Microwave-Assisted Ionic Liquid-Catalyzed Selective Monoesterification of Alkylphosphonic Acids—An Experimental and a Theoretical Study
Abstract
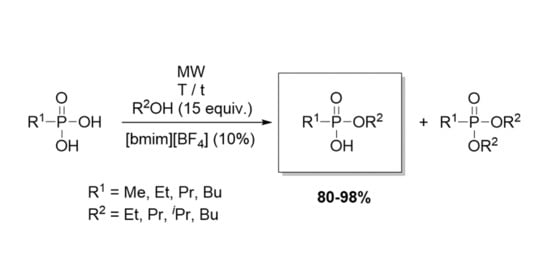
:1. Introduction
2. Results and Discussion
2.1. Preparative Experiments on the Microwave-Assited Monoesterification of Alkylphosphonic Acids
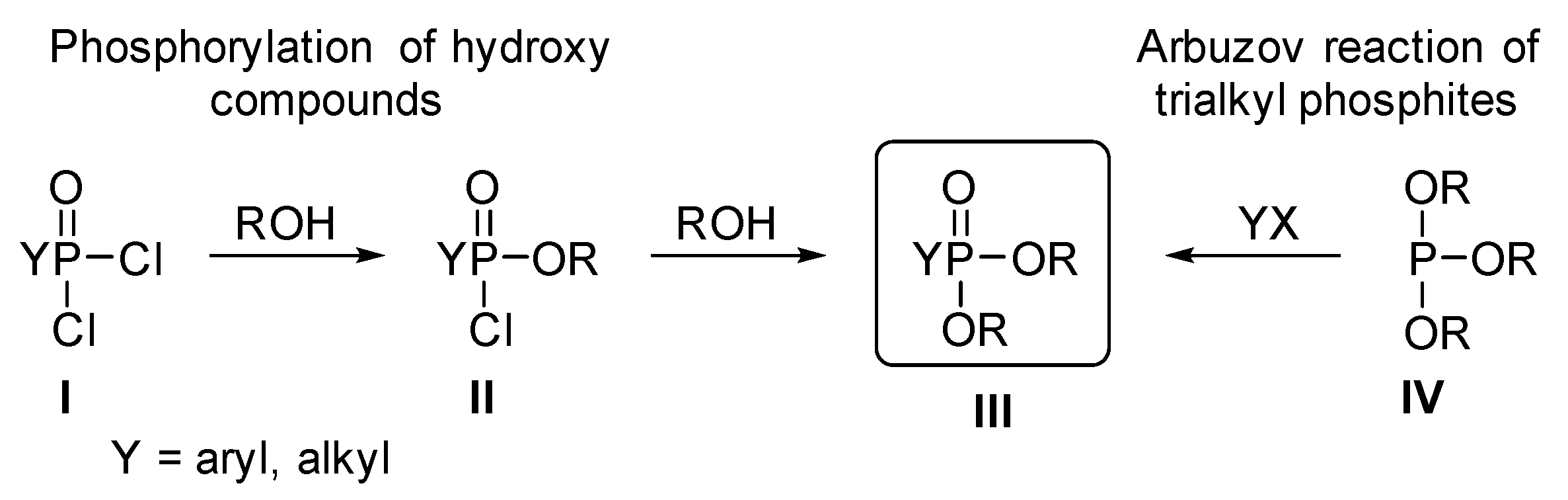
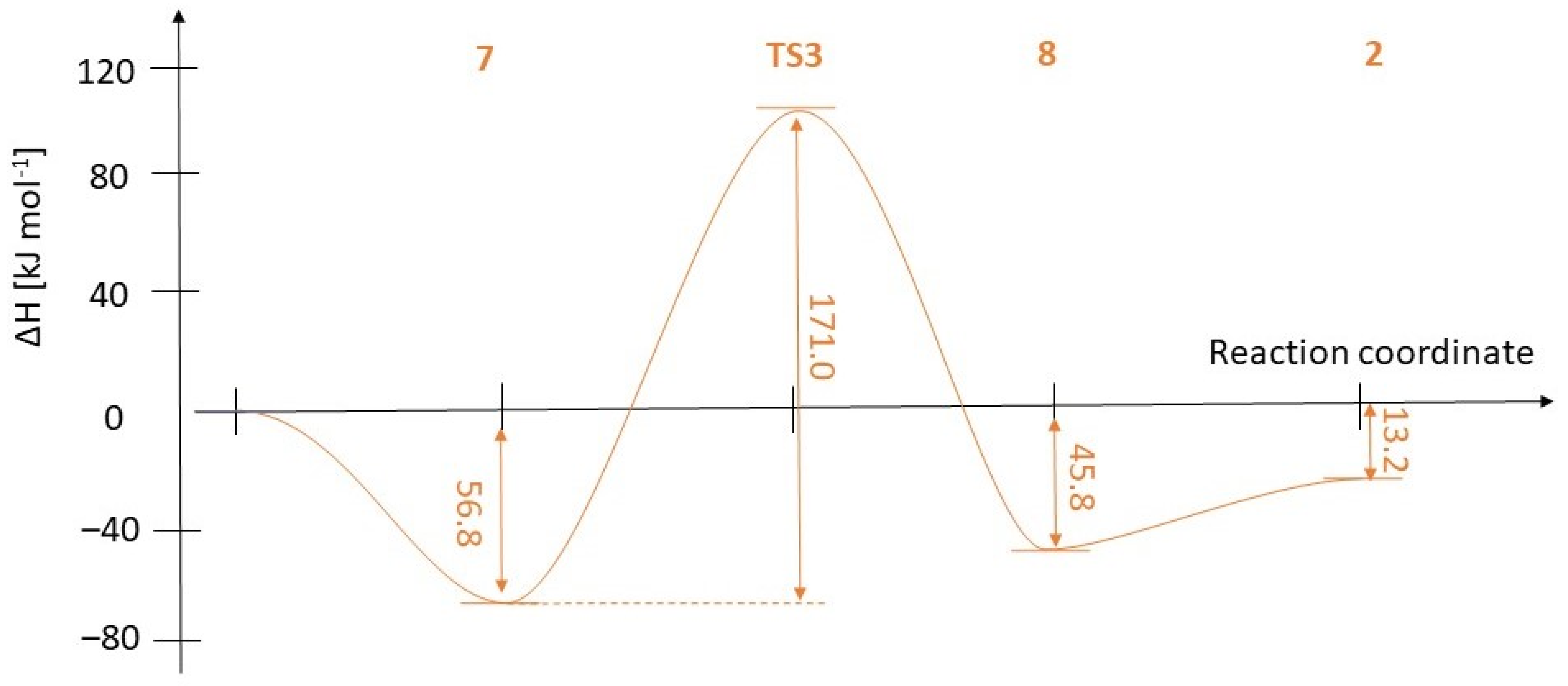
2.2. Theoretical Calculations on the Direct Esterification of Alkylphosphonic Acids
3. Experimental
3.1. General
3.2. Use of the 31P NMR Spectra in Quantitative Analysis
3.3. General Procedure for the Direct Esterification of Akylphosphonic Acids in the Presence of Ionic Liquids
3.4. Additional Spectral Data for the New Ester-Acids
3.4.1. Monopropyl Ethylphosphonate (2Bb)
3.4.2. Monobutyl Ethylphosphonate (2Bd)
3.4.3. Monopropyl Propylphosphonate (2Cb)
3.4.4. Monoethyl Butylphosphonate (2Da)
3.4.5. Monopropyl Butylphosphonate (2Db)
3.4.6. Monoisopropyl Butylphosphonate (2Dc)
3.4.7. Monobutyl Butylphosphonate (2Dd)
3.5. Theoretical Calculations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Quin, L.D. A Guide to Organophosphorus Chemistry; Wiley: New York, NY, USA, 2000. [Google Scholar]
- Horsman, G.P.; Zechel, D.L. Phosphonate Biochemistry. Chem. Rev. 2017, 117, 5704–5783. [Google Scholar] [CrossRef]
- Pradere, U.; Garnier-Amblard, E.C.; Coats, S.J.; Amblard, F.; Schinazi, R.F. Synthesis of nucleoside phosphate and phosphonate prodrugs. Chem. Rev. 2014, 114, 9154–9218. [Google Scholar] [CrossRef]
- Nowack, B. Environmental chemistry of phosphonates. Water Res. 2003, 37, 2533–2546. [Google Scholar] [CrossRef]
- Xiang, H.F.; Xu, H.Y.; Wang, Z.Z.; Chen, C.H. Dimethyl methylphosphonate (DMMP) as an efficient flame retardant additive for the lithium-ion battery electrolytes. J. Power Sources 2007, 173, 562–564. [Google Scholar] [CrossRef]
- Kiss, N.Z.; Keglevich, G. Direct esterification of phosphinic and phosphonic acids enhanced by ionic liquid additives. Pure Appl. Chem. 2019, 91, 59–65. [Google Scholar] [CrossRef]
