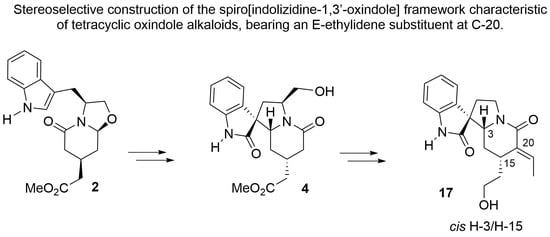
3.3. Removal of the Hydroxymethyl Substituent
(1’R,3′S,7′R,8a’R)-3′-Formyl-7′-(methoxycarbonylmethyl)-2,5′-dioxospiro[indoline-3,1′-indolizidine] (5). Dess-Martin periodinane (DMP, 649 mg, 1.53 mmol) and NaHCO3 (257 mg, 3.06 mmol) were added at room temperature under an argon atmosphere to a solution of spirooxindole 4 (367 mg, 1.02 mmol) in anhydrous CH2Cl2 (38 mL). The resulting mixture was stirred at room temperature for 4 h. Saturated aqueous solutions of Na2S2O3 (30 mL) and NaHCO3 (30 mL) were added and the mixture was stirred for 30 min. The aqueous layer was extracted with CH2Cl2. The combined organic extracts were dried, filtered, and concentrated under reduced pressure. Flash chromatography (1:1 hexane-EtOAc to EtOAc) of the resulting residue gave aldehyde 5 (280 mg, 77%) as a white foam: 1H-NMR (400 MHz, CDCl3, COSY, g-HSQC): δ = 0.75 (q, J = 12.4 Hz, 1H, H-14), 1.73 (dm, J = 12.4 Hz, 1H, H-14), 1.99 (dd, J = 17.8, 12.0 Hz, 1H, H-20), 2.14–2.33 (m, 3H, CH2CO, H-6), 2.44–2.53 (m, 2H, H-6, H-15), 2.70 (ddd, J = 17.8, 5.4, 1.9 Hz, 1H, H-20), 3.63 (s, 3H, CH3O), 4.15 (dd, J = 11.4, 4.2 Hz, 1H, H-3), 4.88 (t, J = 9.6 Hz, 1H, H-5), 6.90 (d, J = 7.4 Hz, 1H, HAR), 6.97 (d, J = 7.8 Hz, 1H, HAR), 7.07 (td, J = 7.5, 1.0 Hz, 1H, HAR), 7.30 (td, J = 7.6, 0.4 Hz, 1H, HAR), 7.69 (br. s, 1H, NH), 9.73 (d, J = 1.7 Hz, 1H, CHO); 13C-NMR (100.6 MHz, CDCl3): δ = 29.5 (C-15), 29.7 (C-14), 34.6 (C-6), 37.2 (C-20), 39.7 (CH2CO), 51.7 (CH3O), 56.7 (C-7), 62.9 (C-5), 65.0 (C-3), 110.8 (CHAR), 123.3 (CHAR), 123.5 (CHAR), 128.8 (CHAR), 129.1 (CAR), 140.2 (CAR), 169.1 (CO), 171.6 (CO), 176.4 (CO), 197.4 (CHO); HRMS (ESI) calcd for [C19H20N2O5 + H+]: 357.1445, found: 357.1450.
(1′R,3′S,7′R,8a’R)-7′-(Methoxycarbonylmethyl)-2,5′-dioxo-3′-(phenylselenocarbonyl)spi-ro[indoline-3,1′-indolizidine] (7). First step: 2-Methyl-2-butene (2 M in hexane, 6.4 mL) and t-BuOH (25.2 mL) were added at room temperature to a solution of aldehyde 5 (321 mg, 0.9 mmol) in CH3CN (8.1 mL). A solution of NaClO2 (472 mg, 5.22 mmol) and NaH2PO4 (733 mg, 5.31 mmol) in distilled H2O (8.7 mL) was added at 0 °C to the above mixture, which was stirred at room temperature for 1 h. Then, 0.1 M Na2S2O3 and 2 N HCl solutions were added until pH = 1, and the resulting mixture was extracted with EtOAc, dried over anhydrous MgSO4, filtered, and concentrated under reduced pressure to give carboxylic acid 6, which was used in the next step without purification. Second step: Diphenyl diselenide [(PhSe)2, 478 mg, 1.53 mmol] and tri-n-butylphosphine (n-Bu3P, 0.63 mL, 2.52 mmol) were added at room temperature under an argon atmosphere to a solution of crude acid 6 in anhydrous CH2Cl2 (5.4 mL). The resulting mixture was stirred at reflux for 16 h. Distilled H2O was added. The aqueous layer was extracted with CH2Cl2, and the combined organic extracts were dried over anhydrous MgSO4, filtered, and concentrated under reduced pressure. Flash chromatography (hexane to 7:3 hexane-EtOAc) of the resulting residue gave seleno ester 7 (216 mg, 50% overall yield for the two steps) as a yellow foam: [α]22D = −4.4 (c 1.04, CHCl3); IR (film): 3242 (NH), 1726, 1698, 1660, 1635 (CO) cm−1; 1H-NMR (400 MHz, CDCl3, COSY, g-HSQC): δ = 0.73 (q, J = 12.8 Hz, 1H, H-14), 1.75–1.82 (dm, J = 12.8 Hz, 1H, H-14), 1.99 (dd, J = 18.0, 12.0 Hz, 1H, H-20), 2.17 (dd, J = 15.6, 7.6 Hz, 1H, CH2CO), 2.30 (dd, J = 15.6, 6.4 Hz, 1H, CH2CO), 2.46 (dd, J = 12.8, 8.4 Hz, 1H, H-6), 2.46–2.58 (m, 1H, H-15), 2.62 (dd, J = 12.8, 8.4 Hz, 1H, H-6), 