Effective Utilisation of Halophyte Biomass from Saline Soils for Biorefinering Processes
Abstract
:1. Introduction
2. Results and Discussion
2.1. Bioethanol Production Process
2.1.1. Halophyte Biomass Pretreatment
2.1.2. Enzyme Complex
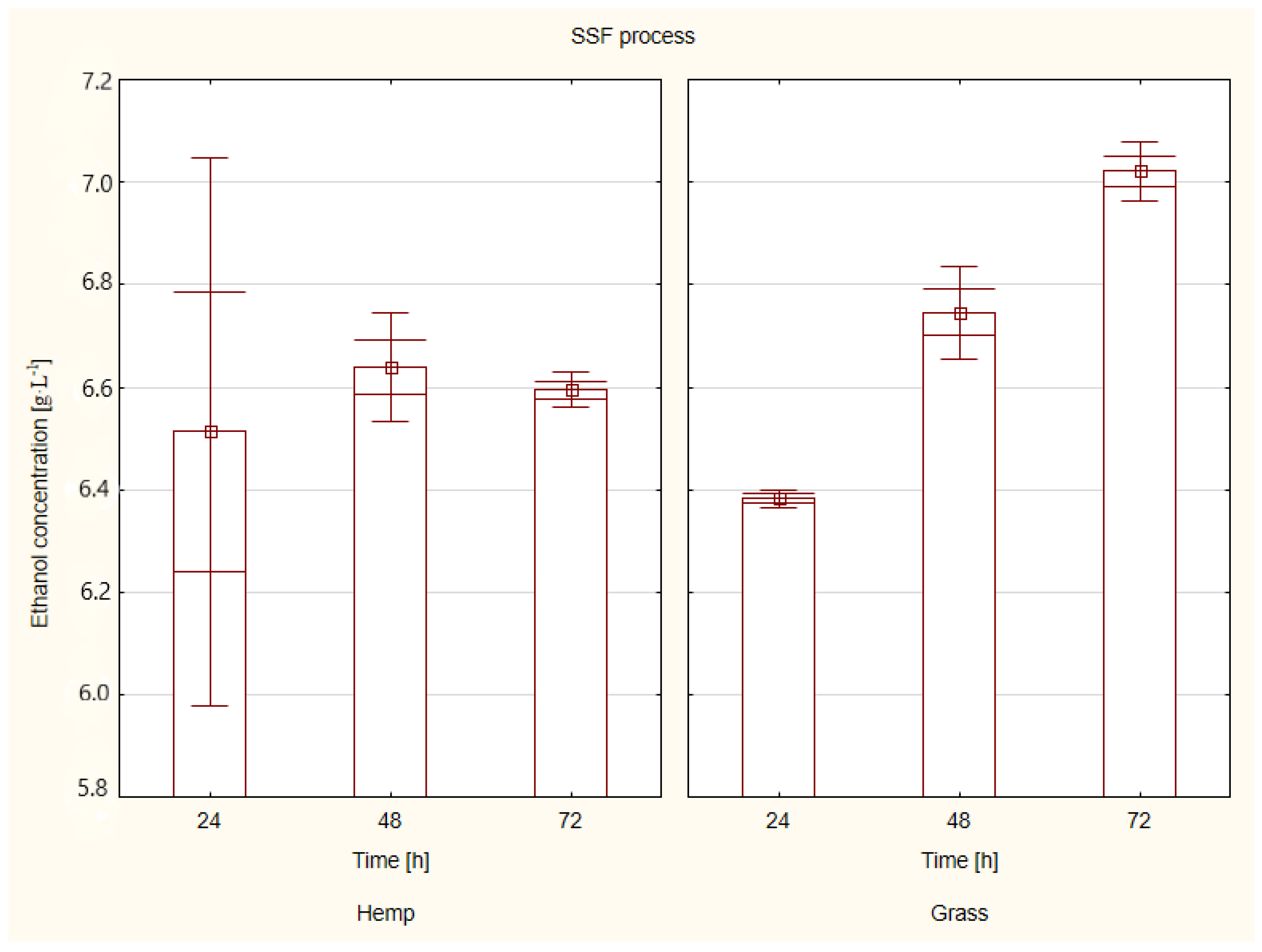
2.1.3. Simultaneous Saccharification and Fermentation (SSF)
2.2. Biocomposite Production Process
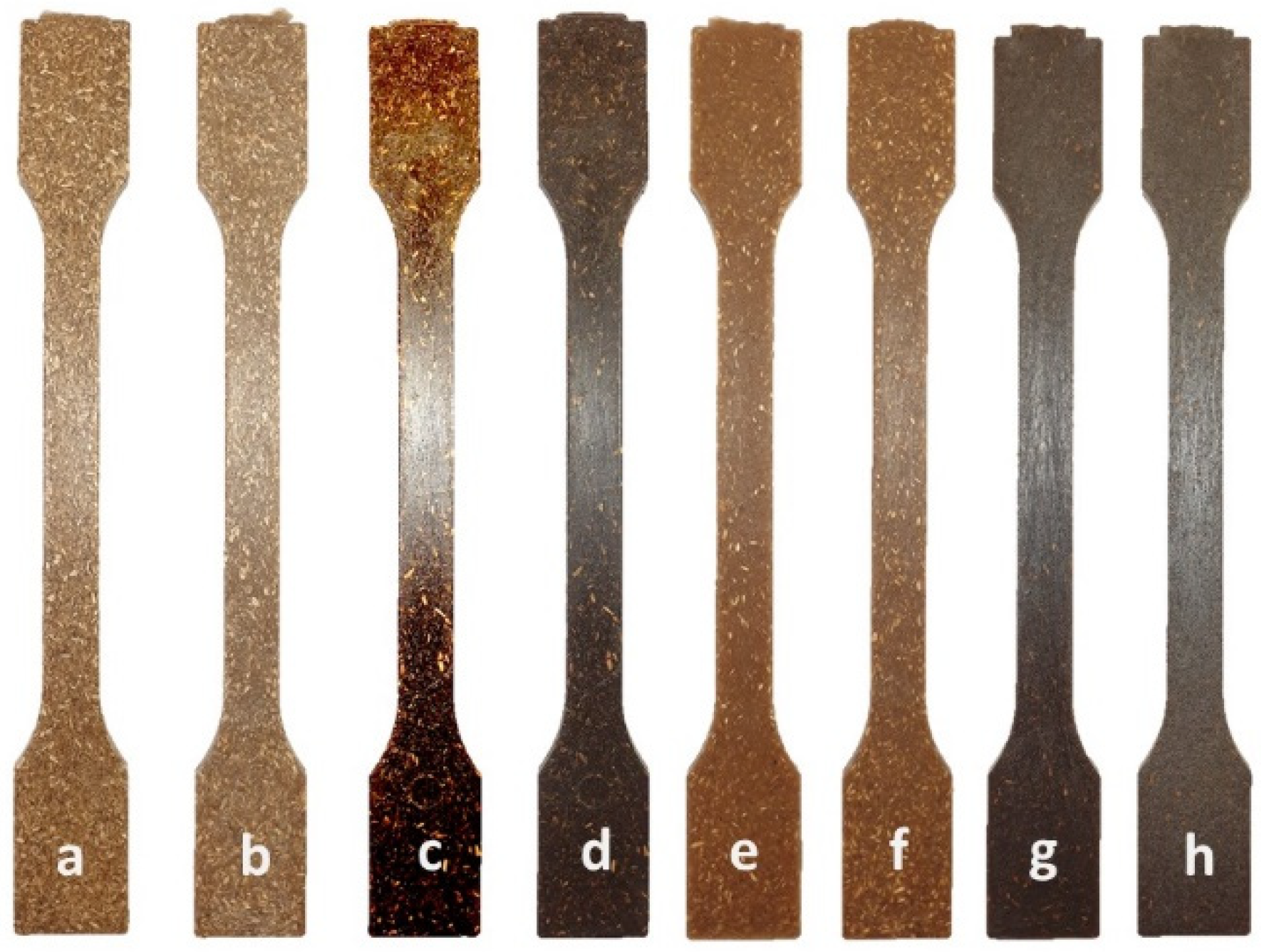
2.2.1. Fillers from Halophyte Biomass
2.2.2. Mechanical Properties of Biocomposites
3. Materials and Methods
3.1. Halophyte Biomass
3.2. Bioethanol Production Process
3.2.1. Halophyte Biomass Pretreatment
3.2.2. Enzyme Complex
3.2.3. Simultaneous Saccharification and Fermentation (SSF)
3.3. Biocomposite Production Process
3.3.1. Natural Fillers from Halophyte Biomass
3.3.2. Polymer Matrix
3.3.3. Preparation of Composites
3.4. Analytical and Testing Methods
3.5. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Hendricks, R.C.; Bushnell, D.M. Atmospheric and soil carbon and halophytes. In Proceedings of the 13th International Symposium on Transport Phenomena and Dynamics of Rotating Machinery (ISROMAC-13), Honolulu, HI, USA, 4–7 April 2010; p. 113. [Google Scholar]
