Syntheses and Characterization of Novel Perovskite-Type LaScO3-Based Lithium Ionic Conductors
Abstract
:1. Introduction
2. Results and Discussion
2.1. Syntheses and Ionic Conductivities of (LixLa1−x/3)ScO3
2.2. Syntheses and Ionic Conductivities of (LixLa1−x/3)ScO3 co-doped with Ce4+, Zr4+, and Nb5+
2.3. Crystal Structure Analysis
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Kamaya, N.; Homma, K.; Yamakawa, Y.; Hirayama, M.; Kanno, R.; Yonemura, M.; Kamiyama, T.; Kato, Y.; Hama, S.; Kawamoto, K.; et al. A lithium superionic conductor. Nat. Mater. 2011, 10, 682–686. [Google Scholar] [CrossRef] [PubMed]
- Kato, Y.; Hori, S.; Saito, T.; Suzuki, K.; Hirayama, M.; Mitsui, A.; Yonemura, M.; Iba, H.; Kanno, R. High-power all-solid-state batteries using sulfide superionic conductors. Nat. Energy 2016, 1, 1–7. [Google Scholar]
- Ma, C.; Chen, K.; Liang, C.; Nan, C.-W.; Ishikawa, R.; Morea, K.; Chi, M. Atomic-scale origin of the large grain-boundary resistance in perovskite Li-ion-conducting solid electrolytes. Energy Environ. Sci. 2014, 7, 1638–1642. [Google Scholar] [CrossRef] [Green Version]
- Cao, C.; Li, Z.-B.; Wang, X.-L.; Zhao, X.-B.; Han, W.-Q. Recent advances in inorganic solid electrolytes for lithium batteries. Front. Energy Res. 2014, 2, 1–10. [Google Scholar]
