2. Results and Discussion
A series of polymerization experiments was conducted in order to obtain a controlled ring-opening polymerization of
d,
l-lactide. In a model reaction, this cyclic ester has been polymerized with cyclooctanol as the initiator and SnOct
2 as the catalyst according to the reaction pathway depicted in
Scheme 1(I). Polymerizations were carried out according to the procedure described in the Experimental Part at monomer-to catalyst ratio ((M)/(C)) between 10/1 and 200/1. All reactions were performed in triplicate to explore and verify the ability of the synthetic method to yield materials with reproducible molecular mass characteristics.
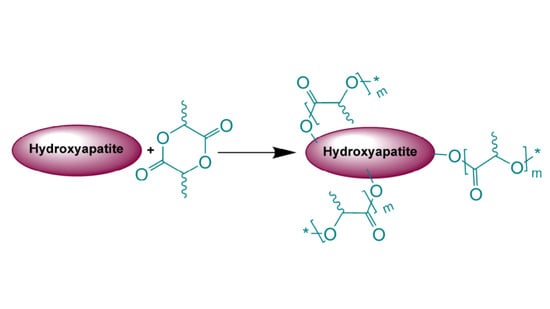
A series of polymerizations under exactly the same conditions was conducted using hydroxyapatite, Ca
10(PO
4)
6(OH)
2, as a source of initiating hydroxyl groups,
Scheme 1(II).
The results obtained show that the cyclooctanol/Sn(Oct)
2 initiating system affords polymers (assigned as “PDLLA #”, the number indicating the monomer to catalyst ratio) with yields between 93% and 97%, and rather reproducible molecular mass characteristics, the molecular mass, M
n, ranging from 540 Da to 4250 Da (
Table 1 and
Figure 1). The polymers are mostly free of oligomer byproducts and their molecular mass increases with the increase in the monomer/catalyst ratio as expected for this type of polymerization system.
During the course of the polymerization initiated by hydroxyapatite (HA), the HA crystals, which were initially insoluble in toluene, went into solution and the reaction appeared to proceed in a homogenous fashion. This could be interpreted as an indication that the propagating poly(
d,
l-lactide) chains, which are freely soluble in the polymerization medium, are grafted onto the HA and, upon reaching certain “critical” mass, lead to its gradual dissolution. We estimate this mass to be above 1000 Da since all HA-PDDLA samples in
Table 1 dissolved during the polymerization. The typical values of M
n for this series are also shown in
Table 1.
Under identical monomer/catalyst ratios and the same reaction conditions, the ring-opening polymerization of
d,
l-lactide with HA as initiator consistently produces polymers in almost quantitative yields (95–97%) and with higher molecular mass, compared to the cyclooctanol-initiated process. The polymers are assigned as “HA-PDLLA #”, the number again indicating the monomer to catalyst ratio. Since this ratio is the same the increase in the molecular mass could be attributed to the presence of multiple initiation sites (OH groups) anchored at the same HA particle. The molecular masses are also quite reproducible, the deviations presumably reflecting the initial heterogeneity of the polymerization system. It should be noted that the change of the initial polymerization solvent from triethylene glycol dimethyl ether (triglyme) to toluene led to a significant improvement in polymer yields (
Table S1 vs.
Table 1).
Figure 1 shows the size exclusion chromatography (SEC) traces of the reaction mixtures of selected cyclooctanol/Sn(Oct)
2 and HA/Sn(Oct)
2 polymerizations. Notably the main polymer peaks in the chromatograms from the HA polymerizations are monodisperse—a clear indication for the simultaneous initiation by all accessible OH groups on the surface of HA particles (see also
Figure S1 in the Supporting Information).
The attachment of the polymer to the surface of the hydroxyapatite particles was further confirmed by differential scanning calorimetry (DSC),
Figure 2 and
Figure 3. The thermograms of poly (
d,
l-lactides) produced with cyclooctanol as initiator showed well defined glass transitions. The observed insignificant difference in the glass transition temperature (T
g) from −6 °C for PDLLA 50 to −7 °C for PDLLA 200 shows a lack of molecular mass effect on the latter (see PDLLA data in
Table 1). However, the observed notable shift in the T
g of all HA-PDLLA polymers compared to their PDLLA counterparts (an example is shown in
Figure S2) could not be explained by a simple increase in the molecular mass as evidenced by the thermal characteristics of the physical mixtures with compositions identical to those of the HA grafts (HA is physically mixed with the corresponding PDLLA to produce a composition identical to the graft),
Figure 2 and
Figure 3. The T
gs of these physical mixtures are practically equal to the T
gs of the corresponding PDLLA. Therefore the consistently higher T
g values for the HA materials are mostly caused by the decreased segmental motion of the poly(
d,
l-lactide) chains covalently bound to the surface of the HA.