2.73–2.79 (dm, J = 18.0 Hz, 1H, H-20), 3.64 (s, 3H, CH3O), 4.37 (dd, J = 11.2, 4.4 Hz, H-3), 5.13 (t, J = 9.2 Hz, 1H, H-5), 6.84 (d, J = 7.6 Hz, 1H, HAR), 6.99 (d, J = 7.6 Hz, 1H, H HAR), 7.05 (td, J = 7.6, 1.2 Hz, 1H, HAR), 7.29 (td, J = 8.0, 1.2 Hz, 1H, HAR), 7.36–7.39 (m, 3H, HAR), 7.52–7.55 (m, 2H, HAR), 8.74 (s, 1H, NH); 13C-NMR (100.6 MHz, CDCl3): δ = 29.3 (C-15), 29.6 (C-14), 37.5 (C-20), 38.0 (C-6), 39.6 (CH2CO), 51.8 (CH3O), 57.1 (C-7), 65.6 (C-3), 66.4 (C-5), 110.8 (CHAR), 123.3 (CHAR), 123.4 (CHAR), 124.8 (CAR), 128.8–129.4 (4CHAR, CAR), 136.1 (2CHAR), 140.2 (CAR), 169.4 (CO), 171.6 (CO), 176.1 (CO), 200.5 (CO); HRMS (ESI) calcd for [C25H24N2O5Se + H+]: 513.0923, found: 513.0927.
(1′R,7′R,8a’R)-7′-(Methoxycarbonylmethyl)-2,5′-dioxospiro[indoline-3,1′-indolizidine] (8). Method A: from seleno derivative 7: Azobisisobutyronitrile (AIBN, 9 mg, 0.05 mmol) was added under an argon atmosphere to a solution of seleno derivative 7 (216 mg, 0.43 mmol) in anhydrous benzene (20 mL). The mixture was heated to reflux, and a solution of tributyltin hydride (TBTH, 180 µL, 0.65 mmol) in anhydrous benzene (4 mL) was added very slowly (over 30 min). The resulting mixture was stirred at reflux for 1 h, and the solvent was evaporated. Flash chromatography (6:4 hexane-EtOAc to 100% EtOAc) of the resulting residue gave spirooxindole 8 (127 mg, 90%). Method B: from aldehyde 5: Argon was bubbled through anhydrous diglyme (3.2 mL) for 30 min. Chloro(1,5-cyclooctadiene)rhodium(I) dimer (3 mg, 0.006 mmol) and 1,3-bis(diphenylphosphino) propane (dppp, 10 mg, 0.023 mmol) were weighed in corning tubes and introduced into the reaction flask under an argon flow using inert glovebox equipment. Anhydrous diglyme (2.2 mL) was transferred into the reaction flask and the bubbling of argon was continued for 15 min. Aldehyde 5 (80 mg, 0.23 mmol) was dissolved in anhydrous diglyme and transferred into the flask. The mixture was stirred at reflux for 24 h. Distilled H2O (2.2 mL) and CH2Cl2 (2.2 mL) were added, the layers were separated, and the aqueous phase was extracted with CH2Cl2. The combined organic extracts were washed with brine, dried over anhydrous MgSO4, filtered, and concentrated under reduced pressure. Flash chromatography (1:1 to 1:9 hexane-EtOAc) of the resulting residue gave compound 8 (57 mg, 75%) as a white foam and minor amounts of its 5,6-dehydro derivative, which was hydrogenated (10% Pd/C, absolute EtOH) to give additional compound 8 (3 mg, 4%) after flash chromatography: [α]22D = + 63.0 (c 0.55, CHCl3); IR (film): 3194 (NH), 1727, 1619 (CO) cm−1; 1H-NMR (400 MHz, CDCl3, COSY, g-HSQC): δ = 0.72 (q, J = 12.4 Hz, 1H, H-14), 1.67 (dm, J = 12.4 Hz, 1H, H-14), 1.95 (dd, J = 17.6, 12.0 Hz, 1H, H-20), 2.09 (dd, J = 12.4, 8.4 Hz, 1H, H-6), 2.14 (dd, J = 15.6, 7.6 Hz, 1H, CH2CO), 2.23 (dd, J = 15.6, 6.4 Hz, 1H, CH2CO), 2.35 (m, 1H, H-15), 2.52 (q, J = 12.4 Hz, 1H, H-6), 2.61 (dd, J = 17.6, 4.8 Hz, 1H, H-20), 3.61 (s, 3H, CH3O), 3.83 (t, J = 11.2 Hz, 1H, H-5), 3.99 (tm, J = 11.2 Hz, 1H, H-5), 4.04 (dd, J = 11.2, 4.4 Hz, 1H, H-3), 6.91 (d, J = 7.2 Hz, 1H, HAR), 6.99 (d, J = 7.6 Hz, 1H, HAR), 7.05 (t, J = 7.6 Hz, 1H, HAR), 7.28 (t, J = 7.6 Hz, 1H, HAR), 8.92 (br. s, 1H, NH); 13C-NMR (100.6 MHz, CDCl3): δ = 29.6 (C-15), 29.7 (C-14), 33.3 (C-6), 37.3 (C-20), 39.9 (CH2CO), 43.9 (C-5), 51.7 (CH3O), 56.9 (C-7), 64.3 (C-3), 110.5 (CHAR), 123.1 (CHAR), 123.7 (CHAR), 128.6 (CHAR), 129.7 (CAR), 140.2 (CAR), 168.4 (CO), 171.7 (CO), 177.5 (CO); HRMS (ESI) calcd for [C18H20N2O4 + H+]: 329.1496, found: 329.1497.