- Bachman, J.C.; Muy, S.; Grimaud, A.; Chang, H.-H.; Pour, N.; Lux, S.F.; Paschos, O.; Maglia, F.; Lupart, S.; Lamp, P.; et al. Inorganic solid-state electrolytes for lithium batteries: Mechanisms and properties governing ion conduction. Chem. Rev. 2016, 116, 140–162. [Google Scholar] [CrossRef]
- Sun, C.; Liu, J.; Gong, Y.; Wilkinson, D.P.; Zhang, J. Recent advances in all-solid-state rechargeable lithium batteries. Nano Energy 2017, 33, 363–386. [Google Scholar] [CrossRef] [Green Version]
- Van den Broek, J.; Afyon, S.; Rupp, J.L.M. Interface-engineered all-solid-state li-ion batteries based on garnet-type fast Li+ conductors. Adv. Energy Mater. 2016, 6, 1–11. [Google Scholar]
- Li, X.; Liang, J.; Yang, X.; Adair, K.R.; Wang, C.; Zhao, F.; Sun, X. Progress and perspectives on halide lithium conductors for all-solid-state lithium batteries. Energy Environ. Sci. 2020, 13, 1429–1461. [Google Scholar] [CrossRef]
- Zhao, Q.; Stalin, S.; Zhao, C.-Z.; Archer, L.A. Designing solid-state electrolytes for safe, energy-dense batteries. Nat. Rev. Mater. 2020, 1, 229–252. [Google Scholar] [CrossRef]
- Hori, S.; Suzuki, K.; Hirayama, M.; Kato, Y.; Saito, T.; Yonemura, M.; Kanno, R. Synthesis, structure, and ionic conductivity of solid solution, Li10+δM1+δP2-δS12 (M = Si, Sn). Faraday Discuss. 2014, 176, 83–94. [Google Scholar] [CrossRef]