- Kiss, N.Z.; Mucsi, Z.; Böttger, É.; Drahos, L.; Keglevich, G. A three-step conversion of phenyl-1H-phosphinic acid to dialkyl phenylphosphonates including two microwave-assisted direct esterification steps. Curr. Org. Synth. 2014, 11, 767–772. [Google Scholar] [CrossRef]
- Henyecz, R.; Kiss, A.; Mórocz, V.; Kiss, N.Z.; Keglevich, G. Synthesis of phosphonates from phenylphosphonic acid and its monoesters. Synth. Commun. 2019, 49, 2642–2650. [Google Scholar] [CrossRef] [Green Version]
- Welton, T. Ionic liquids: A brief history. Biophys. Rev. 2018, 10, 691–706. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, S.K.; Savoy, A. Ionic liquids synthesis and applications: An overview. J. Mol. Liq. 2020, 297, 112038. [Google Scholar] [CrossRef]
- Karmakar, A.; Mukundan, R.; Yang, P.; Batista, E.R. Solubility model of metal complex in ionic liquids from first principle calculations. RSC Adv. 2019, 9, 18506–18526. [Google Scholar] [CrossRef] [Green Version]
- Keaveney, S.T.; Haines, R.S.; Harper, J.B. Ionic liquid solvents: The importance of microscopic interactions in predicting organic reaction outcomes. Pure App. Chem. 2017, 89, 745–757. [Google Scholar] [CrossRef] [Green Version]
- Rádai, Z.; Kiss, N.Z.; Keglevich, G. An Overview of the Applications of Ionic Liquids as Catalyst and Additives in Organic Chemical Reactions. Curr. Org. Chem. 2018, 22, 533–556. [Google Scholar] [CrossRef]
- Barucki, H.; Black, R.M.; Kinnear, K.I.; Holden, I.; Read, R.W.; Timperley, C.M. Solid-phase synthesis of some alkyl hydrogen methylphosphonates. Phosphorus Sulfur Silicon 2003, 178, 2279–2286. [Google Scholar] [CrossRef]
- Raghuraman, K.; Pillarsetty, N.; Prabhu, K.R.; Katti, K.K.; Katti, K.V. Unprecedented rhodium-mediated catalytic transfer hydrogenation of a phosphonate functionalized olefin in ecofriendly media. Inorg. Chim. Acta 2004, 357, 2933–2938. [Google Scholar] [CrossRef]
- Gupta, A.K.; Kumar, R.; Gupta, H.K.; Tak, V.; Dubey, D.K. DCC-Celite hybrid immobilized solid support as a new, highly efficient reagent for the synthesis of O-alkyl hydrogen alkylphosphonates under solvent-free conditions. Tetrahedron Lett. 2008, 49, 1656–1659. [Google Scholar] [CrossRef]
