Main Factors Involved in Thyroid Hormone Action
Abstract
:1. Introduction
2. TH Transporters
3. Thyroid Hormone Receptors and Nuclear Functions
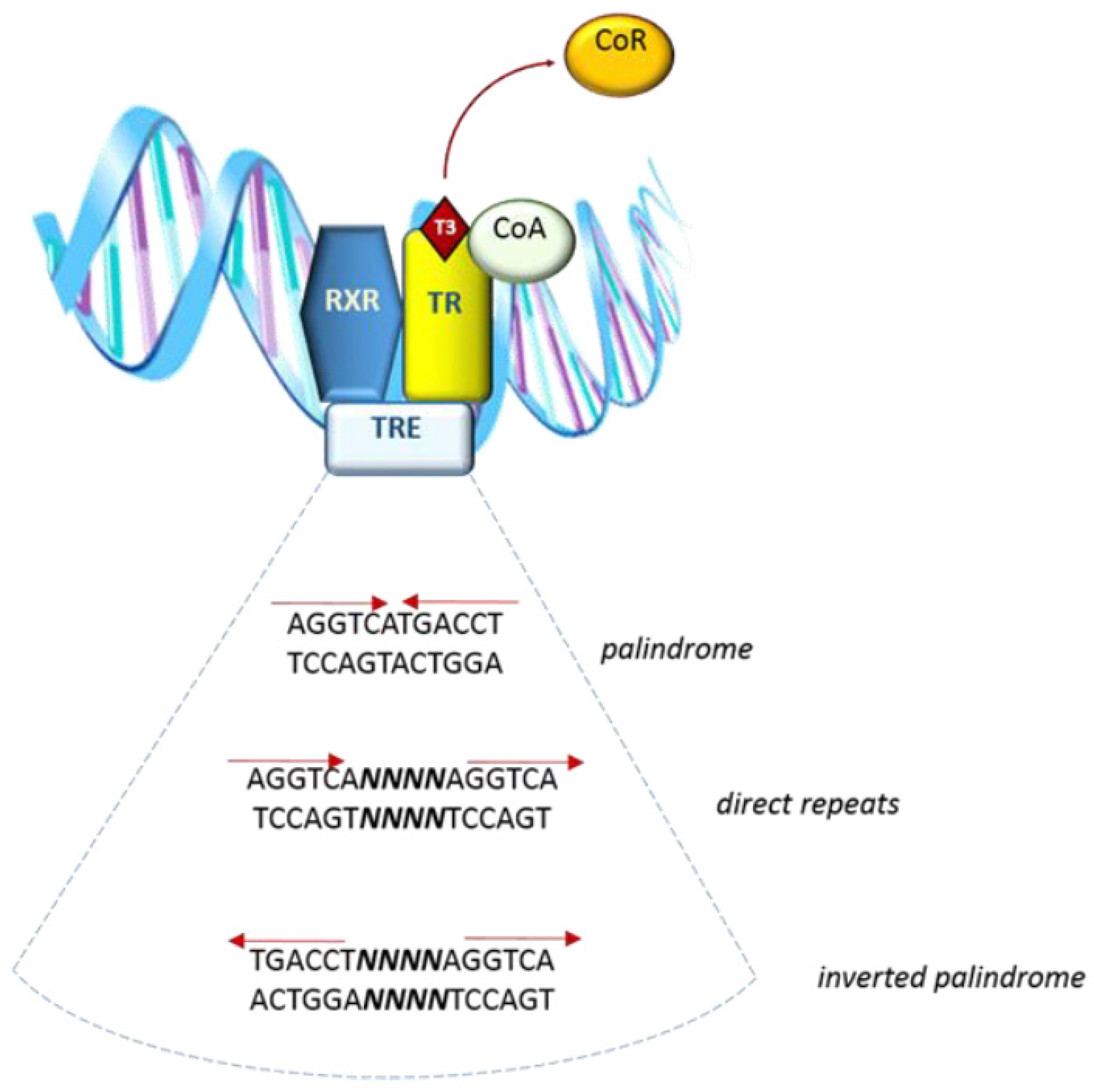
3.1. General Characteristics of Thyroid Hormone Receptors
| Cellular Localization | Ligand | Factor | DNA Interaction | |
|---|---|---|---|---|
| Nuclear receptors | Nucleus | T3 | TRa, TRb | TREs |
| Extra-nuclear receptors | Cytoplasm/Mitochondria | T3 | TRa1 (p46) | mit-TREs |
| p43/p33/p30/p38 | ||||
| Integrin | Cell membrane | T4/T3 | Integrin avb3 | Absent |
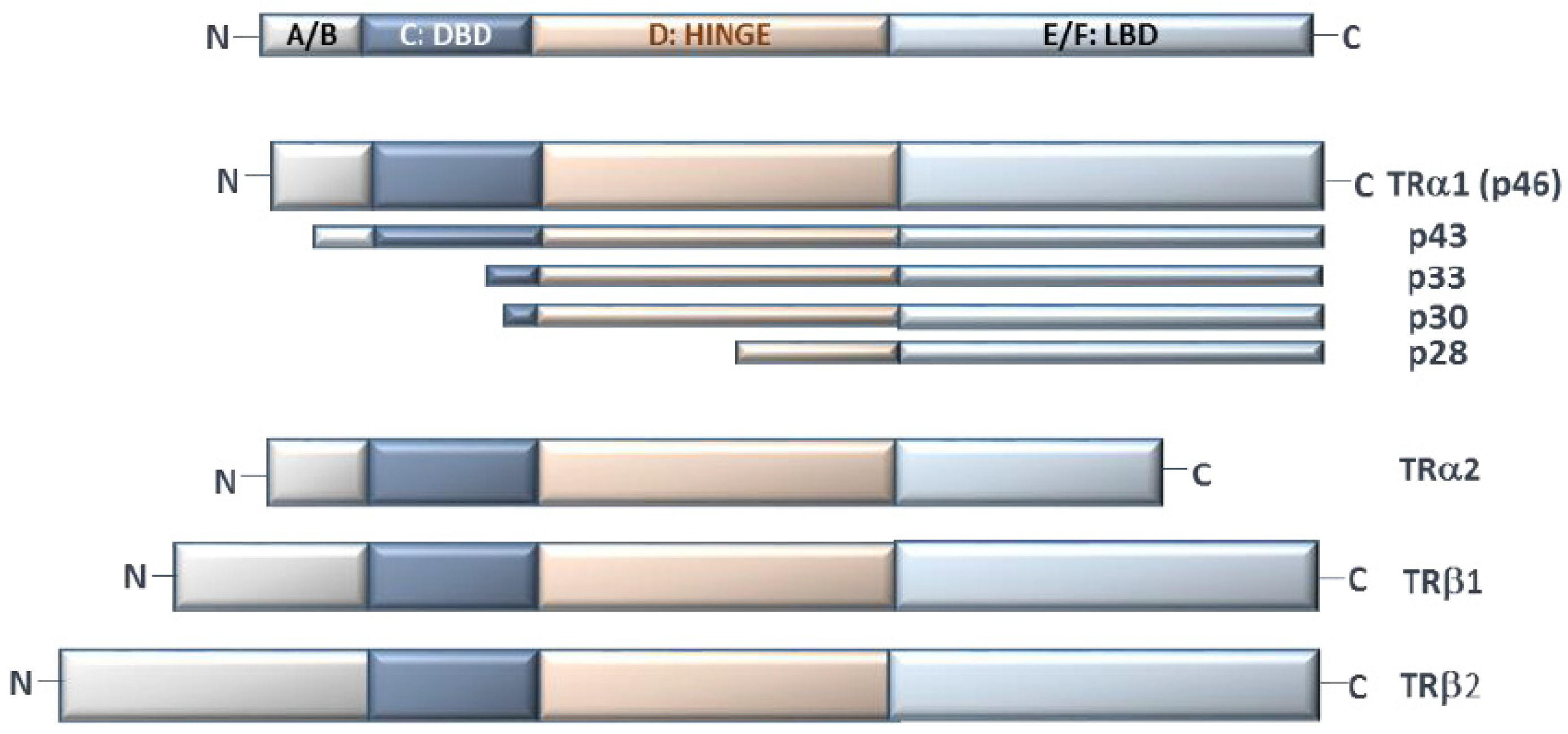
3.2. TRs’ Multidomain Structure
3.3. TR Tissue Distribution
3.4. TR Nucleus–Cytoplasm Shuttling
3.5. TR Extranuclear Functions
4. Integrin αvβ3 Mediation of TH Action
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Brent, G.A. Mechanisms of thyroid hormone action. J. Clin. Investig. 2012, 122, 3035–3043. [Google Scholar] [CrossRef] [Green Version]
- Cheng, S.-Y.; Leonard, J.L.; Davis, P.J. Molecular Aspects of Thyroid Hormone Actions. Endocr. Rev. 2010, 31, 139–170. [Google Scholar] [CrossRef] [Green Version]
- Oetting, A.; Yen, P. New insights into thyroid hormone action. Best Pr. Res. Clin. Endocrinol. Metab. 2007, 21, 193–208. [Google Scholar] [CrossRef]
- Bernal, J. Thyroid hormone receptors in brain development and function. Nat. Clin. Pr. Endocrinol. Metab. 2007, 3, 249–259. [Google Scholar] [CrossRef] [PubMed]
- Taylor, E.; Heyland, A. Evolution of thyroid hormone signaling in animals: Non-genomic and genomic modes of action. Mol. Cell. Endocrinol. 2017, 459, 14–20. [Google Scholar] [CrossRef]
- Flamant, F.; Cheng, S.-Y.; Hollenberg, A.N.; Moeller, L.; Samarut, J.; Wondisford, F.E.; Yen, P.; Refetoff, S. Thyroid Hormone Signaling Pathways: Time for a More Precise Nomenclature. Endocrinology 2017, 158, 2052–2057. [Google Scholar] [CrossRef]
- Bernal, J.; Guadaño-Ferraz, A.; Morte, B. Thyroid hormone transporters—functions and clinical implications. Nat. Rev. Endocrinol. 2015, 11, 406–417, Erratum in Nat. Rev. Endocrinol. 2015, 9, 506; Erratum in Nat. Rev. Endocrinol. 2015, 12, 690. [Google Scholar] [CrossRef] [Green Version]
- Kinne, A.; Schülein, R.; Krause, G. Primary and secondary thyroid hormone transporters. Thyroid. Res. 2011, 4 (Suppl. 1), S7. [Google Scholar] [CrossRef] [Green Version]
