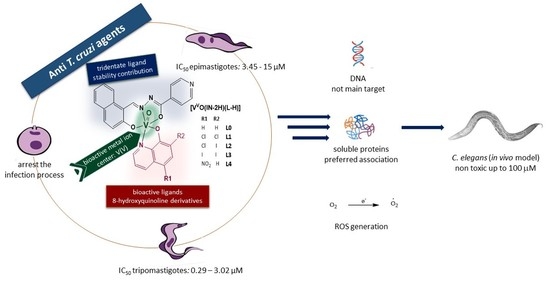
Heteroleptic Oxidovanadium(V) Complexes with Activity against Infective and Non-Infective Stages of Trypanosoma cruzi
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization in the Solid State
2.1.1. FTIR Results
2.1.2. X-ray Diffraction Study of IN and [VVO(IN-2H)(L2-H)]·1.5 THF
2.2. Characterization in Solution
NMR Results
2.3. Biological Results
2.3.1. In Vitro Activity against Epimastigotes and Trypomastigotes of T. cruzi and Selectivity towards the Parasites
2.3.2. Stability Studies and Active Species
2.3.3. Effects on the T. cruzi Infection Process and Persistance
2.3.4. Parasite Recovery Results
2.3.5. Vanadium Uptaken by Epimastigotes and Trypomastigotes
2.3.6. Vanadium Association with Parasite Macromolecules
2.3.7. Generation of Reactive Oxygen Species
2.3.8. DNA Interaction by Fluorescence Studies
2.3.9. In Vivo Toxicity on C. elegans
3. Materials and Methods
3.1. Materials
3.2. Synthesis of the New IN Ligand, the Oxidovanadium(V) Complexes [VVO(IN-2H)(L-H)] and the Dioxidovanadium(V) Complex [VVO2(IN-H)]
3.2.1. Synthesis of the IN Ligand
3.2.2. Syntheses of the Dioxidovanadium(V) Complex, [VVO2(IN-H)]·THF
3.2.3. Synthesis of the Oxidovanadium(V) Complexes [VVO(IN-2H)(L-H)], L = L0–L4
3.3. Physicochemical Characterization
3.4. X-ray Diffraction of IN and [VVO(IN–2H)(L2–H)]·1.5 THF
3.5. Biological Studies
3.5.1. In Vitro Activity against Trypanosoma cruzi and Cytotoxicity on a Mammalian Cell Model (VERO Cells)
Parasite and Culture
Compounds’ Treatment
In Vitro Activity against Epimastigotes and Trypomastigotes of Trypanosoma cruzi
Cytotoxicity on VERO Cells
3.5.2. In Vitro Infection Assays
Effect on the Infection Process
Effect on the Intracellular Parasite Replication
3.5.3. Parasite Recovery Assays
3.5.4. Uptake of Vanadium by Parasites
3.5.5. Vanadium Association with Parasite Macromolecules
3.6. Production of Reactive Oxygen Species (ROS)
3.7. DNA Interaction by Fluorescence Studies
3.8. In Vivo Toxicity on C. elegans
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Chagas Disease (American Trypanosomiasis). Available online: http://www.who.int/chagas/en/ (accessed on 23 April 2021).