- Pienaar, A.; Erasmus, C.M.; Wentzel, M.; Cowley, E.H. Synthesis of alkyl hydrogen alkylphosphonates. Phosphorus Sulfur Silicon 1999, 148, 149–159. [Google Scholar] [CrossRef]
- Mucsi, Z.; Kiss, N.Z.; Keglevich, G. A quantum chemical study on the mechanism and energetics of the direct esterification, thioesterification and amidation of 1-hydroxy-3-methyl-3-phospholene 1-oxide. RSC Adv. 2014, 4, 11948–11954. [Google Scholar] [CrossRef] [Green Version]
- Ansly, E.V.; Dougherty, D.A. Modern Physical Organic Chemistry; University Science Books: Mill Valley, CA, USA, 2006; pp. 361–362. [Google Scholar]
- Keglevich, G.; Kiss, N.Z.; Mucsi, Z.; Körtvélyesi, T. Insights into a surprising reaction: The microwave-assisted direct esterification of phosphinic acids. Org. Biomol. Chem. 2012, 10, 2011–2018. [Google Scholar] [CrossRef]
- Keglevich, G.; Mucsi, Z. 4. Interpretation of the rate enhancing effect of microwaves. In Microwave Chemistry; Giancarlo, C., Diego, C., Eds.; De Gruyter: Berlin, Germany; Boston, MA, USA, 2017; pp. 53–64. [Google Scholar] [CrossRef]
- Hohmann, E.; Keglevich, G.; Greiner, I. The effect of onium salt additives on the Diels Alder reactions of a 1-phenyl-1,2-dihydrophosphinine oxide under microwave conditions. Phosphorus Sulfur Silicon 2007, 182, 2351–2357. [Google Scholar] [CrossRef]
- Harsági, N.; Kiss, N.Z.; Drahos, L.; Keglevich, G. Synthesis of Cyclic Phosphinates by Microwave-Assisted Ionic Liquid-Promoted Alcoholysis. Synthesis 2021, in press. [Google Scholar] [CrossRef]
- Acharya, J.; Shakya, P.D.; Pardasani, D.; Palit, M.; Dubey, D.K.; Gupta, A.K. Surface-mediated solid phase reactions: A simple, efficient and base-free synthesis of phosphonates and phosphates on Al2O3. J. Chem. Res. 2005, 194–196. [Google Scholar] [CrossRef]
- Lai, C.; Xi, C.; Chen, W.; Hua, R. Metallo-phosphorylation of alkenes: A highly regioselective reaction of zirconocene-alkene complexes with chlorophosphate. Tetrahedron 2006, 62, 6295–6302. [Google Scholar] [CrossRef]
- Li, C.; Wang, Q.; Zhang, J.Q.; Ye, J.; Xie, J.; Xu, Q.; Han, L.B. Water determines the products: An unexpected Bronsted acid-catalyzed PO-R cleavage of P(III) esters selectively producing P(O)-H and P(O)-R compounds. Green Chem. 2019, 21, 2916–2922. [Google Scholar] [CrossRef]