- Friesema, E.C.; Grueters, A.; Biebermann, H.; Krude, H.; von Moers, A.; Reeser, M.; Barrett, T.; Mancilla, E.E.; Svensson, J.; Kester, M.H.; et al. Association between mutations in a thyroid hormone transporter and severe X-linked psychomotor retardation. Lancet 2004, 364, 1435–1437. [Google Scholar] [CrossRef]
- Wirth, E.K.; Roth, S.; Blechschmidt, C.; Hölter, S.M.; Becker, L.; Racz, I.; Zimmer, A.; Klopstock, T.; Gailus-Durner, V.; Fuchs, H.; et al. Neuronal 3’,3,5-Triiodothyronine (T3) Uptake and Behavioral Phenotype of Mice Deficient in Mct8, the Neuronal T3 Transporter Mutated in Allan-Herndon-Dudley Syndrome. J. Neurosci. 2009, 29, 9439–9449. [Google Scholar] [CrossRef]
- Ortiga-Carvalho, T.M.; Sidhaye, A.R.; Wondisford, F.E. Thyroid hormone receptors and resistance to thyroid hormone disorders. Nat. Rev. Endocrinol. 2014, 10, 582–591. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alkemade, A.; Vuijst, C.L.; Unmehopa, U.A.; Bakker, O.; Vennström, B.; Wiersinga, W.M.; Swaab, D.F.; Fliers, E. Thyroid hormone receptor expression in the human hypothalamus and anterior pituitary. J. Clin. Endocrinol. Metab. 2005, 2, 904–912. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.-Y.; Brent, G.A. Thyroid hormone crosstalk with nuclear receptor signaling in metabolic regulation. Trends Endocrinol. Metab. 2010, 21, 166–173. [Google Scholar] [CrossRef] [Green Version]
- Schroeder, A.C.; Privalsky, M.L. Thyroid Hormones, T3 and T4, in the Brain. Front. Endocrinol. 2014, 5, 40. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.-K.; Hoffmann, B.; Tran, P.B.-V.; Graupner, G.; Pfahl, M. Retinoid X receptor is an auxiliary protein for thyroid hormone and retinoic acid receptors. Nature 1992, 355, 441–446. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, K.; Cohen, R.N.; Yamada, M.; Markan, K.; Monden, T.; Satoh, T.; Mori, M.; Wondisford, F.E. Cross-talk between Thyroid Hormone Receptor and Liver X Receptor Regulatory Pathways Is Revealed in a Thyroid Hormone Resistance Mouse Model. J. Biol. Chem. 2006, 281, 295–302. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.Y.; Heymann, R.S.; Moatamed, F.; Schultz, J.J.; Sobel, D.; Brent, G.A. A mutant thyroid hormone receptor alpha antagonizes peroxisome proliferator-activated receptor alpha signaling in vivo and impairs fatty acid oxidation. Endocrinology 2007, 148, 1206–1217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aagaard, M.M.; Siersbæk, R.D.; Mandrup, S. Molecular basis for gene-specific transactivation by nuclear receptors. Biochim. Biophys. Acta 2011, 1812, 824–835. [Google Scholar] [CrossRef] [Green Version]