3.4. Introduction of the E-Ethylidene Chain
(1’R,7′S,8a’R)-7′-(2-Hydroxyethyl)-2,5′-dioxospiro[indoline-3,1′-indolizidine] (9): Lithium borohydride (LiBH4, 20 mg, 0.9 mmol) was added at 0 °C under an argon atmosphere to a solution of compound 8 (49 mg, 0.15 mmol) in anhydrous THF (5 mL). The resulting mixture was stirred at room temperature for 72 h. The reaction was quenched at 0 °C by distilled H2O (5 mL), and the mixture was concentrated under reduced pressure using a rotary evaporator with a dry ice condenser. Flash chromatography (95:5 EtOAc-MeOH) of the resulting residue gave oxindole 9 (34 mg, 76%) as a white foam and minor amounts of indoline 10 (3 mg, 5%). Oxindole 9: [α]22D = + 58,0 (c 1.12, MeOH); IR (film): 3100–3600 (NH, OH), 1731, 1714 (CO) cm−1; 1H-NMR (400 MHz, CDCl3, COSY, g-HSQC): δ = 0.64 (q, J = 12.0 Hz, 1H, H-14), 1.43 (m, 2H, CH2CH2O), 1.64 (dm, J = 12.0 Hz, 1H, H-14), 1.90 (dd, J = 17.6, 12.0 Hz, 1H, H-20), 2.05 (m, 2H, H-6, H-15), 2.49 (dt, J = 10.4 Hz, 1H, H-6), 2.58 (dd, J = 17.6, 5.2 Hz, 1H, H-20), 3.60 (t, J = 6.4 Hz, 2H, CH2O), 3.80 (dd, J = 11.6, 9.2 Hz, 1H, H-5), 3.98 (m, 2H, H-5, H-3), 6.91 (d, J = 7.6 Hz, 1H, HAR), 6.97 (d, J = 8.0 Hz, 1H, HAR), 7.04 (t, J = 7.6 Hz, 1H, HAR), 7.26 (td, J = 7.6, 1.2 Hz, 1H, HAR), 8.83 (br. s, 1H, NH); 13C-NMR (100.6 MHz, CDCl3): δ = 29.5 (C-15), 29.9 (C-14), 33.2 (C-6), 37.9 (C-20), 38.4 (CH2CH2O), 43.8 (C-5), 57.0 (C-7), 59.7 (CH2O), 64.6 (C-3), 110.3 (CHAR), 123.1 (CHAR), 123.8 (CHAR), 128.6 (CHAR), 129.9 (CAR), 140.1 (CAR), 169.2 (CO), 177.5 (CO); HRMS (ESI) calcd for [C17H20N2O3 + Na+]: 323.1366, found: 323.1371. (1′S,7′S,8a’R)-7′-(2-Hydroxyethyl)-5′-oxospiro[indoline-3,1′-indolizidine] (10): IR (film): 3346 (NH, OH), 1606 (CO) cm−1; 1H-NMR (400 MHz, CDCl3, COSY, g-HSQC): δ = 0.69 (q, J = 12.4 Hz, 1H, H-14), 1.48 (m, 2H, CH2CH2O), 1.83–1.92 (m, 2H, H-14, H-20), 1.97–2.08 (m, 2H, H-15, H-6), 2.25 (ddd, J = 12.8, 8.4, 2.0 Hz, 1H, H-6), 2.54 (ddd, J = 17.6, 5.2, 1.6 Hz, 1H, H-20), 3.50–3.66 (m, 2H, H-5, H-3), 3.51 (d, J = 9.2 Hz, 1H, H-2), 3.57 (d, J = 9.2 Hz, 1H, H-2), 3.65 (t, J = 6.4 Hz, 2H, CH2CH2O), 3.83–3.91 (m, 1H, H-5), 6.66 (d, J = 7.6 Hz, 1H, HAR), 6.71 (t, J = 7.6 Hz, 1H, HAR), 6.77 (dd, J = 7.6, 1.6 Hz, 1H, HAR), 7.08 (td, J = 7.6, 1.2 Hz, 1H, HAR); 13C-NMR (100.6 MHz, CDCl3): δ = 29.7 (C-15), 30.3 (C-14), 35.9 (C-6), 38.2 (C-20), 38.7 (CH2CH2O), 43.5 (C-5), 54.9 (C-2), 55.4 (C-7), 60.0 (CH2CH2O), 66.1 (C-3), 110.1 (CHAR), 119.5 (CHAR), 124.0 (CHAR), 128.5 (CHAR), 131.0 (CAR), 151.2 (CAR), 169.2 (CO); HRMS (ESI) calcd for [C17H22N2O2 + H+]: 287.1754, found: 287.1758.