- Gaussian 09, Revision D.01; Gaussian, Inc.: Wallingford, CT, USA, 2009.
- Zhao, Y.; Truhlar, D.G. The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: Two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor. Chem. Acc. 2008, 120, 215–241. [Google Scholar] [CrossRef] [Green Version]
- Petersson, G.A.; Bennett, A.; Tensfeldt, T.G.; Al-Laham, M.A.; Shirley, W.A. A complete basis set model chemistry. I. The total energies of closed-shell atoms and hydrides of the first-row elements. J. Chem. Phys. 1988, 89, 2193–2218. [Google Scholar] [CrossRef]





 | |||||||
|---|---|---|---|---|---|---|---|
| Entry | R | Temperature (°C) | Time (h) | Conversion a (%) | Monoester a (2A) (%) | Diester a (3A) (%) | Yield (%) |
| 1 | Et (a) | 160 | 5 | 96 | 94 | 2 | 78 |
| 2 | Pr (b) | 180 | 3 | 97 | 78 | 19 | 62 |
| 3 | iPr (c) | 180 | 3 | 77 | 74 | 3 | – |
| 4 | iPr (c) | 180 | 5 | 100 | 98 | 2 | 75 |
| 5 | Bu (d) | 180 | 3 | 100 | 81 | 19 | 66 |
| 6 | Bu (d) | 200 b,c | 1.25 | 99 | 81 | 18 | 65 |
| 7 | Bu (d) | 200 | 2 | 100 | 45 | 55 | – |
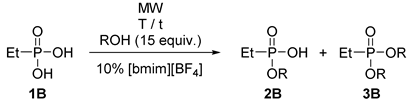 | |||||||
|---|---|---|---|---|---|---|---|
| Entry | R | Temperature (°C) | Time (h) | Conversion a (%) | Monoester a (2B) (%) | Diester a (3B) (%) | Yield (%) |
| 1 | Et (a) | 160 | 6 | 95 | 87 | 8 | 71 |
| 2 | Pr (b) | 180 | 3.5 | 95 | 79 | 16 | 63 |
| 3 | iPr (c) | 180 | 7 | 98 | 91 | 7 | 72 |
| 4 | Bu (d) | 180 | 4 | 96 | 89 | 7 | 69 |
| 5 | Bu (d) | 200 | 2 | 99 | 79 | 20 | 61 |
 | |||||||
|---|---|---|---|---|---|---|---|
| Entry | R | Temperature (°C) | Time (h) | Conversion a (%) | Monoester a (2C) (%) | Diester a (3C) (%) | Yield (%) |
| 1 | Et (a) | 160 | 6 | 99 | 90 | 9 | 79 |
| 2 | Pr (b) | 180 | 3.5 | 94 | 82 | 12 | 65 |
| 3 | iPr (c) | 180 | 7 | 98 | 94 | 4 | 77 |
| 4 | Bu (d) | 180 | 5 | 98 | 83 | 15 | 67 |
| 5 | Bu (d) | 200 | 2 | 97 | 83 | 14 | 65 |
 | |||||||
|---|---|---|---|---|---|---|---|
| Entry | R | Temperature (°C) | Time (h) | Conversion a (%) | Monoester a (2D) (%) | Diester a (3D) (%) | Yield (%) |
| 1 | Et (a) | 165 | 7 | 70 | 67 | 3 | 51 |
| 2 | Pr (b) | 200 | 4 | 99 | 82 | 17 | 66 |