- Shrivastava, P.; Kumar, R. Soil salinity: A serious environmental issue and plant growth promoting bacteria as one of the tools for its alleviation. Saudi J. Biol. Sci. 2015, 22, 123–131. [Google Scholar] [CrossRef] [Green Version]
- Hameed, A.; Khan, M.A. Halophytes: Biology and economic potentials. Karachi Univ. J. Sci. 2011, 39, 40–44. [Google Scholar]
- Zadrożniak, B.; Radwańska, K.; Baranowska, A.; Mystkowska, I. Possibility of industrial hemp cultivation in areas of high nature value. Econ. Reg. Stud. 2017, 10, 114–127. [Google Scholar] [CrossRef] [Green Version]
- Wawro, A.; Batog, J.; Gieparda, W. Chemical and enzymatic treatment of hemp biomass for bioethanol production. Appl. Sci. 2019, 9, 5348. [Google Scholar] [CrossRef] [Green Version]
- Hu, H.; Liu, H.; Liu, F. Seed germination of hemp (Cannabis sativa L.) cultivars responds differently to the stress of salt type and concentration. Ind. Crop. Prod. 2018, 123, 254–261. [Google Scholar] [CrossRef]
- Mohapatra, S.; Mishra, C.; Behera, S.S.; Thatoi, H. Application of pretreatment, fermentation and molecular techniques for enhancing bioethanol production from grass biomass—A review. Renew. Sustain. Energy Rev. 2017, 78, 1007–1032. [Google Scholar] [CrossRef]
- Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=CELEX:32009L0028 (accessed on 8 April 2021).
- Abo, B.O.; Gao, M.; Wang, Y.; Wu, C.; Ma, H.; Wang, Q. Lignocellulosic biomass for bioethanol: An overview on pretreatment, hydrolysis and fermentation processes. Rev. Environ. Health 2019, 34, 57–68. [Google Scholar] [CrossRef]
- Rahmati, S.; Doherty, W.; Dubal, D.; Atanda, L.; Moghaddam, L.; Sonar, P.; Hessel, V.; Ostrikov, K. Pretreatment and fermentation of lignocellulosic biomass: Reaction mechanisms and process engineering. React. Chem. Eng. 2020, 5, 2017–2047. [Google Scholar] [CrossRef]
- Vasić, K.; Knez, Ž.; Leitgeb, M. Bioethanol production by enzymatic hydrolysis from different lignocellulosic sources. Molecules 2021, 26, 753. [Google Scholar] [CrossRef] [PubMed]
- European Council. Directive (EU) 2019/904 of the European Parliament and of the Council of 5 June 2019 on the Reduction of the Impact of Certain Plastic Products on the Environment. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/HTML/?uri=CELEX:32019L0904&from=HU (accessed on 1 February 2021).