- Brindha, J.; Balamurali, M.; Chanda, K. An Overview on the Therapeutics of Neglected Infectious Diseases—Leishmaniasis and Chagas Diseases. Front. Chem. 2021, 9, 1–19. [Google Scholar] [CrossRef]
- Chatelain, E.; Ioset, J.-R. Phenotypic screening approaches for Chagas disease drug discovery. Expert Opin. Drug Discov. 2017, 13, 141–153. [Google Scholar] [CrossRef]
- Kayode, O.T.; Lele, C.K.; Kayode, A.A.A. Trypanosomiasis: Recent advances in strategies for control. Glob. J. Infect. Dis. Clin. Res. 2021, 6, 037–041. [Google Scholar] [CrossRef]
- Paucar, R.; Moreno-Viguri, E.; Pérez-Silanes, S. Challenges in Chagas Disease Drug Discovery: A Review. Curr. Med. Chem. 2016, 23, 3154–3170. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Molina, A.J.; Molina, I. Chagas disease. Lancet 2018, 391, 82–94. [Google Scholar] [CrossRef]
- Francisco, A.F.; Jayawardhana, S.; Olmo, F.; Lewis, M.D.; Wilkinson, S.R.; Taylor, M.C.; Kelly, J.M. Challenges in Chagas Disease Drug Development. Molecules 2020, 25, 2799. [Google Scholar] [CrossRef]
- Santos, S.S.; de Araújo, R.V.; Giarolla, J.; El Seoud, O.; Ferreira, E.I. Searching for drugs for Chagas disease, leishmaniasis and schistosomiasis: A review. Int. J. Antimicrob. Agents 2020, 55, 105906. [Google Scholar] [CrossRef] [PubMed]
- Scarim, C.B.; Jornada, D.H.; Chelucci, R.C.; de Almeida, L.; dos Santos, J.L.; Chung, M.C. Current advances in drug discovery for Chagas disease. Eur. J. Med. Chem. 2018, 155, 824–838. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sánchez-Delgado, R.A.; Anzellotti, A.; Suárez, L. Metal ions and their complexes in medication. In Metal Ions in Biological Systems; Sigel, H., Sigel, A., Eds.; Marcel Dekker: New York, NY, USA, 2004; p. 379. [Google Scholar]
- Brown, R.W.; Hyland, C. Medicinal organometallic chemistry—an emerging strategy for the treatment of neglected tropical diseases. MedChemComm 2015, 6, 1230–1243. [Google Scholar] [CrossRef]
- Camarada, M.B.; Echeverria, C.; Ramirez-Tagle, R. Medicinal organometallic compounds with anti-chagasic activity. MedChemComm 2016, 7, 1307–1315. [Google Scholar] [CrossRef]
- Gambino, D.; Otero, L. Perspectives on what ruthenium-based compounds could offer in the development of potential antiparasitic drugs. Inorg. Chim. Acta 2012, 393, 103–114. [Google Scholar] [CrossRef]
- Navarro, M. Gold complexes as potential anti-parasitic agents. Coord. Chem. Rev. 2009, 253, 1619–1626. [Google Scholar] [CrossRef]
- Navarro, M.; Gabbiani, C.; Messori, L.; Gambino, D. Metal-based drugs for malaria, trypanosomiasis and leishmaniasis: Recent achievements and perspectives. Drug Discov. Today 2010, 15, 1070–1078. [Google Scholar] [CrossRef]
- Tahghighi, A. Importance of metal complexes for development of potential leishmanicidal agents. J. Organomet. Chem. 2014, 770, 51–60. [Google Scholar] [CrossRef]
- Gambino, D. Potentiality of vanadium compounds as anti-parasitic agents. Coord. Chem. Rev. 2011, 255, 2193–2203. [Google Scholar] [CrossRef]
- Gambino, D.; Otero, L. Design of prospective antiparasitic metal-based compounds including selected organometallic cores. Inorg. Chim. Acta 2018, 472, 58–75. [Google Scholar] [CrossRef]