(1′R,7′S,8a’R)-7′-{2-[(tert-Butyldimethylsilyl)oxy]ethyl}-2,5′-dioxospiro[indoline-3,1′-indolizidine] (11). tert-Butyldimethylsilyl chloride (TBDMSCl, 27 mg, 0.18 mmol) and imidazole (25 mg, 0.36 mmol) were added at 0 °C under an argon atmosphere to a solution of alcohol 9 (27 mg, 0.09 mmol) in anhydrous DMF (1 mL). The resulting mixture was stirred at room temperature for 16 h. Brine was added, and the mixture was extracted with EtOAc. The combined organic extracts were dried over anhydrous MgSO4, filtered, and concentrated under reduced pressure. Flash chromatography (7:3 hexane-EtOAc to 1:1 hexane-EtOAc) of the resulting residue gave silyl derivative 11 (30 mg, 80%) as a white foam: [α]22D = + 37.09 (c 0.23, CHCl3); IR (film): 3181 (NH), 1725, 1620 (CO) cm−1; 1H-NMR (400 MHz, CDCl3, COSY, g-HSQC): δ = −0.01 (s, 3H, CH3Si), 0.00 (s, 3H, CH3Si), 0.65 (qd, J = 12.4, 3.6 Hz, 1H, H-14), 0.83 [s, 9H, C(CH3)3], 1.39 (m, 2H, CH2CH2O), 1.65 (dm, J = 12.4 Hz, 1H, H-14), 1.89 (dd, J = 16.4, 11.6 Hz, 1H, H-20), 2.03–2.09 (m, 2H, H-15, H-6), 2.50–2.58 (m, 2H, H-6, H-20), 3.55 (m, 2H, CH2O), 3.81 (t, J = 12.4 Hz, 1H, H-5), 3.95–4.03 (m, 2H, H-5, H-3), 6.91–6.95 (m, 2H, HAR), 7.03–7.07 (m, 1H, HAR), 7.27 (m, 1H, HAR), 7.68 (br. s, 1H, NH); 13C-NMR (100.6 MHz, CDCl3): δ = −5.5 (2CH3Si), 18.2 [C(CH3)3], 25.8 [C(CH3)3], 29.7 (C-15), 30.1 (C-14), 33.3 (C-6), 37.9 (C-20), 38.5 (CH2CH2O), 43.8 (C-5), 57.0 (C-7), 60.1 (CH2O), 64.8 (C-3), 110.2 (CHAR), 123.1 (CHAR), 123.9 (CHAR), 128.5 (CHAR), 129.9 (CAR), 140.0 (CAR), 169.2 (CO), 177.5 (CO); HRMS (ESI) calcd for [C23H34N2O3Si + H+]: 415.2411, found: 415.2419.
(1′R,7′S,8a’R)-7′-{2-[(tert-Butyldimethylsilyl)oxy]ethyl}-1-(methoxymethyl)-2,5′-dioxospiro[indoline-3,1′-indolizidine] (12). A solution of compound 11 (386 mg, 0.93 mmol) in anhydrous THF (2.5 mL) was transferred at 0 °C under an argon atmosphere to a suspension of NaH (95%, 36 mg, 1.4 mmol) in anhydrous DMF (2.5 mL). The resulting mixture was stirred at 0 °C for 30 min. Methoxymethyl chloride (MOMCl, 0.12 mL, 1.4 mmol) was added, and the resulting mixture was stirred at room temperature for 1.5 h. The mixture was cooled to 0 °C and saturated aqueous NaHCO3 (11.2 mL) was added. The mixture was extracted with EtOAc and the combined organic extracts were dried over anhydrous MgSO4, filtered, and concentrated under reduced pressure. Flash chromatography (hexane to 7:3 hexane-EtOAc) of the resulting residue gave the N-MOM derivative 12 (283 mg, 67%) as a white foam: [α]22D = + 50.6 (c 2.26, CHCl3); IR (film): 1725, 1651 (CO) cm−1; 1H-NMR (400 MHz, CDCl3, COSY, g-HSQC): δ = −0.02 (s, 3H, CH3Si), −0.03 (s, 3H, CH3Si), 0.64 (q, J = 12.4 Hz, 1H, H-14), 0.82 [s, 9H, C(CH3)3], 1.37 (dd, J = 12.8, 6.4 Hz, 2H, CH2CH2O), 1.55 (dm, J = 12.4 Hz, 1H, H-14), 1.89 (dd, J = 17.6, 12.0 Hz, 1H, H-20), 2.00–2.07 (m, 2H, H-15, H-6), 2.50–2.57 (m, 2H, H-20, H-6), 3.32 (s, 3H, CH3O), 3.54 (t, J = 6.0 Hz, 2H, CH2CH2O), 3.81 (t, J = 11.6 Hz, 1H, H-5), 3.96–4.03 (m, 2H, H-5, H-3), 5.15 (s, 2H, NCH2O), 6.94 (d, J = 7.6 Hz, 1H, HAR), 7.08–7.11 (m, 2H, HAR), 7.32 (t, J = 7.6 Hz, 1H, HAR); 13C-NMR (100.6 MHz, CDCl3): δ = −5.5 (2CH3Si), 18.2 [C(CH3)3], 25.8 [C(CH3)3], 29.6 (C-15), 30.2 (C-14), 33.7 (C-6), 37.8 (C-20), 38.6 (CH2CH2O), 43.8 (C-5), 56.3, 56.9 (C-7, CH3O), 60.0 (CH2CH2O), 64.8 (C-3), 71.5 (NCH2O), 110.0 (CHAR), 123.6 (CHAR), 123.7 (CHAR), 128.6 (CHAR), 129.1 (CAR), 141.2 (CAR), 169.1 (CO), 176.3 (CO); HRMS (ESI) calcd for [C25H38N2O4Si + H+]: 459.2674, found: 459.2675.