- Inoue, Y.; Suzuki, K.; Matsui, N.; Hirayama, M.; Kanno, R. Synthesis and structure of novel lithium-ion conductor Li7Ge3PS12. J. Solid State Chem. 2017, 246, 334–340. [Google Scholar] [CrossRef]
- Hori, S.; Suzuki, K.; Hirayama, M.; Kato, Y.; Kanno, R. Lithium superionic conductor Li9.42Si1.02P2.1S9.96O2.04 with Li10GeP2S12-type structure in the Li2S–P2S5–SiO2 pseudoternary system: Synthesis, electrochemical properties, and structure–composition relationships. Front. Energy Res. 2016, 4, 1–10. [Google Scholar] [CrossRef]
- Inaguma, Y.; Liquan, C.; Itoh, M.; Nakamura, T. High ionic conductivity in lithium lanthanum titanate. Solid State Commun. 1993, 86, 689–693. [Google Scholar] [CrossRef]
- Hallopeau, L.; Bregiroux, D.; Rousse, G.; Portehault, D.; Stevens, P.; Toussaint, G.; Laberty-Robert, C. Microwave-assisted reactive sintering and lithium ion conductivity of Li1.3Al0.3Ti1.7(PO4)3 solid electrolyte. J. Power Sources 2017, 378, 48–52. [Google Scholar] [CrossRef] [Green Version]
- Kuwano, J.; West, A.R. New Li+ ion conductors in the system, Li4GeO4-Li3VO4. Mater. Res. Bull. 1980, 15, 1661–1667. [Google Scholar] [CrossRef]
- Alpen, U.V.; Bell, M.F.; Wichelhaus, W. Ionic conductivity of Li14Zn(GeO4) (Lisicon). Electrochim. Acta 1978, 23, 1395–1397. [Google Scholar] [CrossRef]