- Gambino, D.; Otero, L. Metal compounds in the developement of antiparasitic agents; rational design from basic chemistry to the clinic. In Essential Metals in Medicine: Therapeutic Use and Toxicity of Metal Ions in the Clinic; Carver, P., Ed.; De Gruyter: Berlin, Germany, 2019; pp. 331–358. [Google Scholar]
- Ong, Y.C.; Roy, S.; Andrews, P.C.; Gasser, G. Metal Compounds against Neglected Tropical Diseases. Chem. Rev. 2019, 119, 730–796. [Google Scholar] [CrossRef] [PubMed]
- Pessoa, J.C.; Etcheverry, S.; Gambino, D. Vanadium compounds in medicine. Coord. Chem. Rev. 2015, 301–302, 24–48. [Google Scholar] [CrossRef] [PubMed]
- Bijelic, A.; Aureliano, M.; Rompel, A. Polyoxometalates as potential next-generation metallodrugs in the combat against cancer. Angew. Chem. Int. Ed. Engl. 2019, 58, 2980–2999. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crans, D.C.; Henry, L.; Cardiff, G.; Posner, B.I. Developing vanadium as an antidiabetic drug: A clinical and historical perspective. In Essential Metals in Medicine: Therapeutic Use and Toxicity of Metal Ions in the Clinic; Carver, P., Ed.; De Gruyter: Berlin, Germany, 2019; pp. 203–230. [Google Scholar]
- Kowalski, S.; Wyrzykowski, D.; Inkielewicz-Stępniak, I. Molecular and Cellular Mechanisms of Cytotoxic Activity of Vanadium Compounds against Cancer Cells. Molecules 2020, 25, 1757. [Google Scholar] [CrossRef]
- Levina, A.; Vieira, A.P.; Wijetunga, A.; Kaur, R.; Koehn, J.T.; Crans, D.C.; Lay, P.A. A Short-Lived but Highly Cytotoxic Vanadium(V) Complex as a Potential Drug Lead for Brain Cancer Treatment by Intratumoral Injections. Angew. Chem. Int. Ed. 2020, 59, 15834–15838. [Google Scholar] [CrossRef]
- Pisano, M.; Arru, C.; Serra, M.; Galleri, G.; Sanna, D.; Garribba, E.; Palmieri, G.; Rozzo, C. Antiproliferative activity of vanadium compounds: Effects on the major malignant melanoma molecular pathways. Metallomics 2019, 11, 1687–1699. [Google Scholar] [CrossRef]
- Scior, T.; Guevara-Garcia, J.A.; Do, Q.-T.; Bernard, H.; Laufer, S. Why antidiabetic vanadium complexes are not in the pipeline of “Big Pharma” drug research? A critical Review. Curr. Med. Chem. 2016, 23, 2874–2891. [Google Scholar] [CrossRef] [Green Version]
- Thompson, K.H.; Lichter, J.; LeBel, C.; Scaife, M.C.; McNeill, J.H.; Orvig, C. Vanadium treatment of type 2 diabetes: A view to the future. J. Inorg. Biochem. 2009, 103, 554–558. [Google Scholar] [CrossRef]
- Willsky, G.R.; Chi, L.-H.; Godzala, M., III; Kostyniak, P.J.; Smee, J.J.; Trujillo, A.M.; Alfano, J.A.; Ding, W.; Hu, Z.; Crans, D.C. Anti-diabetic effects of a series of vanadium dipicolinate complexes in rats with streptozotocin-induced diabetes. Coord. Chem. Rev. 2011, 255, 2258–2269. [Google Scholar] [CrossRef] [Green Version]
- Mosquillo, M.F.; Smircich, P.; Lima, A.; Gehrke, S.A.; Scalese, G.; Machado, I.; Gambino, D.; Garat, B.; Pérez-Díaz, L. High Throughput Approaches to Unravel the Mechanism of Action of a New Vanadium-Based Compound against Trypanosoma cruzi. Bioinorg. Chem. Appl. 2020, 2020, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scalese, G.; Benítez, J.; Rostan, S.; Correia, I.; Bradford, L.; Vieites, M.; Minini, L.; Merlino, A.; Coitiño, E.L.; Birriel, E.; et al. Expanding the family of heteroleptic oxidovanadium(IV) compounds with salicylaldehyde semicarbazones and polypyridyl ligands showing anti-Trypanosoma cruzi activity. J. Inorg. Biochem. 2015, 147, 116–125. [Google Scholar] [CrossRef]
