Nanoscale Wetting of Single Viruses
Abstract
:1. Introduction
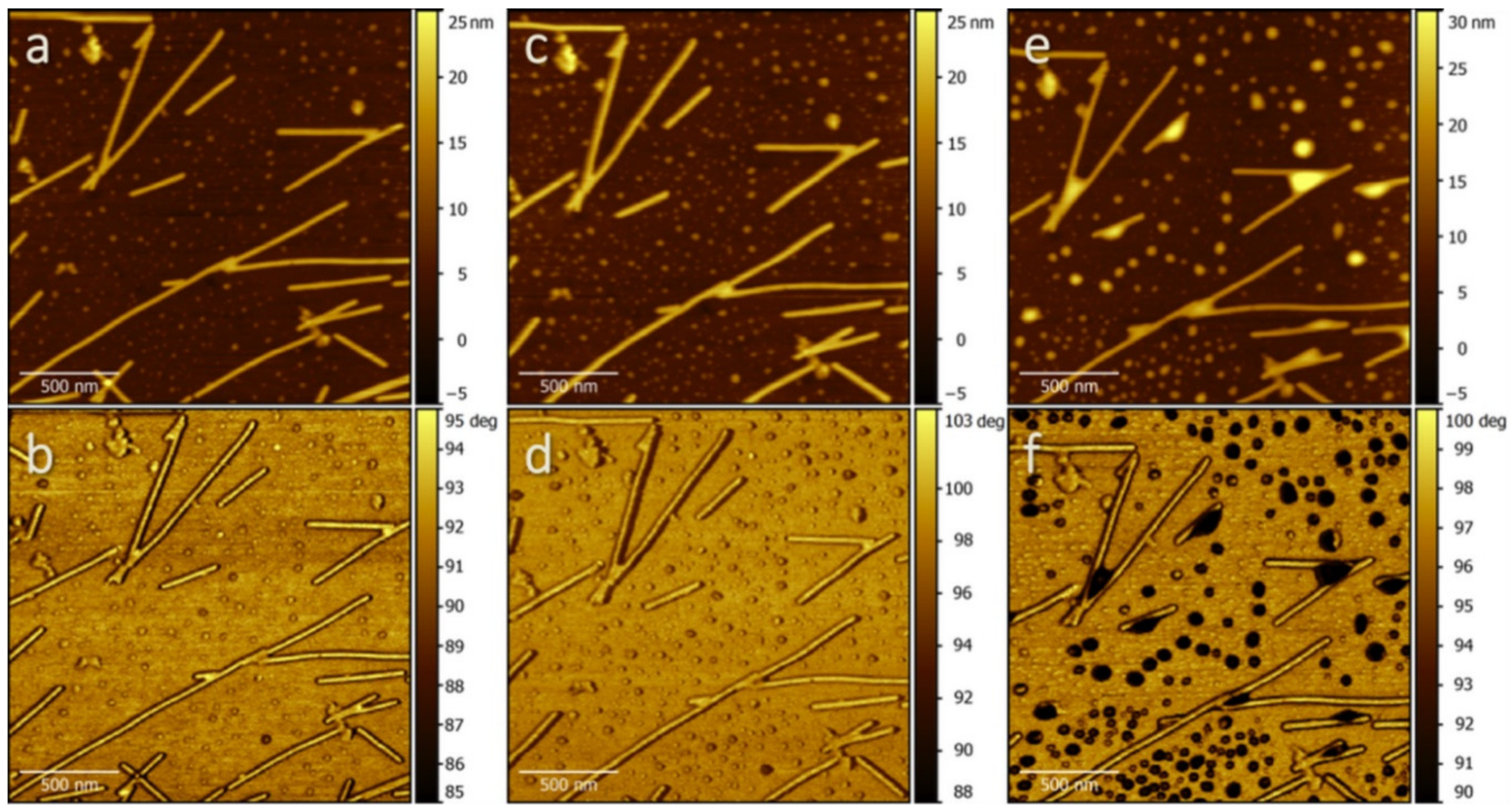
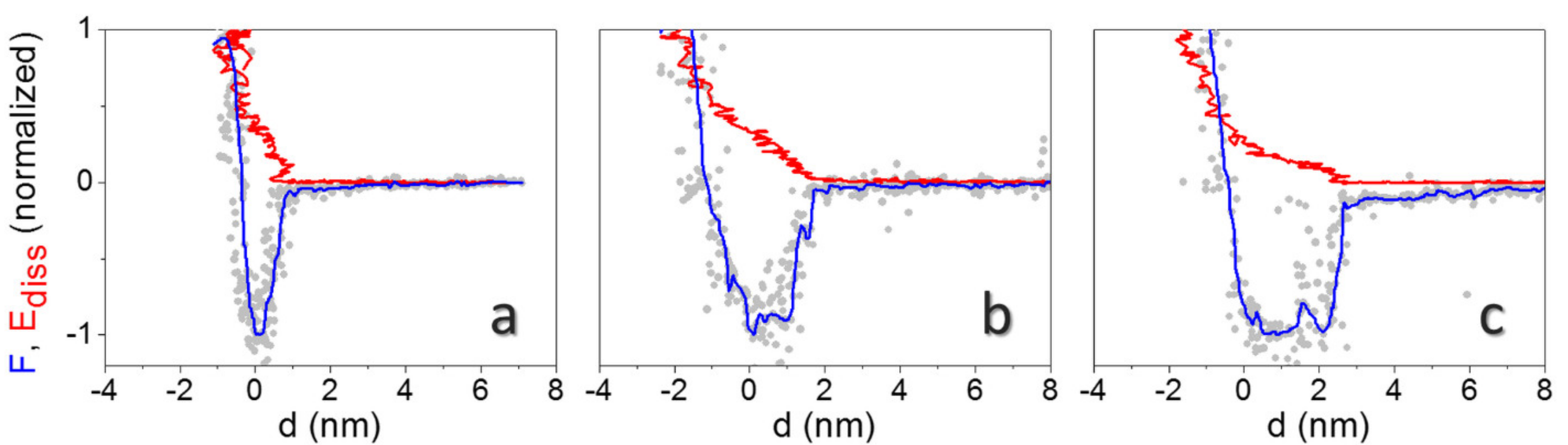
2. Results and Discussion
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Colas de la Noue, A.; Estienney, M.; Aho, S.; Perrier-Cornet, J.-M.; de Rougemont, A.; Pothier, P.; Gervais, P.; Belliot, G. Absolute humidity influences the seasonal persistence and infectivity of human norovirus. Appl. Environ. Microbiol. 2014, 23, 7196–7205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lowen, A.C.; Mubareka, S.; Steel, J.; Palese, P. Influenza virus transmission is dependent on relative humidity and temperature. PLoS Pathog. 2007, 3, 1470–1476. [Google Scholar] [CrossRef]
- Shaman, J.; Kohn, M. Absolute Humidity Modulates influenza Survival, Transmission, and Seasonality. Proc. Natl. Acad. Sci. USA 2009, 106, 3243–3248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stilianakis, N.I.; Drossinos, Y. Dynamics of infectious disease transmission by inhalable respiratory droplets. J. R. Soc. Interface 2010, 7, 1355–1366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sattar, S.A.; Karim, Y.G.; Springthorpe, V.S.; Johnson-Lussenburg, C.M. Survival of Human Rhinovirus Type 14 Dried Onto Nonporous Inanimate Surfaces: Effect of Relative Humidity and Suspending Medium. Can. J. Microbiol. 1987, 33, 802–806. [Google Scholar] [CrossRef] [PubMed]
- Minhaz Ud-Dean, S.M. Structural Explanation for the effect of humidity on persistence of airborne virus: Seasonality of influenza. J. Theor. Biol. 2010, 264, 822–829. [Google Scholar] [CrossRef]
- Yang, W.; Marr, L.C. Mechanism by which ambient humidity may affect viruses in aerosols. Appl. Environ. Microbiol. 2012, 78, 6781–6788. [Google Scholar] [CrossRef] [Green Version]
- Baclayon, M.; Wuite, G.J.L.; Roos, W.H. Imaging and manipulation of single viruses by atomic force microscopy. Soft Matter 2010, 6, 5273–5285. [Google Scholar] [CrossRef]
- Kuznetsov, Y.G.; Malkin, A.J.; Lucas, R.W.; Plomp, M.; McPherson, A. Imaging of viruses by atomic force microscopy. J. Gen. Virol. 2001, 82, 2025–2034. [Google Scholar] [CrossRef]