- McKenna, N.J.; Xu, J.; Nawaz, Z.; Tsai, S.Y.; Tsai, M.-J.; O’Malley, B.W. Nuclear receptor coactivators: Multiple enzymes, multiple complexes, multiple functionsProceedings of Xth International Congress on Hormonal Steroids, Quebec, Canada, 17–21 June 1998. J. Steroid Biochem. Mol. Biol. 1999, 69, 3–12. [Google Scholar] [CrossRef]
- Huang, P.; Chandra, V.; Rastinejad, F. Structural Overview of the Nuclear Receptor Superfamily: Insights into Physiology and Therapeutics. Annu. Rev. Physiol. 2010, 72, 247–272. [Google Scholar] [CrossRef] [Green Version]
- Nascimento, A.S.; Dias, S.M.G.; Nunes, F.M.; Aparício, R.; Ambrosio, A.L.; Bleicher, L.; Figueira, A.C.M.; Santos, M.A.M.; Neto, M.D.O.; Fischer, H.; et al. Structural Rearrangements in the Thyroid Hormone Receptor Hinge Domain and Their Putative Role in the Receptor Function. J. Mol. Biol. 2006, 360, 586–598. [Google Scholar] [CrossRef] [PubMed]
- Lazar, M.A. Thyroid hormone receptors: Multiple forms, multiple possibilities. Endocr. Rev. 1993, 2, 184–193. [Google Scholar]
- Yen, P.M. Physiological and Molecular Basis of Thyroid Hormone Action. Physiol. Rev. 2001, 81, 1097–1142. [Google Scholar] [CrossRef] [Green Version]
- Anyetei-Anum, C.S.; Roggero, V.R.; Allison, L.A. Thyroid hormone receptor localization in target tissues. J. Endocrinol. 2018, 237, R19–R34. [Google Scholar] [CrossRef]
- Plateroti, M.; Gauthier, K.; Domon-Dell, C.; Freund, J.N.; Samarut, J.; Chassande, O. Functional interference between thyroid hormone receptor alpha (TRalpha) and natural truncated TRDeltaalpha isoforms in the control of intestine development. Mol. Cell Biol. 2001, 21, 4761–4772. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harvey, C.B.; Bassett, J.H.; Maruvada, P.; Yen, P.M.; Williams, G.R. The rat thyroid hormone receptor (TR) Deltabeta3 displays cell-, TR isoform-, and thyroid hormone response element-specific actions. Endocrinology 2007, 148, 1764–1773. [Google Scholar] [CrossRef] [Green Version]
- Amma, L.L.; Campos-Barros, A.; Wang, Z.; Vennström, B.; Forrest, D. Distinct Tissue-Specific Roles for Thyroid Hormone Receptors β and α1 in Regulation of Type 1 Deiodinase Expression. Mol. Endocrinol. 2001, 15, 467–475. [Google Scholar] [CrossRef] [Green Version]
- Zandieh-Doulabi, B.; Dop, E.; Schneiders, M.; Schiphorst, M.P.; Mansen, A.; Vennström, B.; Dijkstra, C.D.; Bakker, O.; Wiersinga, W.M. Zonal expression of the thyroid hormone receptor alpha isoforms in rodent liver. J. Endocrinol. 2003, 179, 379–385. [Google Scholar] [CrossRef] [Green Version]