(1′R,7′S,8a’R)-7′-{2-[(tert-Butyldimethylsilyl)oxy]ethyl}-1-(tert-butoxycarbonyl)-2,5′-dioxospiro[indoline-3,1′-indolizidine] (13). NaH (95%, 25 mg, 0.64 mmol) and (Boc)2O (70 mg, 0.32 mmol) were added at 0 °C under an argon atmosphere to a solution of compound 11 (33 mg, 0.08 mmol) in anhydrous DMF (1 mL). The mixture was stirred at room temperature for 16 h. The reaction was quenched by the addition of a few drops of distilled H2O, and the resulting residue was dried over anhydrous MgSO4, filtered, and concentrated under reduced pressure. Flash chromatography (hexane to 4:1 EtOAc-MeOH) gave N-Boc derivative 13 (26 mg, 64%): [α]22D = + 56.7 (c 0.92, CHCl3); IR (film): 1794, 1766, 1733 (CO) cm−1; 1H-NMR (400 MHz, CDCl3, COSY, g-HSQC): δ = −0.28 (s, 3H, CH3Si), −0.21 (s, 3H, CH3Si), 0.60 (q, J = 12.0 Hz, 1H, H-14), 0.82 [s, 9H, SiC(CH3)3], 1.37–1.45 (m, 2H, CH2CH2O), 1.65 [s, 9H, C(CH3)3], 1.65 (masked, 1H, H-14), 1.88 (dd, J = 17.6, 12.0 Hz, 1H, H-20), 2.01 (m, 1H, H-15), 2.08 (dd, J = 12.8, 6.8 Hz, 1H, H-6), 2.52 (m, 2H, H-6, H-20), 3.50–3.57 (m, 2H, CH2CH2O), 3.79 (t, J = 12.0 Hz, 1H, H-5), 3.94–4.03 (m, 2H, H-3, H-5), 6.90 (d, J = 6.8 Hz, 1H, HAR), 7.16 (t, J = 7.6 Hz, 1H, HAR), 7.34 (td, J = 7.2, 1.2 Hz, 1H, HAR), 7.90 (d, J = 8.0 Hz, 1H, HAR); 13C-NMR (100.6 MHz, CDCl3): δ = −5.5 (2CH3Si), 18.1 [SiC(CH3)3], 25.8 [SiC(CH3)3], 28.1 [C(CH3)3], 29.9 (C-15), 30.2 (C-14), 34.5 (C-6), 37.8 (C-20), 38.5 (CH2CH2O), 43.7 (C-5), 56.8 (C-7), 60.2 (CH2CH2O), 65.5 (C-3), 84.9 [C(CH3)3], 115.3 (CHAR), 123.3 (CHAR), 125.1 (CHAR), 128.5 (CAR), 128.7 (CHAR), 138.9 (CAR), 148.9 (CO), 169.1 (CO), 174.6 (CO); HRMS (ESI) calcd for [C28H42N2O5Si + H+]: 515.2936, found: 515.2941.
(1′R,6′R,7′S,8a’R)-6′-Acetyl-7′-{2-[(tert-butyldimethylsilyl)oxy]ethyl}-1-(methoxymtehyl)-2,5′-dioxospiro[indoline-3,1′-indolizidine] (14). Lithium diisopropylamide (LDA, 0.26 mL of a 2.0 M solution in THF/heptane/ethylbenzene, 0.42 mmol) was added at −78 °C under an argon atmosphere to a solution of spiro compound 12 (62 mg, 0.14 mmol) in anhydrous THF (0.7 mL), and the mixture was stirred at −78 °C for 1 h. Methyl acetate (0.05 mL, 0.56 mmol) was added at −78 °C, and the resulting mixture was stirred at room temperature for 4 h. Saturated aqueous NH4Cl was added, and the mixture was extracted with CH2Cl2. The combined organic extracts were dried over anhydrous MgSO4, filtered, and concentrated under reduced pressure. Flash chromatography (hexane to 7:3 hexane-EtOAc) of the resulting residue gave starting material 12 (18 mg, 29%) and acetyl derivative 14 (35 mg, 52%) as a white foam: [α]22D = + 26.2 (c 0.82, CHCl3); IR (film): 1723, 1642, 1613 (CO) cm−1; 1H-NMR (400 MHz, CDCl3, COSY, g-HSQC): δ = −0.07 (s, 3H, CH3Si), −0.06 (s, 3H, CH3Si), 0.70–0.84 (m, 1H, H-14), 0.79 [s, 9H, C(CH3)3], 1.19–1.25 (m, 1H, CH2CH2O), 1.41–1.49 (m, 1H, CH2CH2O), 1.64–1.70 (m, 1H, H-14), 2.05 (dd, J = 12.8, 2.8 Hz, 1H, H-6), 2.37 (s, 3H, COCH3), 2.40–2.46 (m, 1H, H-15), 2.55 (dt, J = 12.8, 10.8 Hz, 1H, H-6), 3.21 (d, J = 10.8 Hz, 1H, H-20), 3.32 (s, 3H, CH3O), 3.47 (t, J = 6.4 Hz, 2H, CH2CH2O), 3.81 (dd, J = 12.8, 10.8 Hz, 1H, H-5), 3.96–4.02 (m, 1H, H-5), 4.09 (dd, J = 11.6, 4.0 Hz, 1H, H-3), 5.13 (d, J = 10.8 Hz, 1H, NCH2O), 5.15 (d, J = 10.8 Hz, 1H, NCH2O), 6.92 (d, J = 7.6 Hz, 1H, HAR), 7.08–7.12 (m, 2H, HAR), 7.33 (td, J = 9.2, 1.2 Hz, 1H, HAR); 13C-NMR (100.6 MHz, CDCl3): δ = −5.6 (CH3Si), −5.5 (CH3Si), 18.1 [C(CH3)3], 25.8 [C(CH3)3], 28.8 (C-14), 31.1 (CH3CO), 32.7 (C-15), 33.7 (C-6), 36.6 (CH2CH2O), 44.3 (C-5), 56.3 (CH3O), 56.9 (C-7), 60.2 (CH2CH2O), 61.7 (C-20), 64.3 (C-3), 71.5 (NCH2O), 110.1 (CHAR), 123.6 (CHAR), 123.7 (CHAR), 128.7 (CAR), 128.8 (CHAR), 141.2 (CAR), 165.8 (CO), 176.0 (CO), 205.2 (CO); HRMS (ESI) calcd for [C27H40N2O5Si + H+]: 501.2779, found: 501.2791.