- Kuznetsov, Y.G.; McPherson, A. Atomic force microscopy in imaging of viruses and virus-infected cells. Microbiol. Mol. Biol. Rev. 2011, 75, 268–285. [Google Scholar] [CrossRef] [Green Version]
- Carrasco, C.; Douas, M.; Miranda, R.; Castellanos, M.; Serena, P.A.; Carrascosa, J.L.; Mateu, M.G.; Marqués, M.I.; de Pablo, P.J. The capillarity of nanometric water menisci confined inside closed-geometry viral cages. Proc. Natl. Acad. Sci. USA 2009, 106, 5475–5480. [Google Scholar] [CrossRef] [Green Version]
- Calò, A.; Eleta-Lopez, A.; Stoliar, P.; De Sancho, D.; Santos, S.; Verdaguer, A.; Bittner, A.M. Multifrequency force microscopy of helical protein assembly on a virus. Sci. Rep. 2016, 6, 21899. [Google Scholar] [CrossRef] [Green Version]
- Knez, M.; Sumser, M.P.; Bittner, A.M.; Wege, C.; Jeske, H.; Hoffmann, D.M.P.; Kuhnke, K.; Kern, K. Binding the tobacco mosaic virus to inorganic surfaces. Langmuir 2004, 20, 441–447. [Google Scholar] [CrossRef] [PubMed]
- Alonso, J.M.; Górzny, M.Ł.; Bittner, A.M. The physics of tobacco mosaic virus and virus-based devices in biotechnology. Trends Biotechnol. 2013, 31, 530–538. [Google Scholar] [CrossRef]
- Zhao, Y.; Ge, Z.; Fang, J. Elastic modulus of viral nanotubes. Phys. Rev. E 2008, 78, 031914. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Narayanasamy, P. Microbial plant pathogens-detection and disease diagnosis. In Viral and Viroid Pathogens; Springer: Heidelberg, Germany, 2011; Volume 3. [Google Scholar]
- Kathiria, P.; Sidler, C.; Golubov, A.; Kalischuk, M.; Kawchuk, L.M.; Kovalchuck, I. Tobacco mosaic virus infection results in an increase in recombination frequency and resistance to viral, bacterial and fungal pathogens in the progeny of infected tobacco plants. Plant Physiol. 2010, 153, 1859–1870. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kramer, S.R.; Goregaoker, S.P.; Culver, J.N. Association of the tobacco mosaic virus 126 kDa replication protein with a GDI protein affects host susceptibility. Virology 2011, 414, 110–118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castello, J.D.; Lakshman, D.K.; Tavantzis, S.M.; Rogers, S.O.; Bachand, G.D.; Jagels, R.; Carlisle, J.; Liu, Y. Detection of infectious tomato mosaic tobamovirus in fog and clouds. Phytopathology 1995, 85, 1409–1412. [Google Scholar] [CrossRef]