| 3 | iPr (c) | 200 | 7 b | 77 | 71 | 6 | 60 |
| 4 | Bu (d) | 200 | 3 | 100 | 87 | 13 | 70 |
| 1 | 4 | TS1 | 5 | TS2 | 6 | 2 | ||
|---|---|---|---|---|---|---|---|---|
| R1 = Me, R2 = Me | ΔH (kJ·mol−1) | 0.0 | −123.0 | −52.3 | −47.2 | 49.1 | −116.8 | −10.6 |
| ΔG (kJ·mol−1) | 0.0 | 66.0 | 154.3 | 151.6 | 245.2 | 63.2 | −5.5 | |
| ΔS (J (mol⋅K)−1) | 0.0 | 68.6 | 54.9 | 61.1 | 63.3 | 76.0 | 49.7 | |
| R1 = Me, R2 = Bu | ΔH (kJ·mol−1) | 0.0 | −146.6 | −52.1 | −48.6 | 47.5 | −120.3 | −15.8 |
| ΔG (kJ·mol−1) | 0.0 | 49.8 | 156.5 | 156.4 | 250.5 | 68.4 | −5.1 | |
| ΔS (J (mol⋅K)−1) | 0.0 | 70.9 | 61.2 | 63.8 | 65.6 | 76.9 | 50.4 | |
| R1 = Et, R2 = Bu | ΔH (kJ·mol−1) | 0.0 | −141. 7 | −47.9 | −42.1 | 18.9 | −122.3 | −13.2 |
| ΔG (kJ·mol−1) | 0.0 | 62.3 | 169.4 | 168.4 | 223.8 | 74.1 | −9.1 | |
| ΔS (J (mol⋅K)−1) | 0.0 | 67.1 | 56.6 | 62.1 | 66.6 | 73.4 | 56.6 | |
| R1 = Bu, R2 = Bu | ΔH (kJ·mol−1) | 0.0 | −138.5 | −42.4 | −39.1 | 36.7 | −117.1 | −8.4 |
| ΔG (kJ·mol−1) | 0.0 | 48.2 | 163.8 | 158.3 | 230.7 | 63.3 | −5.7 | |
| ΔS (J (mol⋅K)−1) | 0.0 | 85.3 | 70.0 | 76.9 | 79.6 | 90.4 | 58.6 | |
| R1 = Ph, R2 = Me | ΔH (kJ·mol−1) | 0.0 | −144.1 | −64.5 | −58.4 | 47.7 | −120.6 | −12.8 |
| ΔG (kJ·mol−1) | 0.0 | 46.0 | 141.8 | 138.4 | 256.6 | 58.4 | −10.2 | |
| ΔS (J (mol⋅K)−1) | 0.0 | 75.2 | 62.3 | 69.9 | 50.2 | 84.0 | 52.4 |
| 1 | 7 | TS3 | 8 | 2 | ||
|---|---|---|---|---|---|---|
| R1 = Me, R2 = Me | ΔH (kJ·mol−1) | 0.0 | −63.4 | 105.8 | −65.7 | −10.6 |
| ΔG (kJ·mol−1) | 0.0 | 69.2 | 248.7 | 58.9 | −5.5 | |
| ΔS (J (mol⋅K)−1) | 0.0 | 59.3 | 51.0 | 65.9 | 49.7 | |
| R1 = Me, R2 = Bu | ΔH (kJ·mol−1) | 0.0 | −58.5 | 103.9 | −66.6 | −15.8 |
| ΔG (kJ·mol−1) | 0.0 | 80.2 | 253.5 | 63.2 | −5.1 | |
| ΔS (J (mol⋅K)−1) | 0.0 | 60.2 | 50.4 | 70.2 | 56.6 | |
| R1 = Et, R2 = Bu | ΔH (kJ·mol−1) | 0.0 | −56.8 | 114.2 | −45.8 | −13.2 |
| ΔG (kJ·mol−1) | 0.0 | 85.6 | 268.9 | 84.0 | −9.1 | |
| ΔS (J (mol⋅K)−1) | 0.0 | 76.2 | 67.3 | 82.9 | 50.4 |
| Compound | δP[found] | δP[lit] | HRMS | |||
|---|---|---|---|---|---|---|
| [M + Na]+found | Formula | [M + Na]+calculated | ||||
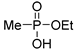 | (2Aa) | 32.8 (CDCl3) | 32.5 [14] (CDCl3) | 147.0190 | C3H9O3PNa | 147.0187 |