A similar trend was also observed in the thermal degradation pattern investigated by thermal gravimetric analyses,
Figure 4. It is seen that the degradation temperature of the HA polymers is higher by 6 °C to 20 °C compared to the values of the linear PDLLA (from 200 °C to 206 °C for PDLLA 200 and HA-PDLLA 200 and from 189 °C to 209 °C for PDLLA 50 and HA-PDLLA 50, respectively).
The attachment of the polymer to the surface of the hydroxyapatite particles could also be verified by
31P magic-angle spinning (MAS) NMR, 1D and 2D NMR. The
31P MAS NMR spectra for the surface-grafted hydroxyapatite and non-modified hydroxyapatite are shown in
Figure 5.
31P NMR spectroscopy is particularly useful in revealing reaction pathways due to the pronounced sensitivity of the phosphorus atom to changes in its environment [
31]. Due to the rather small hydroxyapatite content, a small monomer to catalyst ratio product, HA-PDLLA 10 ((M)/(C) = 10), was used in order to observe the signal. The recorded signal shows a downfield shift from 2.0 ppm in non-modified HA to 2.54 ppm in HA-PDLLA 10 indicating that the chemical environment of the phosphorus atom in the grafted HA crystal has changed. Moreover, the grafting link consists probably of Ca
2+--
−O-C(O)- association rather than a P-O-C(O)- bond, which is more labile and would cause a more dramatic chemical shift [
32]. The observed downfield shift trend has been reported in previous publications [
16], as well.
The grafting of PDLLA onto the surface of HA crystals was further investigated by
1H- and
13C-NMR analyses. The overlaid
1H-NMR spectra shown in
Figure 6 reveal the different chemical environment of the polymer’s methine group in close proximity to the initiator moiety. While the signals for the backbone- and end CH groups have the same chemical shifts (
Figure 6, e and d), the neighboring cyclooctanol group and the surface Ca-O-C(O) groups in hydroxyapatite cause the signals at 4.98 ppm (
Figure 6A, a) and 5.03 ppm (
Figure 6B, c), respectively. In the case of cyclooctanol-PDLLA, however, there is one more characteristic methine group related to the cyclooctanol molecule, which appears at 5.09 ppm (
Figure 6A, b).
Correlation NMR analyses (
Figure 7 and
Figure 8) were used to determine, which peaks originate from the –C(O)-
CH(CH
3)-O-groups next to the initiator moieties (cyclooctanol and hydroxyapatite, respectively). A Distortionless Enhancement of Polarization Transfer Heteronuclear Single Quantum Correlation (DEPT-HSQC) experiment was performed to determine the directly attached
1H-
13C couplings. Four characteristic methine peaks at 4.35 ppm, 4.98 ppm, 5.09 ppm, and 5.15 ppm were detected for cyclooctanol-PDLLA compared to only three –CH peaks for hydroxyapatite-PDLLA at 4.35 ppm, 5.03 ppm, and 5.15 ppm, as shown in the insets of
Figure 7 and
Figure 8. The forth methine peak at 5.09 ppm in the cyclooctanol-PDLLA product is concluded to relate to the –(CH
2)
2-
CH-O-C(O)- group in the initiator cyclooctanol (group b in the structure in
Figure 6A).
The assignment of the different methine peaks in both products was also supported by a long range
1H-
13C heteronuclear correlation experiment, Heteronuclear Multiple Bond Coherence (HMBC),
Figure 9. The methine peak emerging at δ = 4.35 ppm/ δ = 66.7 ppm (peak ‘d’ in
Figure 9) is assigned to the –
CH-OH group, which is the same end-group present in both cyclooctanol-PDLLA and HA-PDLLA. Another –CH- peak observed in both structures at δ = 5.15 ppm/ 69.5 ppm is concluded to be the –C(O)-
CH(CH
3)-O- group in the repeating units of the polymer chains (peak e in
Figure 9). The methine groups in the polymer chain that are directly connected to initiator moieties, however, exhibit a different chemical environment, which is confirmed by the NMR analysis (peaks a and c in
Figure 9). The overlay of the
1H-
13C HMBC NMR spectra presented in
Figure 9 clearly shows the ester linkage between the polymer and the initiator moieties.