- Scalese, G.; Mosquillo, M.F.; Rostán, S.; Castiglioni, J.; Alho, I.; Pérez, L.; Correia, I.; Marques, F.; Pessoa, J.C.; Gambino, D. Heteroleptic oxidovanadium(IV) complexes of 2-hydroxynaphtylaldimine and polypyridyl ligands against Trypanosoma cruzi and prostate cancer cells. J. Inorg. Biochem. 2017, 175, 154–166. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Xu, H.; Chen, W.; Zhan, P.; Liu, X. 8-Hydroxyquinoline: A privileged structure with a broad-ranging pharmacological potential. MedChemComm 2015, 6, 61–74. [Google Scholar] [CrossRef]
- Oliveri, V.; Vecchio, G. 8-Hydroxyquinolines in medicinal chemistry: A structural perspective. Eur. J. Med. Chem. 2016, 120, 252–274. [Google Scholar] [CrossRef]
- Prachayasittikul, V.; Prachayasittikul, V.; Prachayasittikul, S.; Ruchirawat, S. 8-Hydroxyquinolines: A review of their metal chelating properties and medicinal applications. Drug Des. Dev. Ther. 2013, 7, 1157–1178. [Google Scholar] [CrossRef] [Green Version]
- Hofheinz, R.-D.; Dittrich, C.; Jakupec, M.; Drescher, A.; Jaehde, U.; Gneist, M.; Keyserlingk, N.G.V.; Keppler, B.; Hochhaus, A. Early results from a phase I study on orally administered tris(8-quinolinolato)gallium(III) (FFC11, KP46) in patients with solid tumors—A CESAR study (Central European Society for Anticancer Drug Research—EWIV). Int. J. Clin. Pharmacol. Ther. 2005, 43, 590–591. [Google Scholar] [CrossRef]
- Sánchez, A.C.; Martín-Santos, C.; Padrón, J.M.; Mas-Ballesté, R.; Navarro-Ranninger, C.; Alemán, J.; Cabrera, S. Effect of electronic and steric properties of 8-substituted quinolines in gold(III) complexes: Synthesis, electrochemistry, stability, interactions and antiproliferative studies. J. Inorg. Biochem. 2017, 174, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Correia, I.; Adão, P.; Roy, S.; Wahba, M.; Matos, C.; Maurya, M.R.; Marques, F.; Pavan, F.R.; Leite, C.Q.F.; Avecilla, F.; et al. Hydroxyquinoline derived vanadium(IV and V) and copper(II) complexes as potential anti-tuberculosis and anti-tumor agents. J. Inorg. Biochem. 2014, 141, 83–93. [Google Scholar] [CrossRef] [PubMed]
- Duarte, M.C.; Lage, L.M.D.R.; Lage, D.P.; Mesquita, J.T.; Salles, B.C.S.; Lavorato, S.N.; Menezes-Souza, D.; Roatt, B.; Alves, R.J.; Tavares, C.A.P.; et al. An effective in vitro and in vivo antileishmanial activity and mechanism of action of 8-hydroxyquinoline against Leishmania species causing visceral and tegumentary leishmaniasis. Vet. Parasitol. 2016, 217, 81–88. [Google Scholar] [CrossRef]
- Duffin, R.; Blair, V.; Kedzierski, L.; Andrews, P.C. Alkyl gallium(III) quinolinolates: A new class of highly selective anti-leishmanial agents. Eur. J. Med. Chem. 2020, 186, 111895. [Google Scholar] [CrossRef]
- Scalese, G.; Machado, I.; Fontana, C.; Risi, G.; Salinas, G.; Pérez-Díaz, L.; Gambino, D. New heteroleptic oxidovanadium(V) complexes: Synthesis, characterization and biological evaluation as potential agents against Trypanosoma cruzi. JBIC J. Biol. Inorg. Chem. 2018, 23, 1265–1281. [Google Scholar] [CrossRef] [PubMed]
- Scalese, G.; Machado, I.; Correia, I.; Pessoa, J.C.; Bilbao, L.; Pérez-Diaz, L.; Gambino, D. Exploring oxidovanadium(iv) homoleptic complexes with 8-hydroxyquinoline derivatives as prospective antitrypanosomal agents. New J. Chem. 2019, 43, 17756–17773. [Google Scholar] [CrossRef]