- Zucchi, R. Thyroid Hormone Analogues: An Update. Thyroid 2020, 30, 1099–1105. [Google Scholar] [CrossRef] [Green Version]
- Kelly, M.J.; Pietranico-Cole, S.; Larigan, J.D.; Haynes, N.-E.; Reynolds, C.H.; Scott, N.; Vermeulen, J.; Dvorozniak, M.; Conde-Knape, K.; Huang, K.-S.; et al. Discovery of 2-[3,5-Dichloro-4-(5-isopropyl-6-oxo-1,6-dihydropyridazin-3-yloxy)phenyl]-3,5-dioxo-2,3,4,5-tetrahydro[1,2,4]triazine-6-carbonitrile (MGL-3196), a Highly Selective Thyroid Hormone Receptor β Agonist in Clinical Trials for the Treatment of Dyslipidemia. J. Med. Chem. 2014, 57, 3912–3923. [Google Scholar] [CrossRef] [PubMed]
- Forrest, D.; Vennström, B. Functions of thyroid hormone receptors in mice. Thyroid 2000, 10, 41–52. [Google Scholar] [CrossRef]
- Ma, J.; Goryaynov, A.; Yang, W. Super-resolution 3D tomography of interactions and competition in the nuclear pore complex. Nat. Struct. Mol. Biol. 2016, 23, 239–247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roggero, V.R.; Zhang, J.; Parente, L.E.; Doshi, Y.; Dziedzic, R.C.; McGregor, E.L.; Varjabedian, A.D.; Schad, S.E.; Bondzi, C.; Allison, L.A. Nuclear import of the thyroid hormone receptor α1 is mediated by importin 7, importin β1, and adaptor importin α1. Mol. Cell. Endocrinol. 2016, 419, 185–197. [Google Scholar] [CrossRef] [Green Version]
- Subramanian, K.S.; Dziedzic, R.C.; Nelson, H.N.; Stern, M.E.; Roggero, V.R.; Bondzi, C.; Allison, L.A. Multiple exportins influence thyroid hormone receptor localization. Mol. Cell. Endocrinol. 2015, 411, 86–96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodríguez, J.A. Interplay between nuclear transport and ubiquitin/SUMO modifications in the regulation of cancer-related proteins. Semin. Cancer Biol. 2014, 27, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Pacheco, A.; Martínez-Iglesias, O.; Méndez-Pertuz, M.; Aranda, A. Residues K128, 132, and 134 in the Thyroid Hormone Receptor-α Are Essential for Receptor Acetylation and Activity. Endocrinology 2009, 150, 5143–5152. [Google Scholar] [CrossRef]
- Wrutniak-Cabello, C.; Casas, F.; Cabello, G. Thyroid hormone action in mitochondria. J. Mol. Endocrinol. 2001, 26, 67–77. [Google Scholar] [CrossRef] [Green Version]
- Carazo, A.; Levin, J.; Casas, F.; Seyer, P.; Grandemange, S.; Busson, M.; Pessemesse, L.; Wrutniak-Cabello, C.; Cabello, G. Protein sequences involved in the mitochondrial import of the 3,5,3′-L-triiodothyronine receptor p43. J. Cell. Physiol. 2012, 227, 3768–3777. [Google Scholar] [CrossRef]