The final evidence for the successful growth of PDLLA on the surface of the hydroxyapatite is provided by infra-red spectroscopy.
Figure 10 contains the FT-IR spectra of hydroxyapatite and HA-PDLLA synthesized with different (M)/(C) ratios. The HA spectrum (
Figure 10a) shows the typical absorption bands for PO
43− at 600–650 cm
−1 (antisymmetric bending), 960 cm
−1 (symmetric stretching) and 1040–1094 cm
−1 (antisymmetric stretching) [
33] together with a typical combination of a sharp and a broad band between 3560 cm
−1 and 3100 cm
−1 due to hydrogen bonded HO
− [
34]. Interestingly the position of the C=O band in the HA-PDLLA shifts from 1653 cm
−1 for polymers produced at (M)/(C) = 19 and 54 (
Figure S3 and
Figure 10b) to a value more typical of the ungrafted PDLLA (1740 cm
−1) for polymers from (M)/(C) = 217 (
Figure 10c). At the same time the HA absorption bands gradually weaken. This unusual molecular mass dependence of the FT-IR absorbances of the ester groups could possibly be explained by their tethering at the surface of the HA particles due to some selective interaction with the negatively charged HA surface (the zeta potential of HA is estimated to be between −8 and −12 mV [
35,
36]. Moreover, at lower molecular mass (shorter chains) a significant portion of the carbonyl groups belong to the fraction of Ca
2+---
−O-C(O) linkages, characterized by an antisymmetric stretching vibration at 1653 cm
−1 [
36]. At monomer/initiator ratios of 100 and higher their relative content in the HA-graft would diminish and the spectrum would be dominated by the absorption bands, characteristic for the polyester carbonyl sufficiently removed from the HA surface to experience any selective interaction (and immobilization) with the negatively charged HA surface.
HA-PDLLA degradation behavior and phase compatibility in mixtures with commercially available PMMA based bone cement (Simplex P Speedset, Stryker Orthopaedics) were investigated through TGA and DSC, respectively. An example with physical mixtures of 5% weight HA-PDLLA 150 in bone cement is shown in
Figure 11 and
Figure 12a,b. The simple physical mixture of the grafts with Simplex P Speedset does not deteriorate the thermal stability of the cement. Furthermore, the observed DSC curve for the same mixture exhibits the glass transition behavior of the original bone cement formulation (
Figure 12b). Hence, the modification of standard bone cement formulations with 5% weight HA-PDLLA grafts will not compromise the cement stability and storage requirements.
The mechanical properties at different environmental conditions were investigated with two HA-PDLLA nanografts (
Table 1, (M)/(C) = 50 and 150). The compression stress–strain curves of the cement compositions before and after 4 weeks of exposure to an aqueous medium at pH 4 are shown in
Figure 13a,b.
The linear elastic portion of each curve was used for the experimental determination of the compression modulus for the standard and the modified bone cements. The compressive strength was calculated as the failure load (the load at the 2.0% offset) divided by the corresponding cross-sectional area.
The compressive strengths of all three cement formulations in the control group exceeded the minimum of 70 MPa as required by ASTM F451-99a (see Materials and Methods for description),
Figure 14. The compressive strengths for the modified bone cement specimens were observed to be higher than those for the standard cement formulation. After 4 weeks of accelerated degradation in an acidic solution, the observed compressive strengths were 35 ± 3 MPa for the plain bone cement, 75 ± 13 MPa for the cement with 10% weight HA-PDLLA 50, and 65 ± 9 MPa for the formulation with 10% weight HA-PDLLA 150. The exhibited loss of compressive strength was in the amount of 58% for the SimplexP bone cement specimens, 22% for SimplexP + 10% weight HA-PDLLA 50, and 32% for SimplexP + 10% weight HA-PDLLA 150. After 8 weeks of accelerated degradation in an acidic solution, the observed compressive strengths were 31 ± 2 MPa for the plain bone cement, 65 ± 13 MPa for the cement with 10% weight HA-PDLLA 50, and 53 ± 20 MPa for the formulation with 10% weight HA-PDLLA 150. The compressive strength loss was calculated to be 63% for the SimplexP standard formulation, 32% for the SimplexP + 10% weight HA-PDLLA 50 formulation, and 45% for the SimplexP + 10% weight HA-PDLLA 150 formulation. The mean compressive strengths for the three cement formulations were all significantly different from one another (
p < 0.05).