- Gryca, I.; Czerwińska, K.; Machura, B.; Chrobok, A.; Shul’Pina, L.S.; Kuznetsov, M.L.; Nesterov, D.S.; Kozlov, Y.N.; Pombeiro, A.J.L.; Varyan, I.A.; et al. High Catalytic Activity of Vanadium Complexes in Alkane Oxidations with Hydrogen Peroxide: An Effect of 8-Hydroxyquinoline Derivatives as Noninnocent Ligands. Inorg. Chem. 2018, 57, 1824–1839. [Google Scholar] [CrossRef]
- Fernández, M.; Becco, L.; Correia, I.; Benítez, J.; Piro, O.E.; Echeverria, G.A.; Medeiros, A.; Comini, M.; Lavaggi, M.L.; González, M.; et al. Oxidovanadium(IV) and dioxidovanadium(V) complexes of tridentate salicylaldehyde semicarbazones: Searching for prospective antitrypanosomal agents. J. Inorg. Biochem. 2013, 127, 150–160. [Google Scholar] [CrossRef]
- Baran, E.J. Oxovanadium(IV) complexes of halogenated oxines. Chem. Mon. 1997, 128, 323–335. [Google Scholar] [CrossRef]
- Noblía, P.; Vieites, M.; Parajón-Costa, B.S.; Baran, E.J.; Cerecetto, H.; Draper, P.; Gonzalez, M.; Piro, O.E.; Castellano, E.E.; Azqueta, A.; et al. Vanadium(V) complexes with salicylaldehyde semicarbazone derivatives bearing in vitro anti-tumor activity toward kidney tumor cells (TK-10): Crystal structure of [VVO2(5-bromosalicylaldehyde semicarbazone)]. J. Inorg. Biochem. 2005, 99, 443–451. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, M.; Varela, J.; Correia, I.; Birriel, E.; Castiglioni, J.; Moreno, V.; Pessoa, J.C.; Cerecetto, H.; González, M.; Gambino, D. A new series of heteroleptic oxidovanadium(iv) compounds with phenanthroline-derived co-ligands: Selective Trypanosoma cruzi growth inhibitors. Dalton Trans. 2013, 42, 11900–11911. [Google Scholar] [CrossRef] [PubMed]
- Gambino, D.; Fernandez, M.; Santos, D.; Etcheverría, G.A.; Piro, O.E.; Pavan, F.R.; Leite, C.Q.; Tomaz, I.; Marques, F. Searching for gallium bioactive compounds: Gallium(III) complexes of tridentate salicylaldehyde semicarbazone derivatives. Polyhedron 2011, 30, 1360–1366. [Google Scholar] [CrossRef]
- Noblía, P.; Baran, E.J.; Otero, L.; Draper, P.; Cerecetto, H.; Gonzalez, M.; Piro, O.E.; Castellano, E.E.; Inohara, T.; Adachi, Y.; et al. New Vanadium(V) Complexes with Salicylaldehyde Semicarbazone Derivatives: Synthesis, Characterization, and in vitro Insulin-Mimetic Activity–Crystal Structure of [VvO2(salicylaldehyde semicarbazone)]. Eur. J. Inorg. Chem. 2004, 2004, 322–328. [Google Scholar] [CrossRef]
- Nica, S.; Rudolph, M.; Görls, H.; Plass, W. Structural characterization and electrochemical behavior of oxovanadium(V) complexes with N-salicylidene hydrazides. Inorg. Chim. Acta 2007, 360, 1743–1752. [Google Scholar] [CrossRef]
- Benítez, J.; Becco, L.; Correia, I.; Leal, S.M.; Guiset, H.; Pessoa, J.; Lorenzo, J.; Tanco, S.; Escobar, P.; Moreno, V.; et al. Vanadium polypyridyl compounds as potential antiparasitic and antitumoral agents: New achievements. J. Inorg. Biochem. 2011, 105, 303–312. [Google Scholar] [CrossRef]
- Benítez, J.; Guggeri, L.; Tomaz, I.; Pessoa, J.C.; Moreno, V.; Lorenzo, J.; Avilés, F.X.; Garat, B.; Gambino, D. A novel vanadyl complex with a polypyridyl DNA intercalator as ligand: A potential anti-protozoa and anti-tumor agent. J. Inorg. Biochem. 2009, 103, 1386–1394. [Google Scholar] [CrossRef]
- Rivas, F.; Medeiros, A.; Comini, M.; Suescun, L.; Arce, E.R.; Martins, M.; Pinheiro, T.; Marques, F.; Gambino, D. Pt-Fe ferrocenyl compounds with hydroxyquinoline ligands show selective cytotoxicity on highly proliferative cells. J. Inorg. Biochem. 2019, 199, 110779. [Google Scholar] [CrossRef]