(1′R,6′R,7′S,8a’R)-7′-{2-[(tert-Butyldimethylsilyl)oxy]ethyl}-6′-(1R- and 1S-hydroxyethyl)-1-(methoxymethyl)-2,5′-dioxospiro[indoline-3,1′-indolizidine] (15a and 15b). NaBH4 (10 mg, 0.24 mmol) was added at −10 °C under an argon atmosphere to a solution of ketone 14 (60 mg, 0.12 mmol) in anhydrous MeOH (2 mL). The resulting mixture was stirred at −10 °C for 1 h. Saturated aqueous NaHCO3 (1.3 mL) and CH2Cl2 were added, and the mixture was stirred for 5 min. The organic solvent was evaporated, and the resulting aqueous mixture was extracted with CH2Cl2. The combined organic extracts were dried over anhydrous MgSO4, filtered, and concentrated under reduced pressure. Flash chromatography (7:3 hexane-EtOAc to 1:1 hexane-EtOAc) of the resulting residue gave alcohols 15a (28 mg, 46%) and 15b (27 mg, 46%) as white foams. 15a: [α]22D = + 30.3 (c 1.16, CHCl3); IR (film): 3427 (OH), 1725, 1614 (CO) cm−1; 1H-NMR (400 MHz, CDCl3, COSY, g-HSQC): δ = −0.05 (s, 3H, CH3Si), −0.04 (s, 3H, CH3Si), 0.65–0.81 (m, 1H, H-14), 0.81 [s, 9H, C(CH3)3], 1.24–1.32 (m, 1H, CH2CH2O), 1.39 (d, J = 6.4 Hz, 3H, CH3CHOH), 1.58 (dt, J = 13.2, 3.9 Hz, 1H, H-14), 1.76–1.85 (m, 1H, CH2CH2O), 1.92–1.95 (m, 1H, H-15), 2.00–2.05 (m, 1H, H-6), 2.11 (dd, J = 9.2, 4.8 Hz, 1H, H-20), 2.54 (td, J = 12.4, 9.6 Hz, 1H, H-6), 3.32 (s, 3H, CH3O), 3.45–3.56 (m, 2H, CH2CH2O), 3.81 (dd, J = 12.4, 9.6 Hz, 1H, H-5), 3.96–4.03 (m, 3H, H-3, H-5, CHOH), 5.15 (s, 2H, NCH2O), 6.96 (dm, J = 8.0 Hz, 1H, HAR), 7.08–7.12 (m, 2H, HAR), 7.33 (td, J = 8.0, 1.2 Hz, 1H, HAR); 13C-NMR (100.6 MHz, CDCl3): δ = −5.5 (2CH3Si), 18.1 [C(CH3)3], 22.1 (CH3CHOH), 25.8 [C(CH3)3], 30.4 (C-14), 32.4 (C-15), 33.9 (C-6), 38.0 (CH2CH2O), 44.7 (C-5), 53.3 (C-20), 56.3 (CH3O), 57.2 (C-7), 60.7 (CH2CH2O), 63.6 (C-3), 69.7 (CHOH), 71.5 (NCH2O), 110.1 (CHAR), 123.6 (CHAR), 123.8 (CHAR), 128.7 (CHAR, CAR), 141.2 (CAR), 171.2 (CO), 176.2 (CO); HRMS (ESI) calcd for [C27H42N2O5Si + H+]: 503.2936, found: 503.2937. 15b: [α]22D = + 14.0 (c 1.13, CHCl3); IR (film): 3418 (OH), 1725, 1614 (CO) cm−1; 1H-NMR (400 MHz, CDCl3, COSY, g-HSQC): δ = −0.07 (s, 3H, CH3Si), −0.05 (s, 3H, CH3Si), 0.70–0.84 (m, 1H, H-14), 0.80 [s, 9H, C(CH3)3], 1.18–1.20 (m, 1H, CH2CH2O), 1.19 (d, J = 6.4 Hz, 3H, CH3CHOH), 1.61–1.75 (m, 3H, H-14, H-15, CH2CH2O), 2.06 (dd, J = 12.8, 6.4 Hz, 1H, H-6), 2.34 (dd, J = 10.8, 3.6 Hz, 1H, H-20), 2.55 (tm, J = 12.8 Hz, 1H, H-6), 3.32 (s, 3H, CH3O), 3.44–3.54 (m, 2H, CH2CH2O), 3.84 (dd, J = 12.8, 10.8 Hz, 1H, H-5), 3.96–4.01 (m, 3H, H-3, H-5, CHOH), 5.15 (s, 2H, NCH2O), 6.94 (d, J = 7.2 Hz, 1H, HAR), 7.09–7.13 (m, 2H, HAR), 7.34 (t, J = 7.6 Hz, 1H, HAR); 13C-NMR (100.6 MHz, CDCl3): δ = −5.5 (2CH3Si), 18.0 [C(CH3)3], 18.6 (CH3CHOH), 25.8 [C(CH3)3], 29.3 (C-14), 32.2 (C-15), 33.8 (C-6), 36.3 (CH2CH2O), 44.1 (C-5), 52.3 (C-20), 56.3 (C-7), 57.0 (CH3O), 59.9 (CH2CH2O), 64.1 (C-3), 67.1 (CHOH), 71.5 (NCH2O), 110.2 (CHAR), 123.7 (CHAR), 123.8 (CHAR), 128.7 (CAR), 128.8 (CHAR), 141.2 (CAR), 172.2 (CO), 176.1 (CO); HRMS (ESI) calcd for [C27H42N2O5Si + H+]: 503.2936, found: 503.2933.