- Wenzel, S.; Randau, S.; Leichtweiß, T.; Weber, D.A.; Sann, J.; Zeier, W.G.; Janek, J. Direct observation of the interfacial instability of the fast ionic conductor Li10GeP2S12 at the lithium metal anode. Chem. Mater. 2016, 28, 2400–2407. [Google Scholar] [CrossRef]
- Itoh, M.; Inaguma, Y.; Jung, W.-H.; Chen, L.; Nakamura, T. High lithium ion conductivity in the perovskite-type compounds. Solid State Ion. 1994, 70, 203–207. [Google Scholar] [CrossRef]
- García-Martin, S.; García-Alvarado, F.; Robertson, A.D.; West, A.R.; Alario-Franco, M.A. Microstructural study of the Li+ ion substituted perovskites Li0.5−3xNd0.5+xTiO3. J. Solid State Chem. 1997, 128, 97–101. [Google Scholar] [CrossRef]
- Birke, P.; Scharner, S.; Huggins, R.A.; Weppner, W. Electrolytic stability limit and rapid lithium insertion in the fast-ion-conducting Li0.29La0.57TiO3 perovskite-type compound. J. Electrochem. Soc. 1997, 144, L167–L169. [Google Scholar] [CrossRef]
- Kawakami, Y.; Ikuta, H.; Wakihara, M. Ionic conduction of lithium for perovskite type compounds, (Li0.05La0.317)1−xSr0.5xNbO3, (Li0.1La0.3)1−xSr0.5xNbO3 and (Li0.25La0.25)1−xM0.5xNbO3 (M = Ca and Sr). J. Solid State Electrochem. 1998, 110, 187–192. [Google Scholar] [CrossRef]
- Stramare, S.; Thangadura, V.; Weppner, W. Lithium lanthanum titanates: A review. Chem. Mater. 2003, 15, 3974–3990. [Google Scholar] [CrossRef]
