Synthesis and Olfactory Properties of Seco-Analogues of Lilac Aldehydes
Abstract
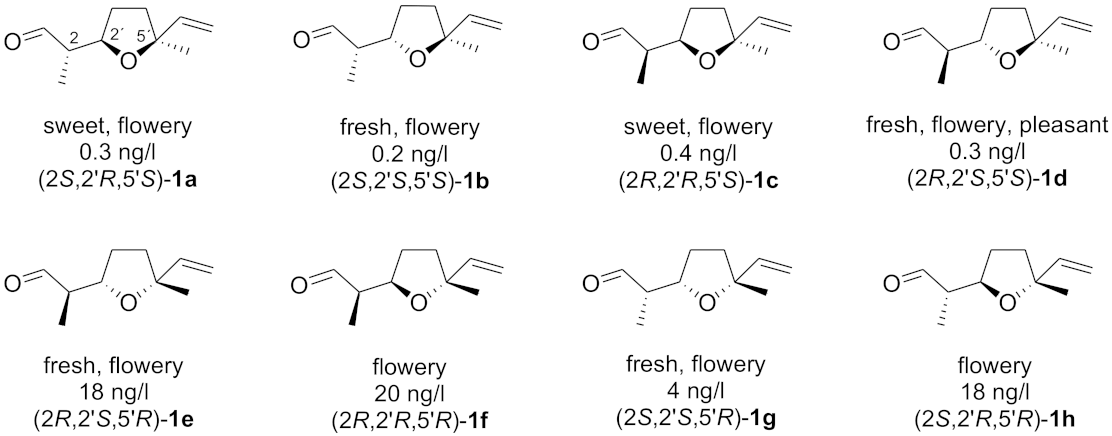
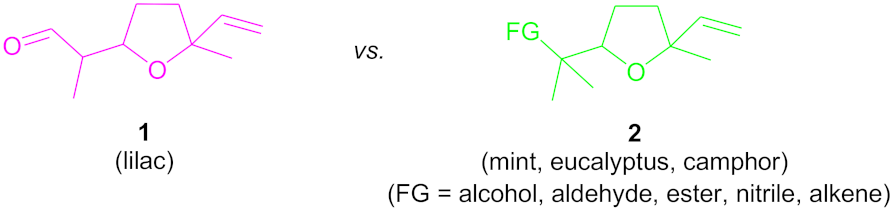
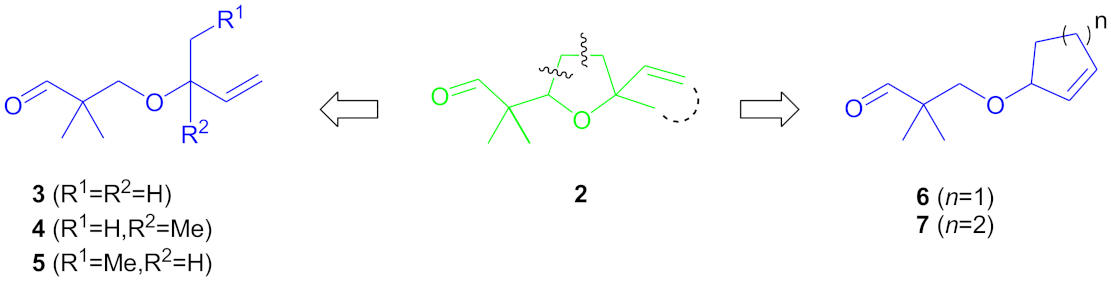
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. Materials and Methods
3.2. Synthetic Procedures and Analytical Data
3.2.1. General Experiment for the Preparation of Chloroketals (12–15)
2-(1-Chloroethyl)-2,5,5-trimethyl-[1,3]dioxane (12)
2-(1-Chloroethyl)-2-ethyl-5,5-dimethyl-[1,3]dioxane (13)
1-Chloro-8,8-dimethyl-6,10-dioxaspiro [4.5] decane (14)
7-Chloro-3,3-dimethyl-1,5-dioxaspiro[5.5]undecane (15)
3.2.2. General Experiment for the Preparation of Alkene Ketals (16–19, 25)
2,5,5-Trimethyl-2-vinyl-[1,3]dioxane (16)
2-Ethyl-5,5-dimethyl-2-vinyl-[1,3]dioxane (17)
8,8-Dimethyl-6,10-dioxaspiro[4.5]dec-1-ene (18)
3,3-Dimethyl-1,5-dioxaspiro[5.5]undec-7-ene (19) and 3,3-Dimethyl-1,5-dioxaspiro[5.5]undec-8-ene (25)
3.2.3. Preparation of Alcohol (21)
2,2-Dimethyl-3-(2-methyl-but-3-en-2-yloxy)propan-1-ol (21)
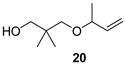
3.2.4. General Experiment for the Preparation of Alcohols (20, 22, 23)
3-(But-3-en-2-yloxy)-2,2-dimethylpropan-1-ol (20)
2,2-Dimethyl-3-(pent-1-en-3-yloxy)propan-1-ol (22)
3-(Cyclopent-2-enyloxy)-2,2-dimethylpropanol (23)
3.2.5. Preparation of Alcohol (24)
3.2.6. General Experiment for the Preparation of Aldehydes (3–7)
3-(But-3-en-2-yloxy)-2,2-dimethylpropan-1-al (3)
2,2-Dimethyl-3-(2-methyl-but-3-en-2-yloxy)propan-1-al (4)
2,2-Dimethyl-3-(pent-1-en-3-yloxy)propan-1-al (5)
3-(Cyclopent-2-enyloxy)-2,2-dimethylpropan-1-al (6)
3-(Cyclohex-2-enyloxy)-2,2-dimethylpropan-1-al (7)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Wakayama, S.; Namba, S. Lilac Aldehydes. Bull. Chem. Soc. Jpn. 1974, 47, 1293–1294. [Google Scholar] [CrossRef] [Green Version]
- Kreck, M.; Mosandl, A. Synthesis, Structure Elucidation, and Olfactometric Analysis of Lilac Aldehyde and Lilac Alcohol Stereoisomers. J. Agric. Food Chem. 2003, 51, 2722–2726. [Google Scholar] [CrossRef] [PubMed]
- Kreck, M.; Püschel, S.; Wüst, M.; Mosandl, A. Biogenetic studies in Syringa vulgaris L.: Synthesis and bioconversion of deuterium-labelled precursors into lilac aldehydes and lilac alcohols. J. Agric. Food Chem. 2003, 51, 463–469. [Google Scholar] [CrossRef] [PubMed]
- Matich, A.J.; Young, H.; Allen, J.M.; Wang, M.Y.; Fielder, S.; McNeilage, M.A.; MacRae, E.A. Actinidia arguta: Volatile compounds in fruit and flowers. Phytochemistry 2003, 63, 285–301. [Google Scholar] [CrossRef]