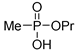 | (2Ab) | 32.6 (CDCl3) | 32.8 [14] (CDCl3) | 161.0341 | C4H11O3PNa | 161.0344 |
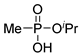 | (2Ac) | 32.5 (CDCl3) | 33.8 [14] (CDCl3) | 161.0343 | C4H11O3PNa | 161.0344 |
 | (2Ad) | 32.6 (CDCl3) | 33.9 [14] (CDCl3) | 175.0504 | C5H13O3PNa | 175.0500 |
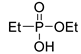 | (2Ba) | 36.3 (CDCl3) | 37.5 [15] (CDCl3) | 161.0341 | C4H11O3PNa | 161.0344 |
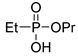 | (2Bb) | 36.1 (CDCl3) | – | 175.0500 | C5H13O3PNa | 175.0500 |
 | (2Bc) | 35.4 (CDCl3) | 33.6 [16] (CD3OD) | 175.0505 | C5H13O3PNa | 175.0500 |
 | (2Bd) | 35.1 (CDCl3) | – | 163.0835 a | C6H16O3P | 163.0837 |
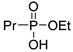 | (2Ca) | 35.0 (CDCl3) | 34.1 [16] (CD3OD) | 175.0501 | C5H13O3PNa | 175.0500 |
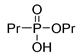 | (2Cb) | 34.9 (CDCl3) | – | 189.0655 | C6H15O3PNar | 189.0657 |
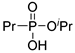 | (2Cc) | 34.4 (CDCl3) | 34.2 b [17] | 189.0658 | C6H15O3PNa | 189.0657 |
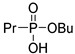 | (2Cd) | 35.2 (CDCl3) | 33.8 [16] (CD3OD) | 203.0815 | C7H17O3PNa | 203.0813 |
 | (2Da) | 35.2 (CDCl3) | – | 189.0662 | C6H15O3PNa | 189.0657 |
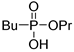 | (2Db) | 35.4 (CDCl3) | – | 203.0815 | C7H17O3PNa | 203.0813 |
 | (2Dc) | 34.4 (CDCl3) | – | 203.0815 | C7H17O3PNa | 203.0813 |
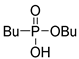 | (2Dd) | 35.6 (CDCl3) | – | 217.0971 | C8H19O3PNa | 217.0970 |
| Compound | δP[found] | δP[lit] | HRMS | |||
|---|---|---|---|---|---|---|
| [M + Na]+found | Formula | [M + Na]+calculated | ||||
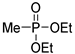 | (3Aa) | 30.1 (DMSO) | 31.1 a [24] | 175.0499 | C5H13O3PNa | 175.0500 |
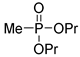 | (3Ab) | 30.2 (DMSO) | 30.8 a [24] | 203.0811 | C7H17O3PNa | 203.0813 |
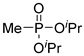 | (3Ac) | 28.2 (DMSO) | 30.9 a [24] | 181 b | C7H18O3P | 181 |
 | (3Ad) | 30.2 (DMSO) | 30.1 a [24] | 231.1132 | C9H21O3PNa | 231.1126 |
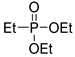 | (3Ba) | 33.1 (DMSO) | 34.3 a [24] | 189.0655 | C6H15O3PNa | 189.0657 |