- Bhalla, A.S.; Guo, R.; Roy, R. The perovskite structure—A review of its role in ceramic science and technology. Mater. Res. Innov. 2000, 4, 3–26. [Google Scholar] [CrossRef]
- Hellstrom, E.E.; Van Gool, W. Li ion conduction in Li2ZrO3, Li4ZrO4, and LiScO2. Solid State Ion. 1981, 2, 59–64. [Google Scholar] [CrossRef]
- Liferovich, R.P.; Mitchell, R.H. A structural study of ternary lanthanide orthoscandate perovskites. J. Solid State Chem. 2004, 177, 2188–2197. [Google Scholar] [CrossRef]
- Zhao, G.; Muhammad, I.; Suzuki, K.; Hirayama, M.; Kanno, R. Synthesis, crystal structure, and the ionic conductivity of new lithium ion conductors, M-doped LiScO2 (M = Zr, Nb, Ta). Mater. Trans. 2016, 57, 1370–1373. [Google Scholar] [CrossRef] [Green Version]
- Muy, S.; Bachman, J.C.; Chang, H.-H.; Giordano, L.; Maglia, F.; Lupart, S.; Lamp, P.; Zeier, W.G.; Shao-Horn, Y. Lithium conductivity and Meyer-Neldel rule in Li3PO4-Li3VO4-Li4GeO4 lithium superionic conductors. Chem. Mater. 2018, 30, 5573–5582. [Google Scholar] [CrossRef]
- Mizuno, Y.; Matsuda, Y.; Suzuki, K.; Yonemura, M.; Hirayama, M.; Kanno, R. Synthesis, crystal structure, and electrochemical properties of Li1.2+xMn0.3Co0.2Ni0.3O2 (x > 0) for lithium-ion battery cathodes. Electrochemistry 2015, 83, 820–823. [Google Scholar] [CrossRef] [Green Version]