- Dötterl, S.; Wolfe, L.M.; Jürgens, A. Qualitative and quantitative analyses of flower scent in Silene latifolia. Phytochemistry 2005, 66, 203–213. [Google Scholar] [CrossRef]
- Plepys, D.; Ibarra, F.; Löfstedt, C. Volatiles from flowers of Platanthera bifolia (Orchidaceae) attractive to the silver Y moth, Autographa gamma (Lepidoptera: Noctuidae). Oikos 2002, 99, 69–74. [Google Scholar] [CrossRef]
- Plepys, D.; Ibarra, F.; Francke, W.; Löfstedt, C. Odour-mediated nectar foraging in the silver Y moth, Autographa gamma (Lepidoptera: Noctuidae): Behavioural and electrophysiological responses to floral volatiles. Oikos 2002, 99, 75–82. [Google Scholar] [CrossRef]
- Dötterl, S.; Jürgens, A.; Seifert, K.; Laube, T.; Weißbecker, B.; Schütz, S. Nursery pollination by a moth in Silene latifolia: The role of odours in eliciting antennal and behavioural responses. New Phytol. 2006, 169, 707–718. [Google Scholar] [CrossRef]
- Dötterl, S.; Burkhardt, D.; Weißbecker, B.; Jürgens, A.; Schütz, S.; Mosandl, A. Linalool and lilac aldehyde/alcohol in flower scents. Electrophysiological detection of lilac aldehyde stereoisomers by a moth. J. Chromatogr. A 2006, 1113, 231–238. [Google Scholar] [CrossRef]
- Andersson, S. Antennal responses to floral scents in the butterflies Inachis io, Aglais urticae (Nymphalidae), and Gonepteryx rhamni (Pieridae). Chemoecology 2003, 13, 13–20. [Google Scholar] [CrossRef]
- Andersson, S.; Dobson, H.E.M. Antennal Responses to Floral Scents in the Butterfly Heliconius melpomene. J. Chem. Ecol. 2003, 29, 2319–2330. [Google Scholar] [CrossRef] [PubMed]
- Jhumur, U.S.; Dötterl, S.; Jürgens, A. Floral Odors of Silene otites: Their Variability and Attractiveness to Mosquitoes. J. Chem. Ecol. 2008, 34, 14–25. [Google Scholar] [CrossRef] [PubMed]
- Nyasembe, V.O.; Torto, B. Volatile phytochemicals as mosquito semiochemicals. Phytochem. Lett. 2014, 8, 196–201. [Google Scholar] [CrossRef] [Green Version]
- Lahondère, C.H.; Vinauger, C.; Okubo, R.P.; Wolff, G.H.; Chan, J.K.; Akbari, O.S.; Riffella, J.A. The olfactory basis of orchid pollination by mosquitoes. Proc. Natl. Acad. Sci. USA 2020, 117, 708–716. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castro-Vázquez, L.; Díaz-Maroto, M.C.; Pérez-Coello, M.S. Aroma composition and new chemical markers of Spanish citrus honeys. Food Chem. 2007, 103, 601–606. [Google Scholar] [CrossRef]
- Špánik, I.; Pažitná, A.; Šiška, P.; Szolcsányi, P. The determination of botanical origin of honeys based on enantiomer distribution of chiral volatile organic compounds. Food Chem. 2014, 158, 497–503. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Battesti, M.-J.; Costa, J.; Dupuy, N.; Paolini, J. Volatile components as chemical markers of the botanical origin of Corsican honeys. Flavour Fragr. J. 2018, 33, 52–62. [Google Scholar] [CrossRef] [Green Version]