The compression modulus for the standard and modified bone cements are presented in
Figure 15. All three cement formulations exhibited a marked decrease in stiffness after 4 weeks of immersion in an acidic solution. The compression modulus was observed to decrease by 46% for the standard bone cement specimens, 43% for the specimens containing 10% weight HA-PDLLA 50, and 40% for the specimens with 10% weight HA-PDLLA 150. After 8 weeks of accelerated degradation, however, the loss of stiffness for the test specimens modified with 10% weight HA-PDLLA 50 was observed to be lower compared to that of either the SimplexP or the SimplexP + 10% weight HA-PDLLA 150 formulation (49% vs. 59% and 60%, respectively). The mean values of the compression modulus for all three cement formulations were significantly different from one another (
p < 0.05).
The results of this study indicate that the compression properties of PMMA-based bone cement are improved by the addition of HA-PDLLA polymers. Both the compressive strength and the compression modulus of SimplexP test specimens measured in our experiments were in accordance with previously published values [
2]. According to the referenced review, the compressive strength of SimplexP specimens, prepared manually and tested 24 h after preparation, is reported to be 89.2 MPa; the reported compression modulus is 2540 MPa. We found that adding HA-PDLLA 50 or HA-PDLLA 150 polymeric grafts to SimplexP resulted in an increase in the observed compressive strength and stiffness of the bone cement specimens. The dissolution of the additives in the monomer liquid prior to the preparation of the cement specimens very likely resulted in their homogeneous distribution in the polymer matrix and prevented the formation of agglomerates. Another factor affecting the regular distribution of the additives in the polymer matrix is the chemical linkage between HA and PDLLA. The PDLLA chains attached to the HA surface are fully compatible with the organic polymer matrix; the organic nature of both the additives and the acrylic cement allowed for a regular mixing of these components.
The compression properties of the standard bone cement and those of the modified cements decreased significantly after being subjected to degradation in extreme conditions (i.e., at pH 4). Interestingly, the observed loss of compression strength for the HA-PDLLA 50 modified bone cements after 8 weeks of accelerated degradation was one half of that measured for the plain bone cement (
Figure 14). One possible explanation for this observation is the sample porosity. The presence of pores is an important factor that significantly affects the compression properties of bone cements. Typically, low porosity materials have stress–strain curves with steep initial slopes of the linear portion (below 2% strain). The stress–strain curves of high porosity materials, by contrast, exhibit more shallow initial slopes. Our results showed similar characteristic features of the stress–strain curves for the modified and the plain bone cements subjected to accelerated degradation (
Figure 13b and
Figure S4c). The pore formation was strongly related to the degradation processes occurring in the polymer matrix. Under acidic conditions, both PMMA and PDLLA would undergo hydrolysis of the ester bonds; however, in the case of PDLLA, the ester linkages subject to hydrolysis were part of the polymer backbone. In the case of PMMA, the product of the acidic hydrolysis, methanol, was miscible in water and would possibly leak out easily from the cement specimens. Another by-product of the PMMA hydrolysis, methacrylic acid, was also miscible in water. HA-PDLLA, on the other hand, was degraded by random chain scission and the initially resulting oligomers with a high molecular weight were not miscible in water.
Our hypothesis is that the leakage of the hydrolysis byproducts would cause the formation of void spaces (micropores) in the cement matrix and would result in the loss of both strength and stiffness of the material. The modified bone cement, however, exhibited a lower decrease in the measured compression properties. One possible explanation for this observation was the presumed tendency of the initially formed oligomers with higher molecular mass to not mix with the water medium and to remain in the cement matrix due to hydrophobic–hydrophobic interactions. Moreover, when the water diffused in the cement bulk, the volume of the PDLLA portion of the polymer additives would notably increase in accordance with the water absorption behavior of PDLLA, as previously reported [
37]. Accordingly, the expanded volume of the polymer grafts would result in “filling” some of the pores in the cement. Representative pictures of plain cement specimens and specimens with 10% weight HA-PDLLA 50 after incubation at pH 4 for 4 weeks are shown in
Figure 16. The difference in number and size of pores in the specimen cross sections is clearly seen. Similar results were observed also for Simplex P + 10% weight HA-PDLLA 150 under identical conditions (
Figure S6).
That is why the compressive strength and stiffness of the modified bone cement specimens were higher compared to those of the standard formulation under the described experimental conditions.
This preliminary investigation of HA-PDLLA modified acrylic bone cement was limited to the mechanical properties of bone cement in compression. The compressive strength measurement is a critical step for determining whether the modified formulations will be strong enough for clinical applications. However, other mechanical tests (i.e., tensile strength and fracture toughness) have to be performed for a more complete and detailed characterization of HA-PDLLA—containing bone cements.