- Phraewphiphat, T.; Iqbal, M.; Suzuki, K.; Hirayama, M.; Kanno, R. Synthesis and Lithium-Ion Conductivity of LiSrB2O6F (B = Nb5+, Ta5+) with a Pyrochlore Structure. J. Jpn. Soc. Powder Powder Metall. 2018, 65, 26–33. [Google Scholar] [CrossRef] [Green Version]
- Izumi, F.; Momma, K. Three-dimensional visualization in powder diffraction. Solid State Phenom. 2007, 130, 15–20. [Google Scholar] [CrossRef]
- Neudecker, B.-J.; Weppner, W. Li9SiAlO8: A lithium ion electrolyte for voltages above 5.4 V. J. Electrochem. Soc. 1996, 143, 2198–2203. [Google Scholar] [CrossRef]

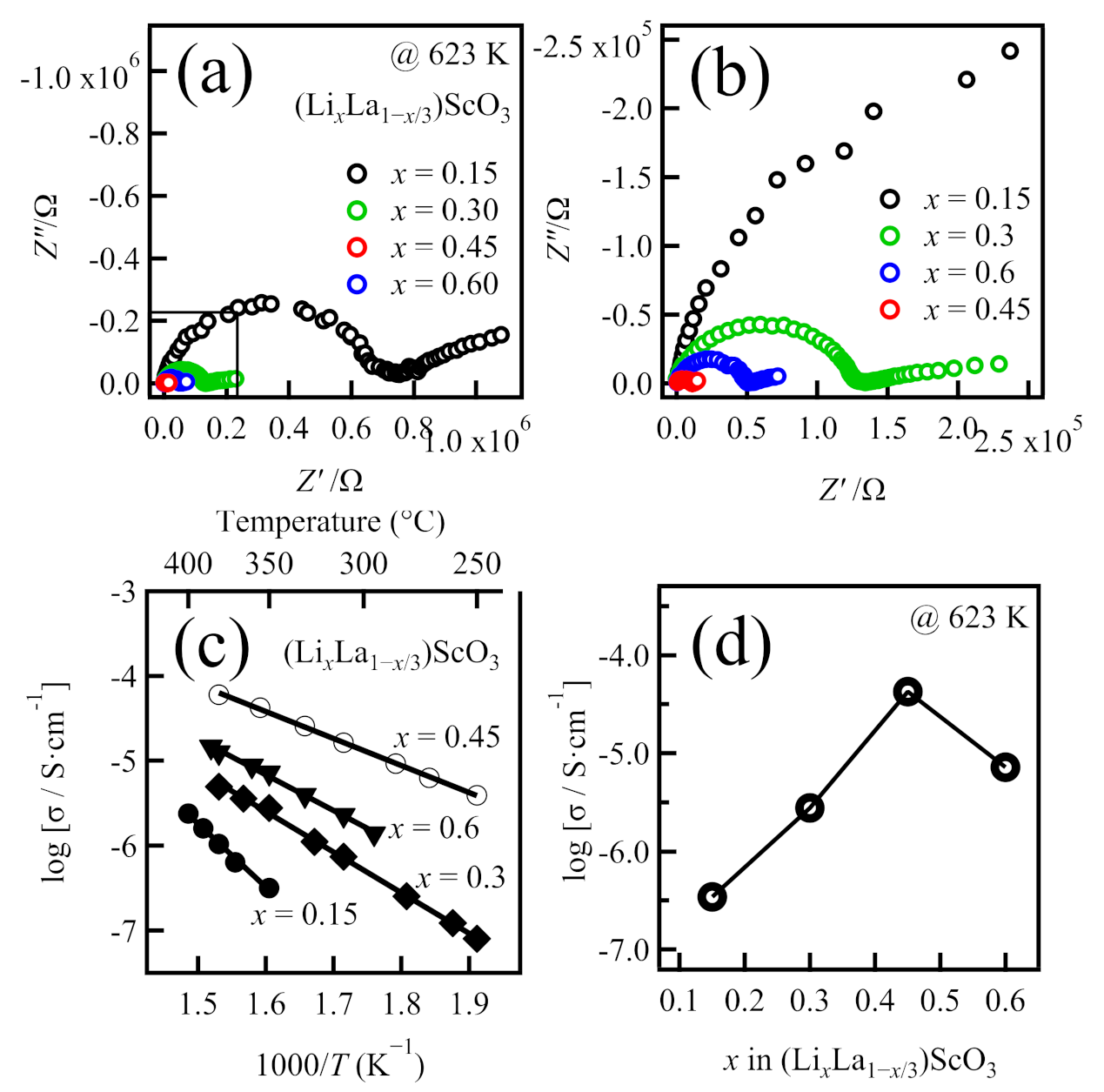
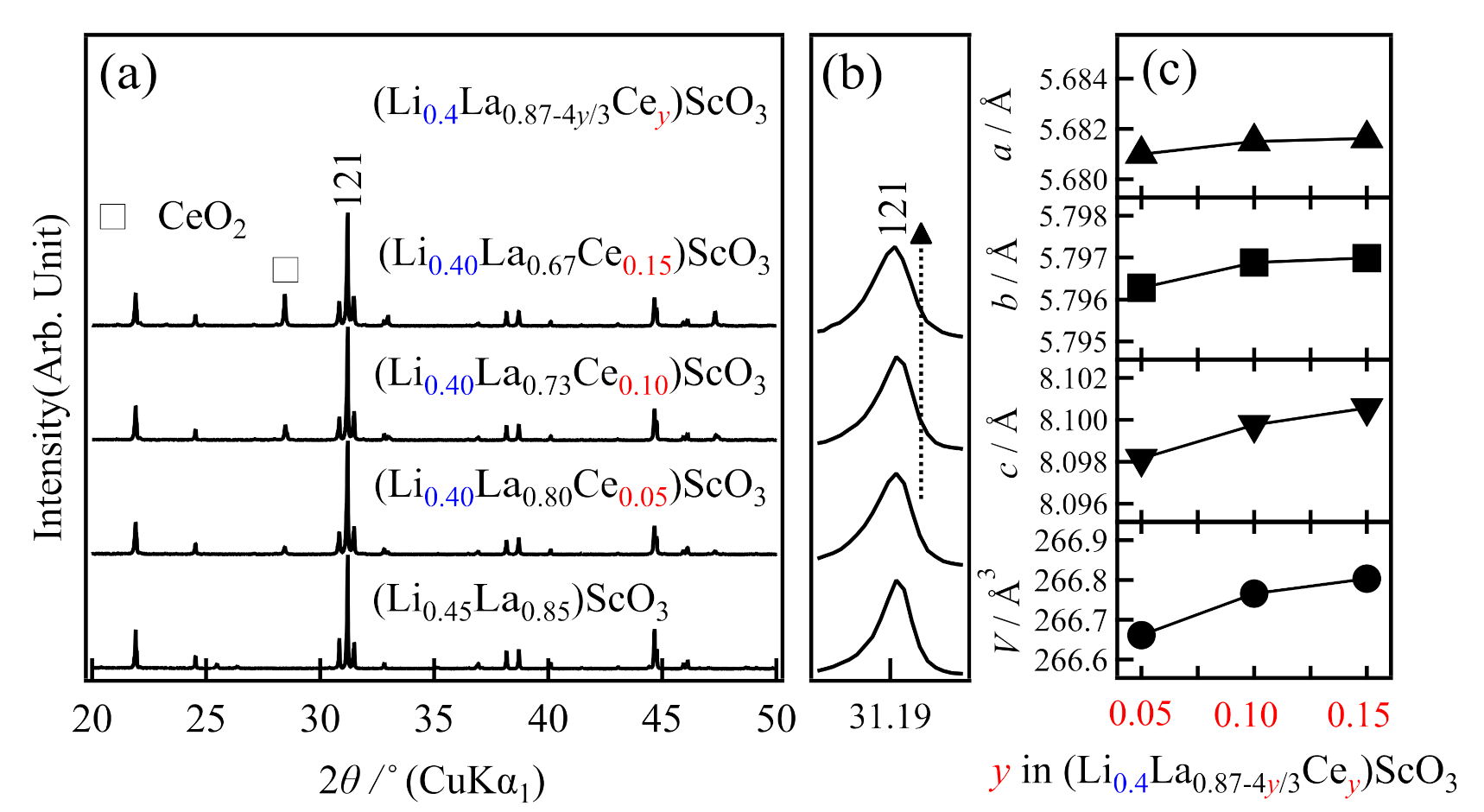
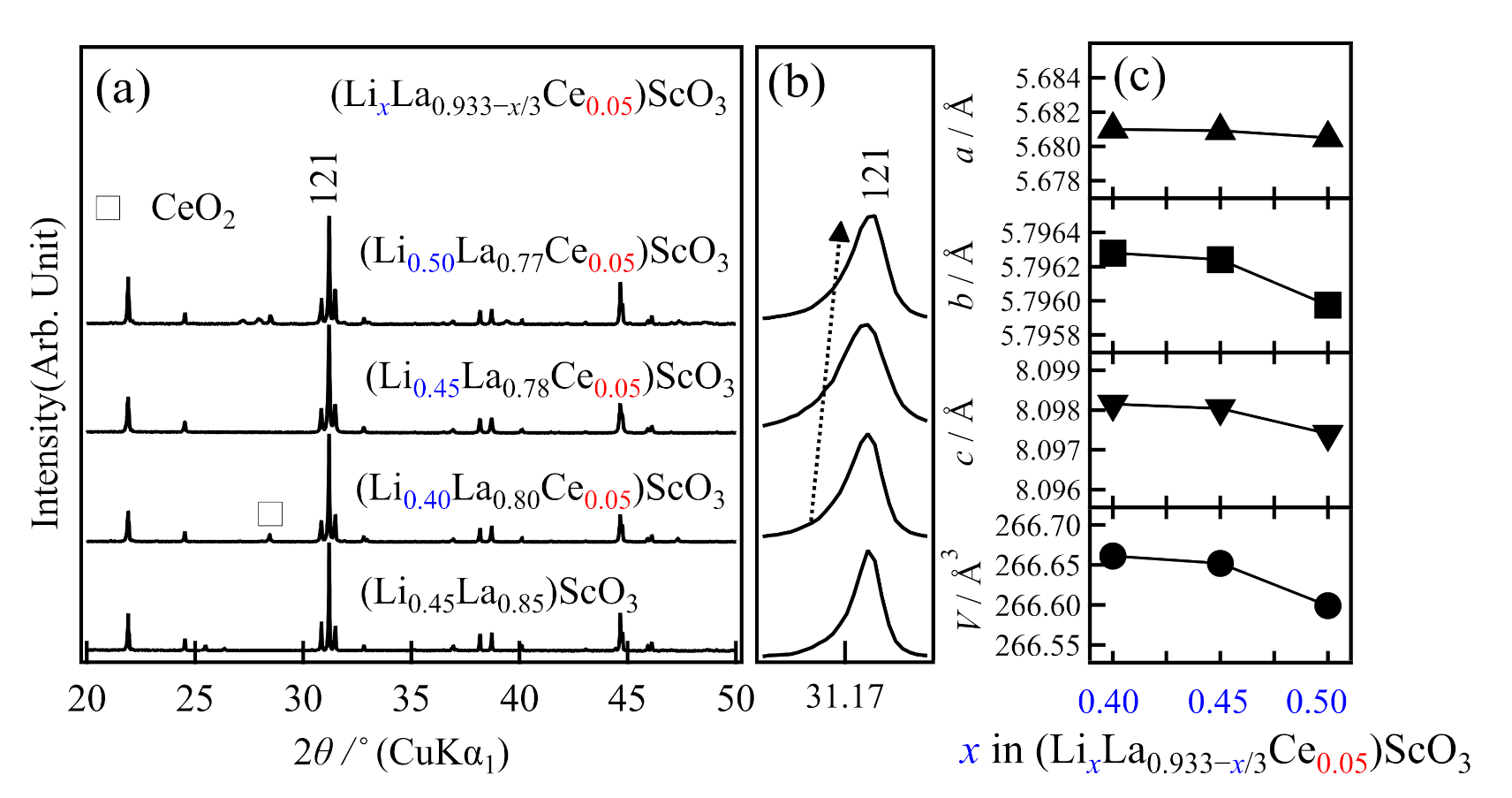
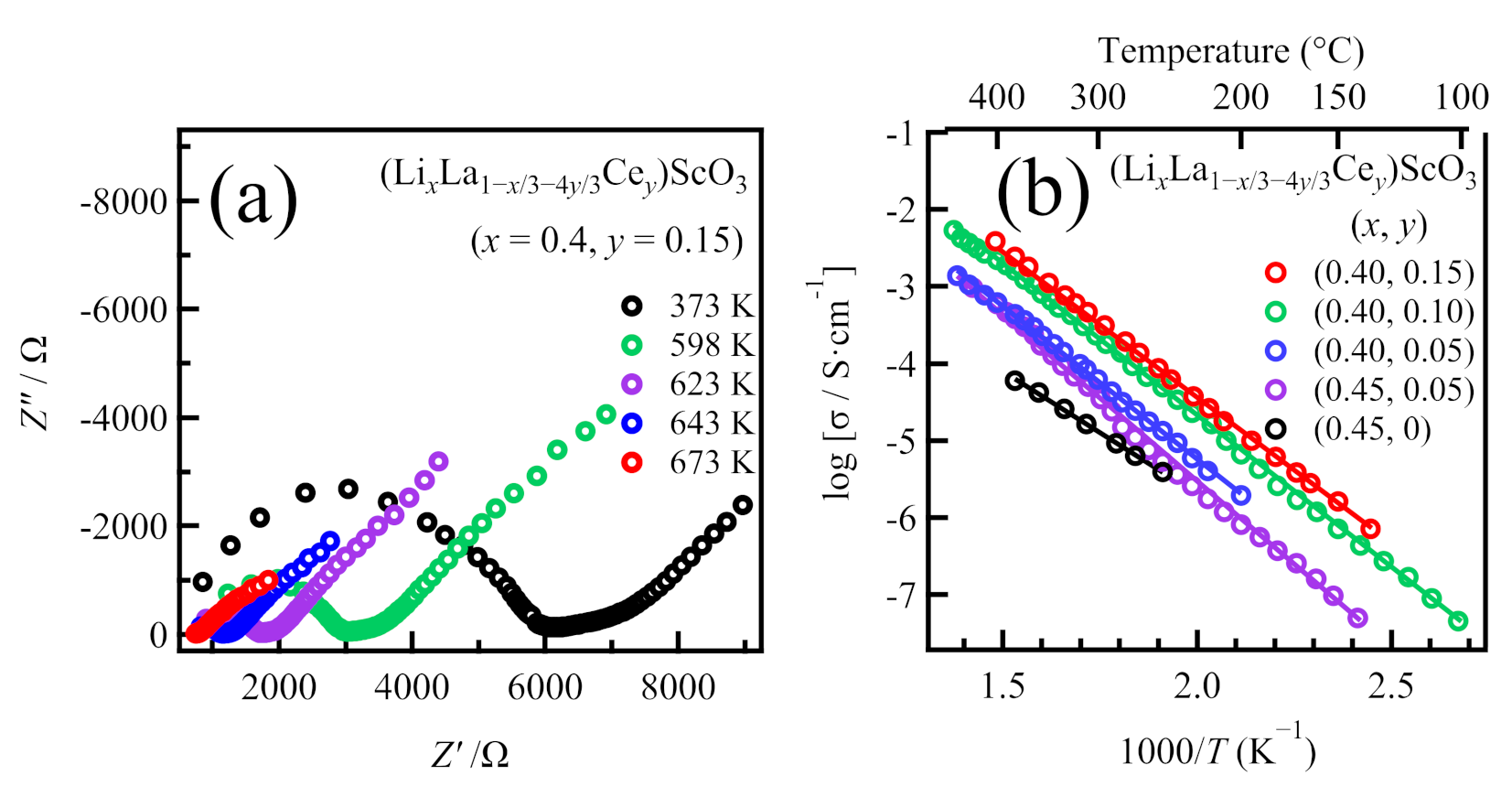

| Formula | Nominal Composition | Identified Phases | σtotal/S cm−1 | Ea/kJ mol−1 |
|---|---|---|---|---|
| (LixLa1−x/3)ScO3 | LaScO3 | LaScO3-type | − | − |
| (Li0.15La0.95)ScO3 | LaScO3-type | 3.4 × 10−7 | 143.0 ± 6.8 | |
| (Li0.30La0.90)ScO3 | LaScO3-type | 2.8 × 10−6 | 91.6 ± 1.7 | |
| (Li0.45La0.85)ScO3 | LaScO3-type | 4.2 × 10−5 | 61.0 ± 0.9 | |
| (Li0.60La0.80)ScO3 | LaScO3-type, LaLiO2 | 7.2 × 10−6 | 80.7 ± 2.2 |
| Chemical Formula | Composition | Identified Phases | σtotal/S cm−1 | Ea/kJ mol−1 |
|---|---|---|---|---|
| (LixLa1−x/3)ScO3 | (Li0.45La0.85)ScO3 | LaScO3-type | 4.2 × 10−5 | 61.0 ± 0.9 |