- Moore, C. Plastic Pollution. Encyclopedia Britannica. Available online: https://www.britannica.com/science/plastic-pollution (accessed on 18 June 2021).
- Getme, A.S.; Patel, B. A review: Bio-fiber’s as reinforcement in composites of polylactic acid (PLA). Mater. Today Proc. 2019, 26, 2116–2122. [Google Scholar] [CrossRef]
- Das, P.P.; Chaudhary, V. Moving towards the era of bio fibre based polymer composites. Clean. Eng. Technol. 2021, 4, 100182. [Google Scholar] [CrossRef]
- Manral, A.; Ahmad, F.; Chaudhary, V. Static and dynamic mechanical properties of PLA bio-composite with hybrid reinforcement of flax and jute. Mater. Today Proc. 2020, 25, 577–580. [Google Scholar] [CrossRef]
- Papadopoulou, E.; Bikiaris, D.; Chrysafis, K.; Władyka-Przybylak, M.; Wesołek, D.; Mankowski, J.; Kołodziej, J.; Baraniecki, P.; Bujnowicz, K.; Gronberg, V. Value-added industrial products from bast fiber crops. Ind. Crop. Prod. 2015, 68, 116–125. [Google Scholar] [CrossRef]
- Gąsiorowski, R.; Rojewski, S.; Wesołek, D.; Maciejewski, H.; Bujnowicz, K. The influence of lignocellulosic filler modification with silicon compounds on flammability and adhesion with a composite polymer matrix. Polym. Process. 2013, 19, 336–339. [Google Scholar]
- Akinshina, N.; Toderich, K.; Azizov, A.; Saito, L.; Ismail, S. Halophyte biomass—A promising source of renewable energy. J. Arid Land Stud. 2014, 24, 231–235. [Google Scholar]
- Turcios, A.E.; Cayenne, A.; Uellendahl, H.; Papenbrock, J. Halophyte plants and their residues as feedstock for biogas production-chances and challenges. Appl. Sci. 2021, 11, 2746. [Google Scholar] [CrossRef]
- Kumar, D.; Murthy, G.S. Impact of pretreatment and downstream processing technologies on economics and energy in cellulosic ethanol production. Biotechnol. Biofuels 2011, 4, 27. [Google Scholar] [CrossRef] [Green Version]
- Stevulova, N.; Cigasova, J.; Estokova, A.; Terpakova, E.; Geert, A.; Kacik, F.; Singovszka, E.; Holub, M. Properties characterization of chemically modified hemp hurds. Materials 2014, 7, 8131–8150. [Google Scholar] [CrossRef] [PubMed]
- Araújo, D.; Vilarinho, M.; Machado, A. Effect of combined dilute-alkaline and green pretreatments on corncob fractionation: Pretreated biomass characterization and regenerated cellulose film production. Ind. Crop. Prod. 2019, 141, 111785. [Google Scholar] [CrossRef]
- Ciolacu, D.; Ciolacu, F.; Popa, V.I. Amorphous cellulose—Structure and characterization. Cellul. Chem. Technol. 2011, 45, 13–21. [Google Scholar]
- Sun, X.F.; Xu, F.; Sun, R.C.; Fowler, P.; Baird, M.S. Characteristics of degraded cellulose obtained from steam-exploded wheat straw. Carbohydr. Res. 2005, 340, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.D.; Pandey, S.; Kumar Jaiswal, V.; Bhadauria, V.; Singh, H. Simultaneous oxidation and esterification of cellulose for use in treatment of water containing Cu (II) ions. Carbohydr. Polym. 2019, 222, 114964. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Xu, Y.; Wang, W.; Griffin, J.; Wang, D. High Ethanol concentration (77 g/L) of industrial hemp biomass achieved through optimizing the relationship between ethanol yield/concentration and solid loading. ACS Omega 2020, 5, 21913–21921. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Xu, Y.; Zhang, M.; Wang, D. Integrating bran starch hydrolysates with alkaline pretreated soft wheat bran to boost sugar concentration. Bioresour. Technol. 2020, 302, 122826. [Google Scholar] [CrossRef] [PubMed]