- Correia, I.; Roy, S.; de Matos, C.P.; Borovic, S.; Butenko, N.; Cavaco, I.; Marques, F.; Lorenzo, J.; Rodríguez, A.; Moreno, V.; et al. Vanadium(IV) and copper(II) complexes of salicylaldimines and aromatic heterocycles: Cytotoxicity, DNA binding and DNA cleavage properties. J. Inorg. Biochem. 2015, 147, 134–146. [Google Scholar] [CrossRef]
- Levina, A.; Crans, D.; Lay, P.A. Speciation of metal drugs, supplements and toxins in media and bodily fluids controls in vitro activities. Co-ord. Chem. Rev. 2017, 352, 473–498. [Google Scholar] [CrossRef]
- Levina, A.; Lay, P.A. Stabilities and Biological Activities of Vanadium Drugs: What is the Nature of the Active Species? Chem. Asian J. 2017, 12, 1692–1699. [Google Scholar] [CrossRef] [PubMed]
- Docampo, R.; Vercesi, A.E. Mitochondrial Ca2+ and Reactive Oxygen Species in Trypanosomatids. Antioxid. Redox Signal. 2021. [Google Scholar] [CrossRef] [PubMed]
- Menna-Barreto, R.F.S. Cell death pathways in pathogenic trypanosomatids: Lessons of (over)kill. Cell Death Dis. 2019, 10, 1–11. [Google Scholar] [CrossRef]
- Wu, J.-X.; Hong, Y.-H.; Yang, X.-G. Bis(acetylacetonato)-oxidovanadium(IV) and sodium metavanadate inhibit cell proliferation via ROS-induced sustained MAPK/ERK activation but with elevated AKT activity in human pancreatic cancer AsPC-1 cells. JBIC J. Biol. Inorg. Chem. 2016, 21, 919–929. [Google Scholar] [CrossRef] [PubMed]
- Bustos, P.L.; Volta, B.J.; Perrone, A.E.; Milduberger, N.; Bua, J. A homolog of cyclophilin D is expressed in Trypanosoma cruzi and is involved in the oxidative stress–damage response. Cell Death Discov. 2017, 3, 16092. [Google Scholar] [CrossRef] [Green Version]
- Lepecq, J.B.; Paoletti, C. A fluorescent complex between ethidium bromide and nucleic acids: Physical-chemical characterization. J. Mol. Biol. 1967, 27, 87–106. [Google Scholar] [CrossRef]
- Cipriani, M.; Toloza, J.; Bradford, L.; Putzu, E.; Vieites, M.; Curbelo, E.; Tomaz, A.I.; Garat, B.; Guerrero, J.; Gancheff, J.S.; et al. Effect of the Metal Ion on the anti T. cruzi Activity and Mechanism of Action of 5-Nitrofuryl-Containing Thiosemicarbazone Metal Complexes. Eur. J. Inorg. Chem. 2014, 2014, 4677–4689. [Google Scholar] [CrossRef]
- Rivas, F.; Medeiros, A.; Arce, E.R.; Comini, M.; Ribeiro, C.M.; Pavan, F.; Gambino, D. New heterobimetallic ferrocenyl derivatives: Evaluation of their potential as prospective agents against trypanosomatid parasites and Mycobacterium tuberculosis. J. Inorg. Biochem. 2018, 187, 73–84. [Google Scholar] [CrossRef] [Green Version]
- Arce, E.R.; Sarniguet, C.; Moraes, T.S.; Vieites, M.; Tomaz, A.I.; Medeiros, A.; Comini, M.; Varela, J.; Cerecetto, H.; González, M.; et al. A new ruthenium cyclopentadienyl azole compound with activity on tumor cell lines and trypanosomatid parasites. J. Co-ord. Chem. 2015, 68, 2923–2937. [Google Scholar] [CrossRef]
- Carretero, M.; Solis, G.M.; Petrascheck, M.C. Elegans as Model for Drug Discovery. Curr. Top. Med. Chem. 2017, 17, 2067–2076. [Google Scholar] [CrossRef]
- Krause, L.; Herbst-Irmer, R.; Sheldrick, G.M.; Stalke, D. Comparison of silver and molybdenum microfocus X-ray sources for single-crystal structure determination. J. Appl. Crystallogr. 2015, 48, 3–10. [Google Scholar] [CrossRef] [Green Version]