- Machado, A.M.; Miguel, M.G.; Vilas-Boas, M.; Figueiredo, A.C. Honey Volatiles as a Fingerprint for Botanical Origin—A Review on their Occurrence on Monofloral Honeys. Molecules 2020, 25, 374. [Google Scholar] [CrossRef] [Green Version]
- ZZhang, J.; Liu, S.; Walton, W.C.; Wang, Y. Volatile organic compounds of Eastern oyster (Crassostrea virginica) cultured by two treatments and their changes during cold storage. Aquac. Res. 2021, 52, 1442–1452. [Google Scholar] [CrossRef]
- Naegeli, P.; Weber, G. The total synthesis of racemic davanone. Tetrahedron Lett. 1970, 12, 959–962. [Google Scholar] [CrossRef]
- Wakayama, S.; Namba, S.; Hosoi, K.; Ohno, M. The Synthesis and Absolute Configurations of Lilac Alcohols. Bull. Chem. Soc. Japan 1971, 44, 875. [Google Scholar] [CrossRef] [Green Version]
- Wakayama, S.; Namba, S.; Hosoi, K.; Ohno, M. The synthesis and the absolute configurations of lilac alcohols, new naturally occurringodorous ingredients of lilac flower. Bull. Chem. Soc. Jpn. 1973, 46, 3183–3187. [Google Scholar] [CrossRef] [Green Version]
- Weerdt, A.J.; Heide, R.; Jägers, P.; Dort, H.M. Narcissus trevithian and Narcissus geranium: Analysis and synthesis of compounds. J. Agric. Food. Chem. 1993, 41, 2063–2075. [Google Scholar]
- Katsuta, Y.; Fujita, A.; Takagi, K. Preparation of Lilac Aldehyde from 4-methyl-4-acetoxy-5-hexenal or Lilac Alcohol. Patent JP0533 9252 1993. [Google Scholar]
- Ito, N.; Azuma, M.; Wada, S.; Imano, H.; Hasebe, A. Preparation of Lilac Aldehyde. Patent JP200033 6083 2000. [Google Scholar]
- Sabitha, G.; Prasad, N.M.; Bhikshapathi, M.; Yadav, J.S. Stereospecific Total Synthesis of (+)-Davana Acid, (+)-Nordavanone and (+)-Davanone. Synthesis 2010, 5, 807–810. [Google Scholar] [CrossRef]
- Schneider, M.-A.; Dötterl, S.; Seifert, K. Diastereoselective Synthesis of a Lilac Aldehyde Isomer and Its Electrophysiological Detection by a Moth. Chem. Biodivers. 2013, 10, 1252–1259. [Google Scholar] [CrossRef]
- Nacsa, E.D.; Fielder, B.C.; Wetzler, S.P.; Srisuknimit, V.; Litz, J.P.; van Vleet, M.J.; Quach, K.; Vosburg, D.A. Direct, Biomimetic Synthesis of (+)-Artemone via a Stereoselective, Organocatalytic Cyclization. Synthesis 2015, 47, 2599–2602. [Google Scholar]
- Šiška, P.; Fodran, P.; Szolcsányi, P. Synthesis and olfactory properties of unnatural derivatives of lilac aldehydes. Tetrahedron 2014, 70, 6420–6427. [Google Scholar] [CrossRef]