- Fillhart, R.C.; Bachand, G.D.; Castello, J.D. Airborne transmission of tomato mosaic tobamovirus and its occurrence in red spruce in the northeastern United States. Can. J. For. Res. 1997, 27, 1176–1181. [Google Scholar] [CrossRef]
- Martinez, N.F.; Patil, S.; Lozano, J.R.; Garcia, R. Enhanced compositional sensitivity in atomic force microscopy by the excitation of the first two flexural modes. Appl. Phys. Lett. 2006, 89, 153115. [Google Scholar] [CrossRef]
- Rodríguez, T.R.; García, R. Compositional mapping of surfaces in atomic force microscopy by excitation of the second normal mode of the microcantilever. Appl. Phys. Lett. 2004, 84, 449–451. [Google Scholar] [CrossRef]
- Calò, A.; Vidal Robles, O.; Santos, S.; Verdaguer, A. Capillary and van der waals interactions on CaF2 crystals from amplitude modulation AFM force reconstruction profiles under ambient conditions. Beilstein J. Nanotechnol. 2015, 6, 809–819. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santos, S.; Amadei, C.A.; Verdaguer, A.; Chiesa, M. Size Dependent Transitions in Nanoscale Dissipation. J. Phys. Chem. C 2013, 117, 10615–10622. [Google Scholar] [CrossRef]
- Calò, A.; Domingo, N.; Santos, S.; Verdaguer, A. Revealing water films structure from force reconstruction in dynamic AFM. J. Phys. Chem. C 2015, 119, 8258–8265. [Google Scholar] [CrossRef]
- Amadei, C.A.; Santos, S.; Pehkonen, S.O.; Verdaguer, A.; Chiesa, M. Minimal invasiveness and spectroscopy-like fingerprints for the characterization of heterogeneous nanoscale wetting in ambient conditions. J. Phys. Chem. C 2013, 117, 20819–20825. [Google Scholar] [CrossRef] [Green Version]
- Amadei, C.A.; Yang, R.; Chiesa, M.; Gleason, K.K.; Santos, S. Revealing amphiphilic nanodomains of anti-biofouling polymer coatings. ACS Appl. Mater. Interfaces 2014, 6, 4705–4712. [Google Scholar] [CrossRef]
- Lozano, J.R.; Garcia, R. Theory of multifrequency atomic force microscopy. Phys. Rev. Lett. 2008, 100, 076102. [Google Scholar] [CrossRef]
- Garcia, R.; San Paulo, A. Amplitude curves and operating regimes in dynamic force microscopy. Ultramicroscopy 2000, 82, 79–83. [Google Scholar] [CrossRef]