- Casas, F.; Rochard, P.; Rodier, A.; Cassar-Malek, I.; Marchal-Victorion, S.; Wiesner, R.J.; Cabello, G.; Wrutniak, C. A variant form of the nuclear triiodothyronine receptor c-ErbAalpha1 plays a direct role in regulation of mitochondrial RNA synthesis. Mol. Cell Biol. 1999, 19, 7913–7924. [Google Scholar] [CrossRef] [Green Version]
- Davis, P.J.; Mousa, S.A.; Cody, V.; Tang, H.-Y.; Lin, H.-Y. Small Molecule Hormone or Hormone-Like Ligands of Integrin αVβ3: Implications for Cancer Cell Behavior. Horm. Cancer 2013, 4, 335–342. [Google Scholar] [CrossRef]
- Bergh, J.J.; Lin, H.Y.; Lansing, L.; Mohamed, S.N.; Davis, F.B.; Mousa, S.; Davis, P.J. Integrin alphaVbeta3 contains a cell surface receptor site for thyroid hormone that is linked to activation of mitogen-activated protein kinase and induction of angiogenesis. Endocrinology 2005, 146, 2864–2871. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.J.; Lin, H.Y.; Luidens, M.K.; Davis, F.B.; Davis, P.J. Cytoplasm-to-nucleus shuttling of thyroid hormone receptor-beta1 (Trbeta1) is directed from a plasma membrane integrin receptor by thyroid hormone. Endocr. Res. 2009, 34, 31–42, Erratum in 2009, 34, 155–157. [Google Scholar] [PubMed]
- Lin, H.-Y.; Sun, M.; Tang, H.-Y.; Lin, C.; Luidens, M.K.; Mousa, S.A.; Incerpi, S.; Drusano, G.L.; Davis, F.B.; Davis, P.J. l-Thyroxine vs. 3,5,3′-triiodo-l-thyronine and cell proliferation: Activation of mitogen-activated protein kinase and phosphatidylinositol 3-kinase. Am. J. Physiol. Physiol. 2009, 296, C980–C991. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chi, H.-C.; Tsai, C.-Y.; Tsai, M.-M.; Yeh, C.-T.; Lin, K.-H. Molecular functions and clinical impact of thyroid hormone-triggered autophagy in liver-related diseases. J. Biomed. Sci. 2019, 26, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Incerpi, S.; Luly, P.; De Vito, P.; Farías, R.N. Short-Term Effects of Thyroid Hormones on the Na/H Antiport in L-6 Myoblasts: High Molecular Specificity for 3,3′,5-Triiodo- l -Thyronine 1. Endocrinology 1999, 140, 683–689. [Google Scholar] [CrossRef]
- Barczyk, M.; Carracedo, S.; Gullberg, D. Integrins. Cell Tissue Res. 2010, 339, 269–280. [Google Scholar] [CrossRef] [Green Version]
- Xiong, J.P.; Stehle, T.; Zhang, R.; Joachimiak, A.; Frech, M.; Goodman, S.L.; Arnaout, M.A. Crystal structure of the extracellular segment of integrin alpha Vbeta3 in complex with an Arg-Gly-Asp ligand. Science 2002, 296, 151–155. [Google Scholar] [CrossRef] [PubMed]