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A Found. Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef] [Green Version]
- Hübschle, C.B.; Sheldrick, G.M.; Dittrich, B. ShelXle: A Qt graphical user interface for SHELXL. J. Appl. Crystallogr. 2011, 44, 1281–1284. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez-Arce, E.; Mosquillo, M.F.; Pérez-Díaz, L.; Echeverría, G.A.; Piro, O.E.; Merlino, A.; Coitiño, E.L.; Ribeiro, C.M.; Leite, C.Q.F.; Pavan, F.; et al. Aromatic amine N-oxide organometallic compounds: Searching for prospective agents against infectious diseases. Dalton Trans. 2015, 44, 14453–14464. [Google Scholar] [CrossRef] [PubMed]
- MacLean, L.M.; Thomas, J.; Lewis, M.D.; Cotillo, I.; Gray, D.W.; De Rycker, M. Development of Trypanosoma cruzi in vitro assays to identify compounds suitable for progression in Chagas’ disease drug discovery. PLoS Negl. Trop. Dis. 2018, 12, e0006612. [Google Scholar] [CrossRef]
- Vieites, M.; Smircich, P.; Parajón-Costa, B.; Rodríguez, J.; Galaz, V.; Olea-Azar, C.; Otero, L.; Aguirre, G.; Cerecetto, H.; González, M.; et al. Potent in vitro anti-Trypanosoma cruzi activity of pyridine-2-thiol N-oxide metal complexes having an inhibitory effect on parasite-specific fumarate reductase. JBIC J. Biol. Inorg. Chem. 2008, 13, 723–735. [Google Scholar] [CrossRef] [PubMed]
- Pavan, F.R.; Maia, P.I.D.S.; Leite, S.R.; Deflon, V.M.; Batista, A.A.; Sato, D.N.; Franzblau, S.G.; Leite, C.Q. Thiosemicarbazones, semicarbazones, dithiocarbazates and hydrazide/hydrazones: Anti-Mycobacterium tuberculosis activity and cytotoxicity. Eur. J. Med. Chem. 2010, 45, 1898–1905. [Google Scholar] [CrossRef] [PubMed]
- Kessler, R.; Soares, M.; Probst, C.M.; Krieger, M.A. Trypanosoma cruzi Response to Sterol Biosynthesis Inhibitors: Morphophysiological Alterations Leading to Cell Death. PLoS ONE 2013, 8, e55497. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mosquillo, M.F.; Bilbao, L.; Hernández, F.; Tissot, F.; Gambino, D.; Garat, B.; Pérez-Díaz, L. Trypanosoma cruzi biochemical changes and cell death induced by an organometallic platinum-based compound. Chem. Biol. Drug Des. 2018, 92, 1657–1669. [Google Scholar] [CrossRef]
- Zuma, A.A.; da Silva, R.B.; Garden, S.J.; de Souza, W. In vitro study of the trypanocidal activity of anilinophenanthrolines against Trypanosoma cruzi. Parasitol. Int. 2021, 83, 102338. [Google Scholar] [CrossRef] [PubMed]
- Barton, J.K.; Goldberg, J.M.; Kumar, C.V.; Turro, N.J. Binding modes and base specificity of tris(phenanthroline)ruthenium(II) enantiomers with nucleic scids: Tuning the stereoselectivity. J. Am. Chem. Soc. 1986, 108, 2081–2088. [Google Scholar] [CrossRef]
- Dighe, S.; Khan, S.; Soni, I.; Jain, P.; Shukla, S.; Yadav, R.; Sen, P.; Meeran, S.M.; Batra, S. Synthesis of β-Carboline-Based N-Heterocyclic Carbenes and Their Antiproliferative and Antimetastatic Activities against Human Breast Cancer Cells. J. Med. Chem. 2015, 58, 3485–3499. [Google Scholar] [CrossRef] [PubMed]
- Lakowicz, J.R. Principles of Fluorescence Spectroscopy, 3rd ed.; Springer: Singapore, 2006. [Google Scholar] [CrossRef]