- Zizler, L.; Herminghaus, S.; Mugele, F. Capillary Forces in tapping mode atomic force microscopy. Phys. Rev. B 2002, 66, 155436. [Google Scholar] [CrossRef] [Green Version]
- Mattia, D.; Starov, V.; Semenov, S. Thickness, stability and contact angle of liquid films on and inside nanofibres, nanotubes and nanochannels. J. Colloid Interf. Sci. 2012, 384, 149–156. [Google Scholar] [CrossRef] [Green Version]
- Alonso, J.M.; Tatti, F.; Chuvilin, A.; Mam, K.; Ondarcuhu, T.; Bittner, A.M. The condensation of water on adsorbed viruses. Langmuir 2013, 29, 14580–14587. [Google Scholar] [CrossRef]
- Alonso, J.M. Ondarçuhu, T.; Parrens, C.; Górzny, N.; Bittner, A.M. Nanoscale wetting of viruses by ionic liquids. J. Molec. Liq. 2019, 276, 667–674. [Google Scholar] [CrossRef] [Green Version]
- Butt, H.-J.; Kappl, M. Normal Capillary Forces. Adv. Colloid Interface Sci. 2009, 146, 48–60. [Google Scholar] [CrossRef]
- Carpick, R.W.; Batteas, J.; de Boer, M.P. Springer Handbook of Nanotechnology; Springer: New York, NY, USA, 2004. [Google Scholar]
- Butt, H.-J.; Golovko, D.S.; Bonaccurso, E. On the Derivation of Young’s Equation for Sessile Drops: Nonequilibrium Effects Due to Evaporation. J. Phys. Chem. B 2007, 111, 5277–5283. [Google Scholar] [CrossRef]
- Wang, H.; Wang, X.; Li, T.; Lee, B. nanomechanical characterization of rod-like superlattices assembled from tobacco mosaic viruses. J. Appl. Phys. 2013, 113, 024308. [Google Scholar] [CrossRef]
- Brakke, K.A. The Surface Evolver. Exp. Math. 1992, 1, 141–165. [Google Scholar] [CrossRef]
- Checco, A.; Cai, Y.; Gang, O.; Ocko, B.M. High resolution non-contact AFM imaging of liquids condensed onto chemically nanopatterned surfaces. Ultramicroscopy 2006, 106, 703–708. [Google Scholar] [CrossRef]
- Katan, A.J.; van Es, M.H.; Oosterkamp, T.H. Quantitative force versus distance measurements in amplitude-modulation afm: A novel force inversion technique. Nanotechnol. 2009, 20, 165703–165711. [Google Scholar] [CrossRef] [PubMed]