- Cody, V.; Davis, P.J.; Davis, F.B. Molecular modeling of the thyroid hormone interactions with alpha v beta 3 integrin. Steroids 2007, 72, 165–170. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, F.; Chen, X. Integrin alpha(v)beta(3)-Targeted Cancer Therapy. Drug Dev. Res. 2008, 69, 329–339. [Google Scholar] [CrossRef] [Green Version]
- Vonlaufen, A.; Wiedle, G.; Borisch, B.; Birrer, S.; Luder, P.; Imhof, B.A. Integrin alpha(v)beta(3) expression in colon carcinoma correlates with survival. Mod. Pathol. 2001, 14, 1126–1132. [Google Scholar] [CrossRef]
- Shi, K.; Wang, S.-L.; Shen, B.; Yu, F.-Q.; Weng, D.-F.; Lin, J.-H. Clinicopathological and prognostic values of fibronectin and integrin αvβ3 expression in primary osteosarcoma. World J. Surg. Oncol. 2019, 17, 1–12. [Google Scholar] [CrossRef]
- McCabe, N.P.; De, S.; Vasanji, A.; Brainard, J.; Byzova, T.V. Prostate cancer specific integrin alphavbeta3 modulates bone metastatic growth and tissue remodeling. Oncogene 2007, 26, 6238–6343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aksorn, N.; Chanvorachote, P. Integrin as a Molecular Target for Anti-cancer Approaches in Lung Cancer. Anticancer Res. 2019, 39, 541–548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davis, F.B.; Tang, H.-Y.; Shih, A.; Keating, T.; Lansing, L.; Hercbergs, A.; Fenstermaker, R.A.; Mousa, A.; Mousa, S.A.; Davis, P.J.; et al. Acting via a Cell Surface Receptor, Thyroid Hormone Is a Growth Factor for Glioma Cells. Cancer Res. 2006, 66, 7270–7275. [Google Scholar] [CrossRef] [Green Version]
- Shinderman-Maman, E.; Cohen, K.J.; Weingarten, C.; Nabriski, D.; Twito, O.; Baraf, L.; Hercbergs, A.; Davis, P.J.; Werner, H.; Ellis, M.J.; et al. The thyroid hormone-αvβ3 integrin axis in ovarian cancer: Regulation of gene transcription and MAPK-dependent proliferation. Oncogene 2016, 35, 1977–1987. [Google Scholar] [CrossRef]
- Cohen, K.; Ellis, M.; Khoury, S.; Davis, P.J.; Hercbergs, A.; Ashur-Fabian, O. Thyroid Hormone Is a MAPK-Dependent Growth Factor for Human Myeloma Cells Acting via αvβ3 Integrin. Mol. Cancer Res. 2011, 9, 1385–1394. [Google Scholar] [CrossRef] [Green Version]
- Hercbergs, A.; Davis, P.J.; Lin, H.-Y.; Keating, K.A.; Mousa, S.A. Thyroid Hormone Replacement Therapy in Patients with Various Types of Cancer. Intechopen.com. 2019. Available online: https://www.intechopen.com/chapters/67201 (accessed on 15 September 2021).