3. Materials and Methods
Materials. Toluene (‘Baker analyzed® HPLC solvent, 0.01% H2O) was purchased from Mallinckrodt Baker, Inc. (Phillipsburg, NJ, USA) and freshly distilled over CaH2 under argon before use. Hydroxyapatite, HA, (synthetic powder, reagent grade), cyclooctanol (94%), and stannous 2-ethylhexanoate (SnOct2) were purchased from Sigma-Aldrich, Inc. (St. Louis, MO, USA); d,l-lactide (min. 99.5%) was purchased from Polysciences, Inc. All reagents were stored in a dry glovebox system operating on inert gas (Ar) upon opening and used without further purification. Tetrahydrofuran (THF, HPLC grade, stabilized with BHT, 0.05% H2O) was obtained from Pharmco-Aaper. Chloroform-d (D, 99.8%) was purchased from Cambridge Isotope Laboratories, Inc. (Andover, MA, USA) and stored refrigerated on molecular sieves. Radio-opaque bone cement “Simplex P Speedset” from Stryker Orthopaedics (Mahwah, NJ, USA) was stored and processed according to company’s instructions.
Polymerization procedure. All reaction mixtures were prepared inside a dry glovebox SYS1-2GB (Innovative Technology, Inc., Newburyport, MA, USA). All polymerizations were conducted in dry toluene for 3 h at reflux (~111−112 °C). Two series of ring-opening polymerizations of d,l-lactide were conducted: (a) d,l-lactide + SnOct2 + cyclooctanol with variation of the monomer to catalyst (M/C) ratio; (b) d,l-lactide + SnOct2 + hydroxyapatite with variation of the M/C ratio.
A typical polymerization procedure was performed as follows:
d,
l-lactide (0.5693 g, 3.95 mmol) and hydroxyapatite (0.03 g, 5.97 × 10
−2 mmol) were weighed into a double neck round bottom flask (50 mL), equipped with a magnetic stirring bar. The flask was attached to a condenser with a Dean-Stark trap and an inlet adaptor for inert gas, and the side neck was closed with a rubber septum. A flow of dry argon was continuously passed through the reaction mixture. The solvent (toluene, 10 weight %) and the catalyst (SnOct
2, 7.9 × 10
−2 mmol) were transferred to the flask by a gas tight syringe. The temperature of the oil bath was increased to 111 °C and kept in the range of 111–112 °C for 3 h. The resulting reaction mixture was a viscous liquid. The material was precipitated in cold methanol and the residual solvent was evaporated using a rotary evaporator. The obtained product was a white-colored foamy material. Yield: 0.6034 g (94%). The molecular mass characteristics of the material were determined by size exclusion chromatography. M
n = 3800 Da, Ð = 2.51 (
Table 1).
Cement Preparation. Three different cement compositions were prepared using Simplex™ P SpeedSet™ cement (Mahwah, NJ, USA) and HA-PDLLA additives: (a) Simplex™ P SpeedSet™ bone cement; (b) Simplex™ P SpeedSet™ with 10% v/v HA-PDLLA 50; (c) Simplex™ P SpeedSet™ with 10% w/w HA-PDLLA 150. The cement specimens were prepared according to the instructions provided by the manufacturer. The polymer additives in the amount of 10% w/w were first dissolved in the liquid monomer at room temperature. Then, the polymer powder and the monomer liquid were hand-mixed in an inert container and stirred with a spatula for 2 min. When a dough-like mass was formed, the mixture was manipulated by hand for 2 more minutes and packed into a cylindrical-shaped mold.
Preparation of Samples for Mechanical Testing. The casting of compression samples was performed according to ASTM standard F451-99a [
38]. The three cement compositions were prepared and put into a Teflon mold consisting of cylindrical holes (each 6 mm in diameter and 12 mm in height). The specimens were allowed to polymerize in the mold for 1 h, followed by 24 h of curing out of the mold. All specimens were polished with 280, 320, and 600 grit sand papers. The specimens were visually examined for surface defects and only acceptable specimens with a uniform appearance and no visible surface defects were used in the study (See
Figure S4 for an example).