- Knowles, J.P.; Whiting, A. The Effects of Ring Size and Substituents on the Rates of Acid-Catalysed Hydrolysis of Five- and Six-Membered Ring Cyclic Ketone Acetals. Eur. J. Org. Chem. 2007, 2007, 3365–3368. [Google Scholar] [CrossRef]
- Newitt, L.A.; Steel, P.G. Evidence for oxacarbenium ion intermediates in the Lewis acid promoted cleavage of spirocyclic dioxanes. J. Chem. Soc. Perkin Trans. 1997, 1, 2033–2036. [Google Scholar] [CrossRef]
- Kumar, V.S.; Floreancig, P.E. Electron Transfer Initiated Cyclizations: Cyclic Acetal Synthesis through Carbon−Carbon σ-Bond Activation. J. Am. Chem. Soc. 2001, 123, 3842–3843. [Google Scholar] [CrossRef] [PubMed]
- Bluhm, N.; Kalesse, M. Synthesis of a C20-Deoxygenated Spirangien Derivative for Target Identification. Synlett 2015, 26, 797–801. [Google Scholar]
- Lautens, M.; Colucci, J.T.; Hiebert, S.; Smith, N.D.; Bouchain, G. Total Synthesis of Ionomycin Using Ring-Opening Strategies. Org. Lett. 2002, 4, 1879–1882. [Google Scholar] [CrossRef] [PubMed]
- Mursakulov, I.G.; Guseinov, M.M.; Kasumov, N.K. Stereochemical Studies—XXVI. Conformational Equilibria of Ketals of 2-Substituted Cyclohexanones. Tetrahedron 1982, 38, 2213–2220. [Google Scholar] [CrossRef]

| Alcohol | Aroma | Aldehyde | Aroma |
|---|---|---|---|
 | Sweet, herbal, minty, eucalypty | 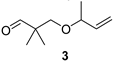 | Spicy, terpenic, pungent |
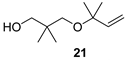 | Balsamic, pleasant, minty, eucalypty | 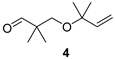 | Spicy, camphor, sharp, with green notes |
 | Oily, sweetish, turpentine-like | 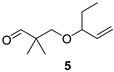 | Herbal, green, sweetish, ethereal, sharp |
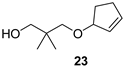 | Fruity-sweetish, fatty, turpentine | 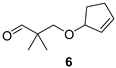 | Sweet, fruity, with eucalyptus notes |
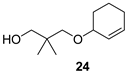 | Bitter-sweet, turpentine-like |  | Herbal, slightly green, with earthy notes |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dacho, V.; Szolcsányi, P. Synthesis and Olfactory Properties of Seco-Analogues of Lilac Aldehydes. Molecules 2021, 26, 7086. https://doi.org/10.3390/molecules26237086
Dacho V, Szolcsányi P. Synthesis and Olfactory Properties of Seco-Analogues of Lilac Aldehydes. Molecules. 2021; 26(23):7086. https://doi.org/10.3390/molecules26237086
Chicago/Turabian StyleDacho, Vladimír, and Peter Szolcsányi. 2021. "Synthesis and Olfactory Properties of Seco-Analogues of Lilac Aldehydes" Molecules 26, no. 23: 7086. https://doi.org/10.3390/molecules26237086