(1′R,6′E,7′S,8a’R)-7′-{2-[(tert-Butyldimethylsilyl)oxy]ethyl}-6′-ethylidene-1-(methoxymethyl)-2,5′-dioxospiro[indoline-3,1′-indolizidine] (16). From alcohol 15a: N,N′-Dicyclohexylcarbodiimide (DCC, 54 mg, 0.26 mmol) and copper(I) chloride (CuCl, 52 mg, 0.52 mmol) were added under an argon atmosphere to a solution of alcohol 15a (26 mg, 0.05 mmol) in anhydrous toluene (1.6 mL), and the resulting mixture was stirred at reflux for 5 h. The suspension was filtered through Celite®, and the residue was washed with CH3CN. The resulting filtrate was kept in the freezer overnight and filtered again through Celite®, washing with minimal amounts of cold CH3CN. The organic filtrate was concentrated under reduced pressure. Flash chromatography (1:9 hexane-EtOAc) of the resulting residue gave the E-ethylidene derivative 16 (22 mg, 91%). From alcohol 15b: First step: Et3N (18 μL, 0.13 mmol) and mesyl chloride (MsCl, 9 μL, 0.11 mmol) were added at 0 °C under an argon atmosphere to a solution of alcohol 15b (21 mg, 0.04 mmol) in anhydrous CH2Cl2 (0.6 mL). The resulting mixture was stirred at 0 °C for 4 h. Saturated aqueous NH4Cl (1.2 mL) was added, and the mixture was extracted with CH2Cl2. The combined organic extracts were dried over anhydrous MgSO4, filtered, and concentrated under reduced pressure to give the corresponding mesylate, which was used in the next step without further purification. Second step: Diazabicycloundecene (DBU, 27 μL, 0.18 mmol) was added under an argon atmosphere to a solution of the above mesylate in anhydrous THF (0.6 mL), and the resulting mixture was stirred at reflux overnight. Distilled H2O was added, and the mixture was extracted with EtOAc. The combined organic extracts were dried over anhydrous MgSO4, filtered, and concentrated under reduced pressure. Flash chromatography (hexane to 7:3 hexane-EtOAc) of the residue gave the Z isomer of compound 16 (4 mg, 20%) and the E-ethylidene derivative 16 (12 mg, 60%) as white foams. 16: [α]22D = −2.9 (c 0.76, CHCl3); IR (film): 1725, 1613 (CO) cm−1; 1H-NMR (400 MHz, CDCl3, COSY, g-HSQC): δ = −0.1 (s, 3H, CH3Si), −0.07 (s, 3H, CH3Si), 0.78 [s, 9H, C(CH3)3], 0.94–1.05 (q, J = 12.0 Hz, 1H, H-14), 1.29–1.36 (m, 1H, CH2CH2O), 1.65–1.70 (m, 1H, H-14), 1.77–1.85 (m, 4H, CH2CH2O, =CHCH3), 1.87–1.95 (dm, J = 12.4 Hz, 1H, H-6), 2.51 (m, 1H, H-6), 2.87–2.96 (m, 1H, H-15), 3.32 (s, 3H, CH3O), 3.36–3.52 (m, 2H, CH2CH2O), 3.88–4.30 (m, 3H, H-5, H-3), 5.14 (d, J = 10.8 Hz, 1H, NCH2O), 5.17 (d, J = 10.8 Hz, 1H, NCH2O), 6.67 (m, 1H, =CHCH3), 7.09–7.16 (m, 3H, HAR), 7.33 (td, J = 7.2, 1.6 Hz, HAR); 13C-NMR (100.6 MHz, CDCl3): δ = −5.5 (2CH3Si), 14.4 (=CHCH3), 18.1 [C(CH3)3], 25.8 [C(CH3)3], 30.8 (C-15), 31.1 (C-14), 34.8 (C-6), 39.4 (CH2CH2O), 44.6 (C-5), 56.3 (CH3O), 57.4 (C-7), 60.4 (CH2CH2O), 61.6 (C-3), 71.5 (NCH2O), 110.1 (CHAR), 123.5 (CHAR), 124.3 (CHAR), 128.7 (CHAR), 128.8 (CAR), 133.5 (=CHCH3), 135.7 (C-20), 141.2 (CAR), 167.6 (CO), 176.6 (CO); HRMS (ESI) calcd for [C27H40N2O4Si + H+]: 485.2830, found: 485.2825. Z-isomer