- Abraham, R.E.; Barrow, C.J.; Puri, M. Relationship to reducing sugar production and scanning electron microscope structure to pretreated hemp hurd biomass (Cannabis sativa). Biomass Bioenerg. 2013, 58, 180–187. [Google Scholar] [CrossRef]
- Kumar, D.; Murthy, G.S. Pretreatments and enzymatic hydrolysis of grass straws for ethanol production in the Pacific Northwest U.S. Biol. Eng. 2011, 3, 97–110. [Google Scholar] [CrossRef]
- Wawro, A.; Batog, J.; Gieparda, W. Efektywność enzymatycznej konwersji biomasy sorgo i konopi do glukozy. Przemysł Chem. 2020, 99, 1731–1734. [Google Scholar]
- Robak, K.; Balcerek, M. Current state-of-the-art in ethanol production from lignocellulosic feedstocks. Microbiol. Res. 2020, 240, 126534. [Google Scholar] [CrossRef]
- Taufikurahman, T.; Xie, S. Production of Bioethanol and Crude Cellulase Enzyme Extract from Napier Grass (Pennisetum purpureum S.) through Simultaneous Saccharification and Fermentation. Bio J. Biol. Sci. Technol. Man. 2020, 2, 18–29. [Google Scholar] [CrossRef]
- Riadi, L.; Hansen, Y.; Pratiwi, J.; Goretti, M.; Purwanto, M. Mild Alkaline Pretreatment on Sugarcane Bagasse: Effects of Pretreatment Time and Lime to Dry Bagasse Ratio. B Life Environ. Sci. 2020, 57, 43–50. [Google Scholar]
- Orlygsson, J. Ethanol production from biomass by a moderate thermophile, Clostridium AK1. Icel. Agric. Sci. 2012, 25, 25–35. [Google Scholar]
- International Standards Organization. PN-EN ISO 3167:2014, Plastics—Multipurpose Test Specimens; Polish Committee for Standardization: Geneva, Switzerland, 2014. [Google Scholar]
- Yan, Z.L.; Wang, H.; Lau, K.T.; Pather, S.; Zhang, J.C.; Lin, G.; Ding, Y. Reinforcement of polypropylene with hemp fibres. Compos. Part B Eng. 2013, 46, 221–226. [Google Scholar] [CrossRef]
- Yu, T.; Jiang, N.; Li, Y. Study on short ramie fiber/poly(lactic acid) composites compatibilized by maleic anhydride. Compos. Part A Appl. Sci. Manuf. 2014, 64, 139–146. [Google Scholar] [CrossRef]
- Batog, J.; Wawro, A. Chemical and biological deconstruction in the conversion process of sorghum biomass for bioethanol. J. Nat. Fibers 2021, 1–12. [Google Scholar] [CrossRef]
- Miller, G.L. Use of dinitrosalicylic acid reagent for determination of reducing sugars. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Kawa-Rygielska, J.; Pietrzak, W. Zagospodarowanie odpadowe pieczywa do produkcji bioetanolu. Żywność Nauka Technol. Jakość 2011, 79, 105–118. [Google Scholar]
- TAPPI 17 m-55. In Cellulose in Wood; TAPPI Press: Atlanta, GA, USA, 1955.
- TAPPI T9 m-54. In Holocellulose in Wood; TAPPI Press: Atlanta, GA, USA, 1998.