Degradation Experiments. The effect of the physiological medium on the properties of the standard and modified bone cements was investigated by exposing the specimens to a solution with fixed acidic pH (pH = 4, in house preparation). The test specimens were grouped according to the duration of accelerated degradation as follows: Group 1, control, degradation time 0 weeks; Group 2, degradation time 4 weeks; Group 3, degradation time 8 weeks. Six specimens of each group were immersed in a 5 mL acidic solution and incubated in an incubator shaker at 37 °C for 4 and 8 weeks, respectively. At the end of each degradation period the specimens were dried to a constant weight and subjected to a compression test.
Methods. Size exclusion chromatography (SEC) was performed on a system (Waters Corporation, Milford, MA, USA) consisting of a Waters M510 HPLC pump, Waters U6K injector and Viscotek 250 detector (Viscotek, Malvern Panalytical Inc., Westborough, MA, USA). The separation was achieved at 40 °C on a set of three 5 µm PLgel columns (Polymer Laboratories, Inc., Amherst, MA, USA) with pore sizes of 50 Ǻ, 1000 Ǻ, and mixed C and eluent flow rate of 0.8 mL/min. Typically, 5 mg of material were dissolved in 1 mL freshly distilled THF and then filtered through a 0.45 µm PTFE filter before injection. The columns were calibrated with a set of 41 poly(styrene), PSt, standards (Polymer Standards Service-USA, Inc., Warwick, RI) with narrow dispersity and molecular masses between 162 and 1,670,000 Da. All chromatograms were acquired by the OmniSEC 3.1 software package (Viscotek, Malvern Panalytical Inc., Westborough, MA, USA) and the molecular masses were calculated using conventional PSt calibration. The chromatograms shown in
Figure 1 and
Figure S1 were produced using a set containing Waters Styragel linear 4E and 5E columns in series under identical analysis and calculation protocols.
Thermogravimetric analysis, TGA, was performed on a Hi-Res TGA 2950 thermogravimetric analyzer (TA Instruments, Waters Corporation, Milford, MA, USA) under a nitrogen atmosphere between 25 °C and 600 °C at a heating gradient of 20 °C/min. The degradation temperature was calculated at the onset of the weight loss.
Differential scanning calorimetry, DSC, was conducted using a differential scanning calorimeter DSC Q200 V24.4 (TA instruments, Waters Corporation, Milford, MA, USA), equipped with a refrigerated cooling system. Samples (5–8 mg) were crimped in Tzero aluminum pans and scanned at 10 °C/min in the range 25–140 °C under a continuously purged dry nitrogen atmosphere (flow rate of 50 mL/min). A heating–cooling–heating cycle was used. The data were collected and analyzed using TA Instruments Universal Analysis 2000 V4.3A software (TA Instruments, Waters Corporation, Milford, MA, USA). The glass transition temperatures (Tg) were calculated at the inflection point of the two baseline tangents before and after the transition using the second heating stage.
FT-IR analyses were performed on Nicolet Model 400 spectrophotometer (Nicolet, ThermoFisher Scientific, Waltham, MA, USA) for soluble samples by depositing a drop of polymer solution in dry CHCl3 on a ZnSe Infracal plate (Wilks, Spectro Inc., Littleton, MA, USA).
Nuclear magnetic resonance (NMR) spectra were obtained using a Bruker AVANCE 600 MHz spectrometer (Bruker, Inc., Billerica MA, USA) operating at 25 °C, with chloroform-
d1 (CDCl
3) as the solvent and internal standard. The
31P-NMR spectra were obtained in solid state using 4 mm DSI CPMAS probe rotors (DOTY Scientific, Columbia, SC, USA) under proton decoupling of 35 kHz, a recycle delay of 2s, and magic-angle spinning (MAS) at a frequency of 6.2 kHz. The DEPT edited HSQC analyses used previously described protocol [
39]: delay time 2 s, obtaining 256 increments with 16 scans per increment, within spectral widths of 7212 Hz (
1H) and 25 000 Hz (
13C) and operating at 125 and 600 MHz. The HMBC analyses were executed using a delay time of 1.2 s, obtaining 256 increments with 40 scans per increment, within spectral widths of 4807 Hz (
1H) and 36,000 Hz (
13C) operating at 125 and 600 MHz.
The compression test was carried out using a screw-driven mechanical load frame with a 500 kg capacity load cell (Q-test, MTS Systems, Eden Prarie, MN, USA). Six specimens (
Figure S4) of each composition (standard and modified at 10%
w/
w additive bone cements) were tested in air at room temperature at a displacement rate of the cross head of 25.4 mm/min. The compressive strength and modulus were calculated using TestWorks 4.0 software (MTS Systems Co.7, Eden Prairie, MN, USA).