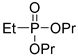 | (3Bb) | 33.1 (DMSO) | 33.0 a [24] | 217.0966 | C8H19O3PNa | 217.0970 |
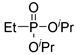 | (3Bc) | 31.2 (DMSO) | 32.9 a [24] | 195 b | C8H20O3P | 195 |
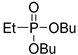 | (3Bd) | 33.1 (DMSO) | 31.5 a [24] | 245.1279 | C10H23O3PNa | 245.1283 |
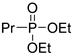 | (3Ca) | 31.7 (DMSO) | 31.8 a [24] | 203.0815 | C7H17O3PNa | 203.0813 |
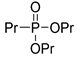 | (3Cb) | 31.1 (DMSO) | 31.1 a [24] | 231.1126 | C9H21O3PNa | 231.1126 |
 | (3Cc) | 29.8 (DMSO) | 30.9 a [24] | 209 b | C9H22O3P | 209 |
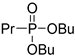 | (3Cd) | 31.7 (DMSO) | 30.7 a [24] | 259.1440 | C11H25O3PNa | 259.1439 |
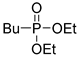 | (3Da) | 32.1 (DMSO) | 33.5 [25] (CDCl3) | 217.0963 | C8H19O3PNa | 217.0970 |
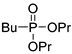 | (3Db) | 32.0 (DMSO) | – | 245.1282 | C10H23O3PNa | 245.1283 |
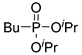 | (3Dc) | 30.1 (DMSO) | – | 245.1283 | C10H23O3PNa | 245.1283 |
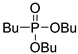 | (3Dd) | 32.1 (DMSO) | 33.1 [26] (CDCl3) | 273.1598 | C12H27O3PNa | 273.1596 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Harsági, N.; Henyecz, R.; Ábrányi-Balogh, P.; Drahos, L.; Keglevich, G. Microwave-Assisted Ionic Liquid-Catalyzed Selective Monoesterification of Alkylphosphonic Acids—An Experimental and a Theoretical Study. Molecules 2021, 26, 5303. https://doi.org/10.3390/molecules26175303
Harsági N, Henyecz R, Ábrányi-Balogh P, Drahos L, Keglevich G. Microwave-Assisted Ionic Liquid-Catalyzed Selective Monoesterification of Alkylphosphonic Acids—An Experimental and a Theoretical Study. Molecules. 2021; 26(17):5303. https://doi.org/10.3390/molecules26175303
Chicago/Turabian StyleHarsági, Nikoletta, Réka Henyecz, Péter Ábrányi-Balogh, László Drahos, and György Keglevich. 2021. "Microwave-Assisted Ionic Liquid-Catalyzed Selective Monoesterification of Alkylphosphonic Acids—An Experimental and a Theoretical Study" Molecules 26, no. 17: 5303. https://doi.org/10.3390/molecules26175303