- Lin, H.-Y.; Landersdorfer, C.B.; London, D.; Meng, R.; Lim, C.-U.; Lin, C.; Lin, S.; Tang, H.-Y.; Brown, D.; Van Scoy, B.; et al. Pharmacodynamic Modeling of Anti-Cancer Activity of Tetraiodothyroacetic Acid in a Perfused Cell Culture System. PLoS Comput. Biol. 2011, 7, e1001073. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, H.-Y.; Chin, Y.-T.; Nana, A.W.; Shih, Y.-J.; Lai, H.-Y.; Tang, H.-Y.; Leinung, M.; Mousa, S.; Davis, P.J. Actions of l-thyroxine and Nano-diamino-tetrac (Nanotetrac) on PD-L1 in cancer cells. Steroids 2016, 114, 59–67. [Google Scholar] [CrossRef] [Green Version]
- Sudha, T.; Bharali, D.J.; Sell, S.; Darwish, N.H.E.; Davis, P.J.; Mousa, S.A. Nanoparticulate Tetrac Inhibits Growth and Vascularity of Glioblastoma Xenografts. Horm. Cancer 2017, 8, 157–165. [Google Scholar] [CrossRef] [Green Version]
- Mousa, S.A.; Yalcin, M.; Bharali, D.J.; Meng, R.; Tang, H.-Y.; Lin, H.-Y.; Davis, F.B.; Davis, P.J. Tetraiodothyroacetic acid and its nanoformulation inhibit thyroid hormone stimulation of non-small cell lung cancer cells in vitro and its growth in xenografts. Lung Cancer 2012, 76, 39–45. [Google Scholar] [CrossRef]
- Glinskii, A.B.; Glinsky, G.V.; Lin, H.-Y.; Tang, H.-Y.; Sun, M.; Davis, F.B.; Luidens, M.K.; Mousa, S.; Hercbergs, A.H.; Davis, P.J. Modification of survival pathway gene expression in human breast cancer cells by tetraiodothyroacetic acid (tetrac). Cell Cycle 2009, 8, 3562–3570. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hercbergs, A.; Johnson, R.E.; Ashur-Fabian, O.; Garfield, D.H.; Davis, P.J. Medically Induced Euthyroid Hypothyroxinemia May Extend Survival in Compassionate Need Cancer Patients: An Observational Study. Oncologist 2015, 20, 72–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tedeschi, L.; Vassalle, C.; Iervasi, G.; Sabatino, L. Main Factors Involved in Thyroid Hormone Action. Molecules 2021, 26, 7337. https://doi.org/10.3390/molecules26237337
Tedeschi L, Vassalle C, Iervasi G, Sabatino L. Main Factors Involved in Thyroid Hormone Action. Molecules. 2021; 26(23):7337. https://doi.org/10.3390/molecules26237337
Chicago/Turabian StyleTedeschi, Lorena, Cristina Vassalle, Giorgio Iervasi, and Laura Sabatino. 2021. "Main Factors Involved in Thyroid Hormone Action" Molecules 26, no. 23: 7337. https://doi.org/10.3390/molecules26237337