- Darabi, F.; Hadadzadeh, H.; Ebrahimi, M.; Khayamian, T.; Rudbari, H.A. The piroxicam complex of cobalt(II): Synthesis in two different ionic liquids, structure, DNA- and BSA interaction and molecular modeling. Inorg. Chim. Acta 2014, 409, 379–389. [Google Scholar] [CrossRef]
- Brenner, S. The genetics of Caenorhabditis elegans. Genetics 1974, 77, 71–94. [Google Scholar] [CrossRef]
- Risi, G.; Aguilera, E.; Ladós, E.; Suárez, G.; Carrera, I.; Álvarez, G.; Salinas, G. Caenorhabditis elegans Infrared-Based Motility Assay Identified New Hits for Nematicide Drug Development. Vet. Sci. 2019, 6, 29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simonetta, S.H.; Golombek, D.A. An automated tracking system for Caenorhabditis elegans locomotor behavior and circadian studies application. J. Neurosci. Methods 2007, 161, 273–280. [Google Scholar] [CrossRef] [PubMed]









| Compound | IC50 VERO ± SD (μM) | IC50 Epimastigotes T. cruzi ± SD (μM) | SI a | IC50 Trypomastigotes T. cruzi ± SD (μM) | SI b |
|---|---|---|---|---|---|
| Nifurtimox | 998.5 ± 90.6 c | 3.68 ± 0.71 | 271 | 20.10 ± 2.86 | 49.6 |
| L0 | 10.1 ± 3.9 c | 9.42 ± 2.23 | 1.1 | 0.47 ± 0.07 | 21.5 |
| L1 | 66.5 ± 13.1 c | 7.17 ± 1.07 | 9.3 | 2.25 ± 0.95 | 29.5 |
| L2 | 64.7 ± 9.1 c | 2.76 ± 0.52 | 23.4 | 0.40 ± 0.08 | 162 |
| L3 | 75.5 ± 16.3 c | 2.17 ± 0.35 | 34.8 | 1.10 ± 0.88 | 68.6 |
| L4 | 12.5 ± 4.5 c | 2.58 ± 0.56 | 4.8 | 1.01 ± 0.73 | 12.3 |
| IN | 22.64 ± 3.7 | >20 | ≤1 | >20 | ≤1 |
| [VVO(IN–2H)(L0–H)] | 10.99 ± 2.1 | >15 | ≤1 | 1.92 ± 0.33 | 5.7 |
| [VVO(IN–2H)(L1–H)] | 22.03 ± 7.4 | 3.45 ± 0.48 | 6.4 | 1.46 ± 0.05 | 15.1 |
| [VVO(IN–2H)(L2–H)] | 31.54 ± 3.4 | 3.99 ± 1.30 | 7.9 | 0.29 ± 0.08 | 109 |
| [VVO(IN–2H)(L3–H)] | 54.18 ± 4.0 | 7.70 ± 0.67 | 7.0 | 1.69 ± 0.07 | 32.1 |
| [VVO(IN–2H)(L4–H)] | 42.09 ± 9.4 | 5.55 ± 1.41 | 7.6 | 3.02 ± 0.98 | 14.0 |
| [VVO2(IN-H)] | >50 | >20 | nd | >20 | nd |
| Compound Concencentration (μM) b | % Entry a ± SD | |||
|---|---|---|---|---|
| Epimastigotes | Trypomastigotes | |||
| 4 h | 24 h | 4 h | 24 h | |
| 1 × IC50 | 4.5 ± 0.4 | 4.4 ± 0.7 | 7.1 ± 0.4 | 8.6 ± 0.4 |
| 5 × IC50 | 2.4 ± 0.2 | 3.4 ± 0.1 | 3.0 ± 0.1 | 4.3 ± 0.1 |
| 10 × IC50 | 2.0 ± 0.4 | 3.6 ± 0.05 | 3.1 ± 0.1 | 5.0 ± 0.1 |
| Compound | log (KSV) |
|---|---|
| IN | 4.29 |
| L2 | 4.68 |
| [VVO(IN-2H)(L2-H)] | 4.51 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scalese, G.; Machado, I.; Salinas, G.; Pérez-Díaz, L.; Gambino, D. Heteroleptic Oxidovanadium(V) Complexes with Activity against Infective and Non-Infective Stages of Trypanosoma cruzi. Molecules 2021, 26, 5375. https://doi.org/10.3390/molecules26175375
Scalese G, Machado I, Salinas G, Pérez-Díaz L, Gambino D. Heteroleptic Oxidovanadium(V) Complexes with Activity against Infective and Non-Infective Stages of Trypanosoma cruzi. Molecules. 2021; 26(17):5375. https://doi.org/10.3390/molecules26175375
Chicago/Turabian StyleScalese, Gonzalo, Ignacio Machado, Gustavo Salinas, Leticia Pérez-Díaz, and Dinorah Gambino. 2021. "Heteroleptic Oxidovanadium(V) Complexes with Activity against Infective and Non-Infective Stages of Trypanosoma cruzi" Molecules 26, no. 17: 5375. https://doi.org/10.3390/molecules26175375
APA StyleScalese, G., Machado, I., Salinas, G., Pérez-Díaz, L., & Gambino, D. (2021). Heteroleptic Oxidovanadium(V) Complexes with Activity against Infective and Non-Infective Stages of Trypanosoma cruzi. Molecules, 26(17), 5375. https://doi.org/10.3390/molecules26175375