- Sader, J.E.; Uchihashi, T.; Higgins, M.J.; Farrell, A.; Nakayama, Y.; Jarvis, S.P. Quantitative Force Measurements Using Frequency Modulation Atomic Force Microscopy: Theoretical Foundations. Nanotechnology 2005, 16, S94–S101. [Google Scholar] [CrossRef]
- Cleveland, J.P.; Anczykowski, B.; Schmid, A.E.; Elings, V.B. Energy dissipation in tapping-mode atomic force microscopy. Appl. Phys. Lett. 1998, 72, 2613–2615. [Google Scholar] [CrossRef] [Green Version]
- Barcons, V.; Verdaguer, A.; Font, J.; Chiesa, M.; Santos, S. Nanoscale capillary Interactions in dynamic atomic force microscopy. J. Phys. Chem. C 2012, 116, 7757–7766. [Google Scholar] [CrossRef]
- Fabié, L.; Durou, H.; Ondarçuhu, T. Capillary forces during liquid nanodispensing. Langmuir 2010, 26, 1870–1878. [Google Scholar] [CrossRef] [PubMed]
- Santos, S.; Verdaguer, A.; Souier, T.; Thomson, N.H.; Chiesa, M. Measuring the true height of water films on surfaces. Nanotechnology 2011, 22, 465705. [Google Scholar] [CrossRef]
- Verdaguer, A.; Weis, C.; Oncins, G.; Ketteler, G.; Bluhm, H.; Salmeron, M. growth and structure of water on SiO2 films on Si investigated by kelvin probe microscopy and in situ x-ray spectroscopies. Langmuir 2007, 23, 9699–9703. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hegner, M.; Wagner, P.; Semenza, G. Ultralarge Atomically flat template stripped au surfaces for scanning probe microscopy. Surf. Sci. 1993, 291, 39–46. [Google Scholar] [CrossRef]
- Horcas, I.; Fernández, R.; Gómez-Rodríguez, J.M.; Colchero, J.; Gómez-Herrero, J.; Baro, A.M. WSXM: A software for scanning probe microscopy and a tool for nanotechnology. Rev. Sci. Instr. 2007, 78, 013705. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calò, A.; Eleta-Lopez, A.; Ondarçuhu, T.; Verdaguer, A.; Bittner, A.M. Nanoscale Wetting of Single Viruses. Molecules 2021, 26, 5184. https://doi.org/10.3390/molecules26175184
Calò A, Eleta-Lopez A, Ondarçuhu T, Verdaguer A, Bittner AM. Nanoscale Wetting of Single Viruses. Molecules. 2021; 26(17):5184. https://doi.org/10.3390/molecules26175184
Chicago/Turabian StyleCalò, Annalisa, Aitziber Eleta-Lopez, Thierry Ondarçuhu, Albert Verdaguer, and Alexander M. Bittner. 2021. "Nanoscale Wetting of Single Viruses" Molecules 26, no. 17: 5184. https://doi.org/10.3390/molecules26175184