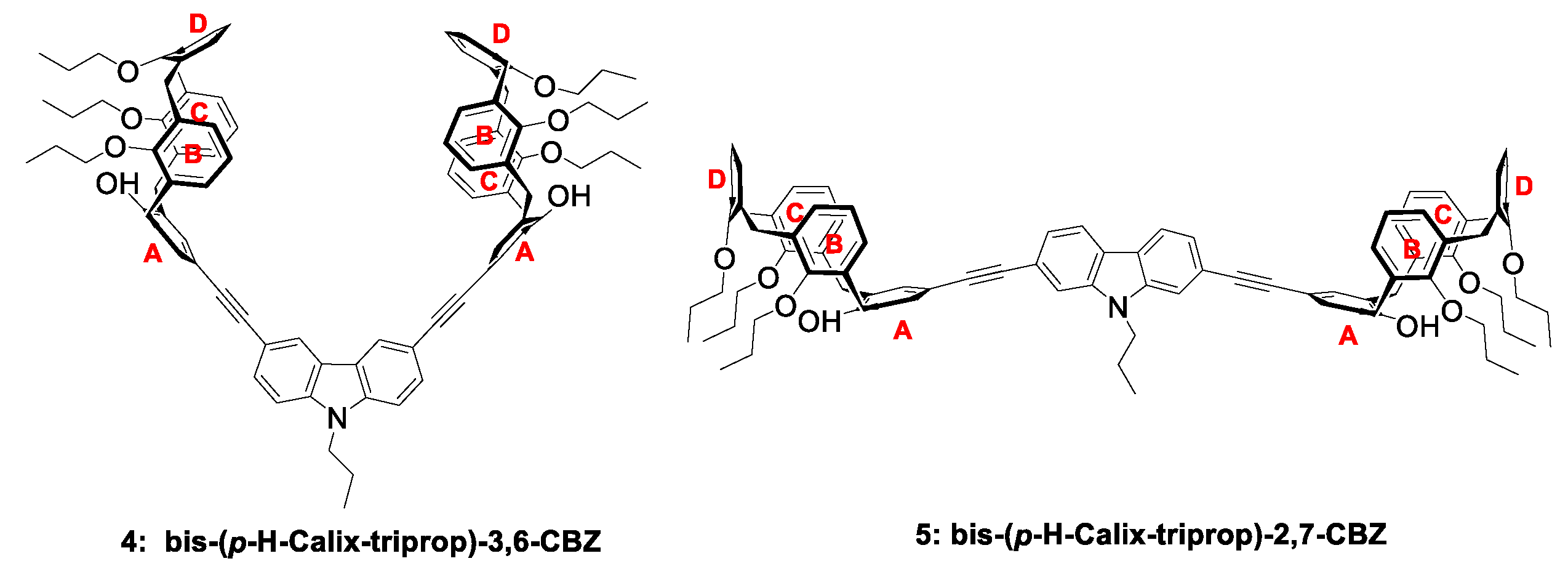







| Compound | λabs max/nm (logεmax) | Eg2/eV | λem max/nm | Stokes Shift 3/nm | ΦF4 |
|---|---|---|---|---|---|
| 4 | 319 (4.675) | 3.37 | 404 | 85 | 0.17 |
| 5 | 358 (4.621) | 3.08 | 394 | 36 | 0.40 |
| C60 | C70 | |||
|---|---|---|---|---|
| Host | Ka/M−1 | ΔG 1/kcal/mol | Ka/M−1 | ΔG 1/kcal/mol |
| 4 | 42,400 ± 3500 (R2 = 0.9988) | −6.31 | 55,800 ± 1800 (R2 = 0.9998) | −6.48 |
| 5 | 36,800 ± 1900 (R2 = 0.9996) | −6.23 | 44,000 ± 900 (R2 = 0.9999) | −6.33 |
| C60@4 | C70 @4 | |
|---|---|---|
| H Location | Δδ/ppm | Δδ/ppm |
| CBZ4,5 | 0.0020 | 0.0043 |
| CBZ2,7 | 0.0022 | 0.0066 |
| Calixring A | 0.0017 | 0.0036 |
| CBZ1,8 | 3 | −0.0056 |
| Calixrings B, C 4 | 0.0071 | 0.0091 |
| Calixring A-phenolic | 0.0099 | 0.0178 |
| Calixring D-OCH2CH2CH3 | −0.0040 | −0.0040 |
| CBZ-OCH2CH2CH3 | −0.0030 | −0.0040 |
| Calixring B, C-OCH2CH2CH3 | −0.0027 | −0.0034 |
| D3(BJ)-B3LYP Functional | B97M-V Functional | |
|---|---|---|
| Complex | ΔE/kcal/mol | ΔE/kcal/mol |
| C60@4 | −36.92 | −39.31 |
| C70@4 | −39.28 | −42.92 |
| ΔΔE | −2.36 | −3.60 |
| Complex | ΔE 1/ kcal/mol | ΔGRRHO 2/ kcal/mol | ΔG1M 3/ kcal/mol | ΔΔGsolv 4/ kcal/mol | ΔGth-sol/ kcal/mol |
|---|---|---|---|---|---|
| C60@4 | −39.31 | +29.57 | −11.64 | +5.74 | −5.9 |
| C70@4 | −42.92 | +32.09 | −12.73 | +6.00 | −6.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barata, P.D.; Costa, A.I.; Costa, S.; Prata, J.V. Fluorescent Bis-Calix[4]arene-Carbazole Conjugates: Synthesis and Inclusion Complexation Studies with Fullerenes C60 and C70. Molecules 2021, 26, 5000. https://doi.org/10.3390/molecules26165000
Barata PD, Costa AI, Costa S, Prata JV. Fluorescent Bis-Calix[4]arene-Carbazole Conjugates: Synthesis and Inclusion Complexation Studies with Fullerenes C60 and C70. Molecules. 2021; 26(16):5000. https://doi.org/10.3390/molecules26165000
Chicago/Turabian StyleBarata, Patrícia D., Alexandra I. Costa, Sérgio Costa, and José V. Prata. 2021. "Fluorescent Bis-Calix[4]arene-Carbazole Conjugates: Synthesis and Inclusion Complexation Studies with Fullerenes C60 and C70" Molecules 26, no. 16: 5000. https://doi.org/10.3390/molecules26165000