| (LixLa1−x/3−4y/3Cey)ScO3 | x = 0.35 (fixed), y = 0.05 (Li0.35La0.82Ce0.05)ScO3 | LaScO3-type, CeO2, unknown | 2.1 × 10−5 | 88.9 ± 0.7 |
| x = 0.35 (fixed), y = 0.10 (Li0.35La0.75Ce0.10)ScO3 | LaScO3-type, CeO2 | 2.4 × 10−5 | 87.9 ± 0.3 | |
| x = 0.40 (fixed), y = 0.10 (Li0.40La0.73Ce0.10)ScO3 | LaScO3-type, CeO2 | 8.8 × 10−4 | 75.5 ± 0.6 | |
| x = 0.40 (fixed), y = 0.15 (Li0.40La0.67Ce0.15)ScO3 | LaScO3-type, CeO2 | 1.1 × 10−3 | 75.4 ± 0.1 | |
| x = 0.40, y = 0.05 (fixed) (Li0.50La0.77Ce0.05)ScO3 | LaScO3-type, CeO2 | 5.5 × 10−5 | 78.2 ± 2.5 | |
| x = 0.45, y = 0.05 (fixed) (Li0.45La0.78Ce0.05)ScO3 | LaScO3-type | 1.9 × 10−4 | 82.9 ± 2.1 | |
| x = 0.50, y = 0.05 (fixed) (Li0.50La0.72Ce0.10)ScO3 | LaScO3-type, CeO2, unknown | 1.3 × 10−5 | 86.5 ± 0.3 | |
| (Li0.45La0.85−0.33z)(Sc1−zZrz)O3 | (Li0.45La0.833)(Sc0.95Zr0.05)O3 | LaScO3-type, LiLaO2 | 7.36 × 10−6 | 77.9 ± 2.0 |
| (Li0.45La0.817)(Sc0.90Zr0.10)O3 | LaScO3-type, LiLaO2 | 8.03× 10−6 | 75.6 ± 1.8 | |
| (Li0.45La0.85−0.67z)(Sc1−zNbz)O3 | (Li0.45La0.817)(Sc0.95Nb0.05)O3 | LaScO3-type, LiLaO2 | 3.67 × 10−6 | 81.1 ± 0.9 |
| (Li0.45La0.783)(Sc0.90Nb0.10)O3 | LaScO3-type, LiLaO2 | 9.50 × 10−6 | 82.0 ± 1.9 |
| Atom | Site | g | x | y | z | Biso/Å2 |
|---|---|---|---|---|---|---|
| La | 4c | 0.938(2) | 0.95637(10) | 0.25 | 0.01062(13) | 0.235(9) |