of 16: IR (film): 1726, 1614 (CO) cm−1; 1H-NMR (400 MHz, CDCl3, COSY, g-HSQC, selected resonances): δ = −0.05 (s, 3H, CH3Si), −0.04 (s, 3H, CH3Si), 0.81 [s, 9H, C(CH3)3], 1.54–1.64 (m, 1H, H-14), 1.83 (m, 1H, CH2CH2O), 2.04 (ddd, J = 12.4, 7.6, 1.6 Hz, 1H, H-6), 2.20 (dd, J = 7.2, 2.0 Hz, 3H, =CHCH3), 2.54–2.60 (m, 1H, H-6), 3.32 (s, 3H, CH3O), 3.48–3.60 (m, 2H, CH2CH2O), 3.83–3.89 (m, 1H, H-5), 4.02–4.08 (m, 2H, H-3, H-5), 5.15 (s, 2H, NCH2O), 5.94 (qd, J = 7.2, 2.0 Hz, 1H, =CHCH3), 6.99 (dd, J = 8.0, 1.2 Hz, 1H, HAR), 7.07–7.11 (m, 2H, HAR), 7.31 (td, J = 7.6, 1.2 Hz, 1H, HAR); 13C-NMR (100.6 MHz, CDCl3, selected resonances): δ = −5.5 (CH3Si), −5.4 (CH3Si), 15.8 (=CHCH3), 18.1 [C(CH3)3], 25.8 [C(CH3)3], 29.8 (C-14), 34.1 (C-6), 36.2 (CH2CH2O), 44.0 (C-5), 56.3 (CH3O), 57.0 (C-7), 60.3 (CH2CH2O), 63.9 (C-3), 71.4 (NCH2O), 109.9 (CHAR), 123.6 (CHAR), 123.9 (CHAR), 128.6 (CHAR), 132.1 (CAR), 134.2 (=CHCH3), 141.2 (CAR), 165.5 (CO), 176.4 (CO); HRMS (ESI) calcd for [C27H40N2O4Si + H+]: 485.2830, found: 485.2825.
(1′R,6′E,7′S,8a’R)-6′-Ethylidene-7′-(2-hydroxyethyl)-2,5′-dioxospiro[indoline-3,1′-indolizidine] (17). TMSCl (30 μL, 0.23 mmol) and sodium iodide (35 mg, 0.23 mmol) were added at 0 °C under an argon atmosphere to a solution of compound 16 (25 mg, 0.05 mg) in anhydrous CH3CN (0.9 mL). The resulting mixture was stirred at 0 °C for 2 h. Saturated aqueous NaHCO3 was added and the mixture was extracted with EtOAc. The combined organic extracts were washed with brine, dried over anhydrous MgSO4, filtered, and concentrated under reduced pressure. The resulting residue was taken in MeOH (4.7 mL), Et3N (22 μL, 0.15 mmol) was added to the solution, and the mixture was stirred at 55 °C for 1 h. Saturated aqueous NH4Cl (1.7 mL) was added, the organic solvent was evaporated, and the aqueous mixture was extracted with EtOAc. The combined organic extracts were washed with brine, dried over anhydrous MgSO4, filtered, and concentrated under reduced pressure. Flash chromatography (hexane to 1:1 hexane-EtOAc) of the residue gave compound 17 (11 mg, 68%) as a white foam: [α]22D = −4.3 (c 0.44, CHCl3); IR (film): 3500–3000 (NH, OH), 1721, 1651 (CO) cm−1; 1H-NMR (400 MHz, CDCl3, COSY, g-HSQC): δ = 1.06 (q, J = 12.0 Hz, 1H, H-14), 1.39–1.48 (m, 1H, CH2CH2O), 1.70–1.76 (m, 1H, H-14), 1.80 (dd, J = 7.2, 1.2 Hz, 1H, =CHCH3), 1.83–1.91 (m, 1H, CH2CH2O), 2.01–2.06 (m, 1H, H-6), 2.46–2.54 (m, 1H, H-6), 2.93–3.00 (m, 1H, H-15), 3.43–3.49 (m, 1H, CH2CH2O), 3.53–3.59 (m, 1H, CH2CH2O), 3.88–4.02 (m, 3H, H-5, H-3), 6.71 (qd, J = 7.2, 2.0 Hz, 1H, =CHCH3), 6.97 (d, J = 7.6 Hz, 1H, HAR), 7.05–7.13 (m, 2H, HAR), 7.26–7.30 (m, 1H, HAR), 8.01 (br. s, 1H, NH); 13C-NMR (100.6 MHz, CDCl3): δ = 14.5 (=CHCH3), 30.5 (C-14), 30.9 (C-15), 34.4 (C-6), 38.5 (CH2CH2O), 44.8 (C-5), 57.4 (C-7), 59.9 (CH2CH2O), 61.5 (C-3), 110.5 (CHAR), 122.9 (CHAR), 124.4 (CHAR), 128.6 (CHAR), 129.7 (CAR), 133.6 (=CHCH3), 134.7 (C-20), 140.2 (CAR), 167.1 (CO), 177.7 (CO); HRMS (ESI) calcd for [C19H22N2O3 + H+]: 327.1703, found: 327.1702.