- Bagby, M.O.; Nelson, G.H.; Helman, E.G.; Clark, T.F. Determination of lignin non-wood plant fiber sources. TAPPI J. 1971, 54, 11. [Google Scholar]
- International Standards Organization. PN-EN ISO 527-1:2020, Plastics—Determination of Tensile Properties—Part 1: General Principles; Polish Committee for Standardization: Geneva, Switzerland, 2020. [Google Scholar]
- International Standards Organization. PN-EN ISO 527-2:2012, Plastics—Determination of Tensile Properties—Part 2: Test Conditions for Moulding and Extrusion Plastics; Polish Committee for Standardization: Geneva, Switzerland, 2012. [Google Scholar]
- International Standards Organization. PN-EN ISO 178:2019, Plastics—Determination of Flexural Properties; Polish Committee for Standardization: Geneva, Switzerland, 2019. [Google Scholar]



| Halophyte | Sample | Reducing Sugars (mg·g−1) |
|---|---|---|
| Grass | BP | 100.20 ± 0.09 |
| AP | 354.59 ± 0.01 | |
| Hemp | BP | 62.95 ± 0.10 |
| AP | 187.95 ± 0.13 |
| Halophyte | Sample | Cellulose (%) | Hemicellulose (%) | Lignin (%) |
|---|---|---|---|---|
| Grass | BP | 33.69 ± 0.40 | 34.74 ± 0.39 | 17.08 ± 0.16 |
| AP | 50.41 ± 0.18 | 25.23 ± 0.37 | 12.35 ± 0.07 | |
| Hemp | BP | 47.34 ± 0.40 | 33.49 ± 0.68 | 13.94 ± 0.05 |
| AP | 58.46 ± 0.29 | 22.12 ± 0.13 | 17.35 ± 0.26 |
| Enzyme | Reducing Sugar (mg·g−1) | |
|---|---|---|
| Hemp | Grass | |
| Flashzyme Plus 200 | 338 ± 0.04 | 846 ± 1.00 |
| Celluclast 1.5 L | 342 ± 0.05 | 696 ± 0.52 |
| Flashzyme/Celluclast 1.5 L (70/30) | 420 ± 0.06 | 892 ± 0.02 |
| Flashzyme/Celluclast 1.5 L (50/50) | 430 ± 0.05 | 800 ± 0.44 |
| Flashzyme/Celluclast 1.5 L (30/70) | 355 ± 0.38 | 810 ± 0.34 |
| Flashzyme/Celluclast 1.5 L (50/50)/β-glucosidase | 351 ± 0.14 | - |
| Flashzyme/Celluclast 1.5 L (50/50)/xylanase | 324 ± 0.65 | - |
| Flashzyme/Celluclast 1.5 L (50/50)/β-glucosidase/xylanase | 343 ± 0.16 | - |
| Flashzyme/Celluclast 1.5 L (70/30)/β-glucosidase | - | 472 ± 3.29 |
| Flashzyme/Celluclast 1.5 L (70/30)/xylanase | - | 458 ± 1.81 |
| Flashzyme/Celluclast 1.5 L (70/30)/β-glucosidase/xylanase | - | 735 ± 1.86 |
| Plant Biomass | Humidity (%) | Particle Size Distribution (%) | ||||||
|---|---|---|---|---|---|---|---|---|
| 1 mm | 0.5 mm | 0.4 mm | 0.25 mm | 0.2 mm | 0.1 mm | Below 0.1 mm | ||
| Grass | 8.73 | 1.2 | 48.3 | 8.3 | 30.5 | 2.5 | 2.7 | 6.5 |
| Hemp | 7.65 | 1.1 | 53.8 | 14.7 | 15.8 | 8.5 | 2.6 | 3.5 |