| Li | 4c | =1–g(La) | =x (La) | =y (La) | =z (La) | =B (La) |
| Sc | 4b | 1.0 | 0.5 | 0 | 0 | 0.908(7) |
| O1 | 4c | 1.0 | 0.53871(1) | 0.25 | 0.90136(4) | 0.788(6) |
| O2 | 8d | 1.0 | 0.29065(7) | 0.94553(6) | 0.70253(7) | 0.848 (3) |
| Atom | Site | g | x | y | z | Biso/ Å2 |
|---|---|---|---|---|---|---|
| La | 4c | 0.8212(5) | 0.95622(4) | 0.25 | 0.00892(6) | 0.178(3) |
| Li | 4c | =1–g(La) | =x (La) | =y (La) | =z (La) | =B (La) |
| Sc | 4b | 1.0 | 0.5 | 0 | 0 | 1.209(4) |
| O1 | 4c | 1.0 | 0.53153(4) | 0.25 | 0.90757(5) | 0.699(3) |
| O2 | 8d | 1.0 | 0.2951(6) | 0.9419(3) | 0.70075(3) | 1.825(4) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, G.; Suzuki, K.; Hirayama, M.; Kanno, R. Syntheses and Characterization of Novel Perovskite-Type LaScO3-Based Lithium Ionic Conductors. Molecules 2021, 26, 299. https://doi.org/10.3390/molecules26020299
Zhao G, Suzuki K, Hirayama M, Kanno R. Syntheses and Characterization of Novel Perovskite-Type LaScO3-Based Lithium Ionic Conductors. Molecules. 2021; 26(2):299. https://doi.org/10.3390/molecules26020299
Chicago/Turabian StyleZhao, Guowei, Kota Suzuki, Masaaki Hirayama, and Ryoji Kanno. 2021. "Syntheses and Characterization of Novel Perovskite-Type LaScO3-Based Lithium Ionic Conductors" Molecules 26, no. 2: 299. https://doi.org/10.3390/molecules26020299