| Sample | Tensile Strength δM (MPa) | Tensile Modulus Et (GPa) | Flexular Strength δfM (MPa) | Flexural Modulus Ef (GPa) |
|---|---|---|---|---|
| PP HP648T | 31.0 ± 0.27 | 1.5 ± 0.02 | 41.5 ± 0.48 | 1.2 ± 0.13 |
| PP-H20 | 23.3 ± 0.25 | 1.7 ± 0.04 | 39.5 ± 0.60 | 1.8 ± 0.17 |
| PP-H20S5 | 27.5 ± 0.57 | 1.8 ± 0.05 | 40.7 ± 0.75 | 2.7 ± 0.16 |
| PP-H30 | 19.3 ± 0.21 | 2.3 ± 0.03 | 37.0 ± 0.70 | 2.5 ± 0.22 |
| PP-H30S5 | 31.4 ± 0.21 | 2.8 ± 0.05 | 51.6 ± 0.77 | 3.1 ± 0.14 |
| Sample | Tensile Strength δM (MPa) | Tensile Modulus Et (GPa) | Flexular Strength δfM (MPa) | Flexural Modulus Ef (GPa) |
|---|---|---|---|---|
| PP HP648T | 31.0 ± 0.27 | 1.5 ± 0.02 | 41.5 ± 0.48 | 1.2 ± 0.13 |
| PP-G20 | 23.6 ± 0.19 | 1.7 ± 0.04 | 39.5 ± 0.27 | 1.3 ± 0.08 |
| PP-G20S5 | 28.1 ± 0.25 | 1.8 ± 0.07 | 40.3 ± 0.35 | 1.4 ± 0.09 |
| PP-G30 | 20.4 ± 0.14 | 2.0 ± 0.03 | 41.0 ± 0.40 | 1.9 ± 0.17 |
| PP-G30S5 | 24.4 ± 0.27 | 2.0 ± 0.05 | 44.4 ± 0.27 | 1.9 ± 0.11 |
| Sample | Tensile Strength δM (MPa) | Tensile Modulus Et (GPa) | Flexular Strength δfM (MPa) | Flexural Modulus Ef (GPa) |
|---|---|---|---|---|
| PLA 3260HP | 64.5 ± 1.25 | 3.5 ± 0.07 | 108.6 ± 0.99 | 3.4 ± 0.14 |
| PLA-H20 | 51.4 ± 0.99 | 5.5 ± 0.04 | 88.0 ± 2.37 | 4.9 ± 0.21 |
| PLA-H30 | 53.0 ± 1.17 | 6.7 ± 0.05 | 94.7 ± 1.76 | 6.6 ± 0.18 |
| Sample | Tensile Strength δM (MPa) | Tensile Modulus Et (GPa) | Flexular Strength δfM (MPa) | Flexural Modulus Ef (GPa) |
|---|---|---|---|---|
| PLA 3260HP | 64.5 ± 1.25 | 3.5 ± 0.07 | 108.6 ± 0.99 | 3.4 ± 0.14 |
| PLA-G20 | 49.1 ± 0.30 | 3.9 ± 0.02 | 83.6 ± 0.43 | 3.8 ± 0.11 |
| PLA-G30 | 41.2 ± 1.22 | 4.1 ± 0.02 | 78.1 ± 0.93 | 4.0 ± 0.20 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Batog, J.; Bujnowicz, K.; Gieparda, W.; Wawro, A.; Rojewski, S. Effective Utilisation of Halophyte Biomass from Saline Soils for Biorefinering Processes. Molecules 2021, 26, 5393. https://doi.org/10.3390/molecules26175393
Batog J, Bujnowicz K, Gieparda W, Wawro A, Rojewski S. Effective Utilisation of Halophyte Biomass from Saline Soils for Biorefinering Processes. Molecules. 2021; 26(17):5393. https://doi.org/10.3390/molecules26175393
Chicago/Turabian StyleBatog, Jolanta, Krzysztof Bujnowicz, Weronika Gieparda, Aleksandra Wawro, and Szymon Rojewski. 2021. "Effective Utilisation of Halophyte Biomass from Saline Soils for Biorefinering Processes" Molecules 26, no. 17: 5393. https://doi.org/10.3390/molecules26175393






