Fabrication and Characterization of Lignin/Dendrimer Electrospun Blended Fiber Mats
Abstract
:1. Introduction
2. Results and Discussion
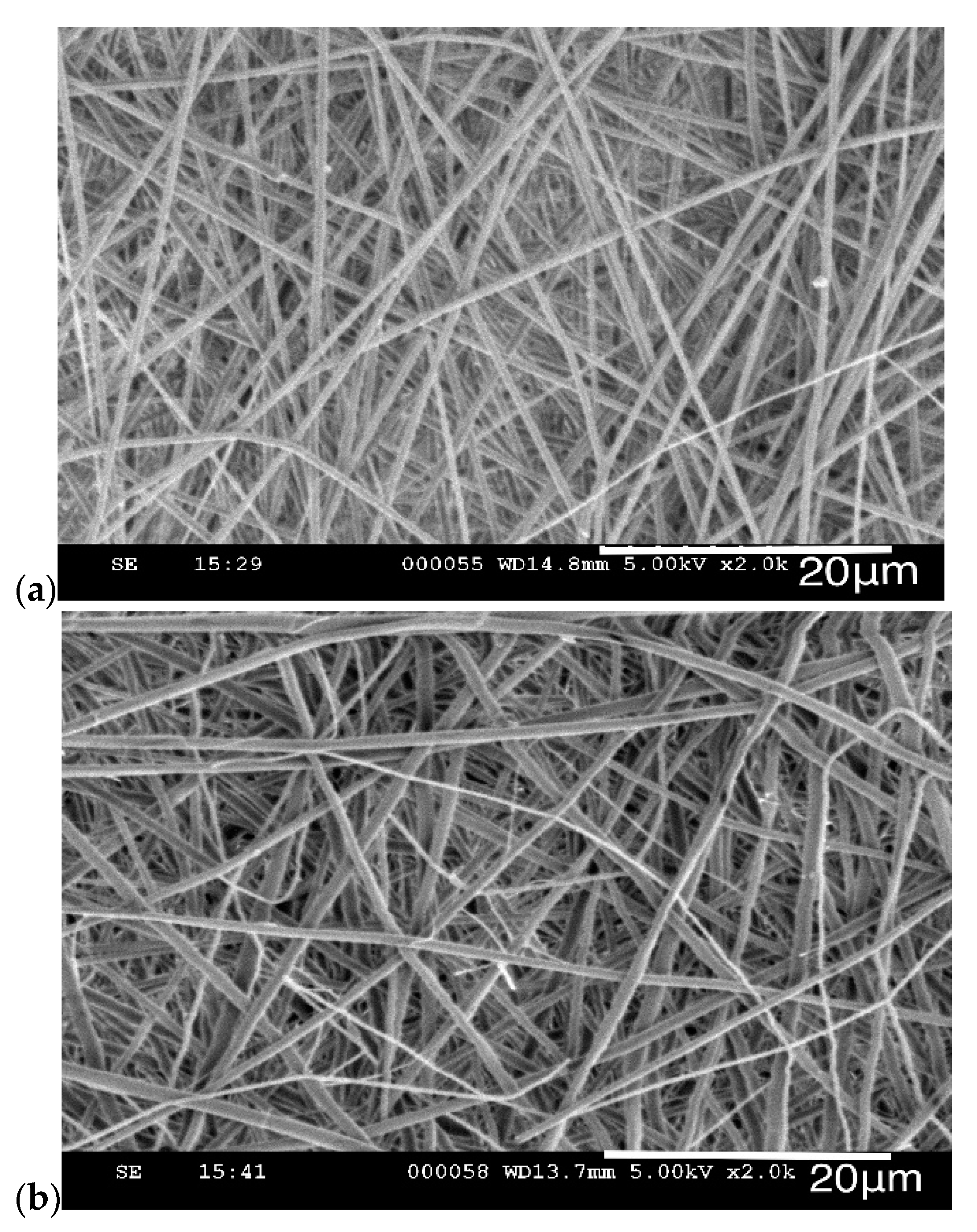
2.1. Morphology Analysis
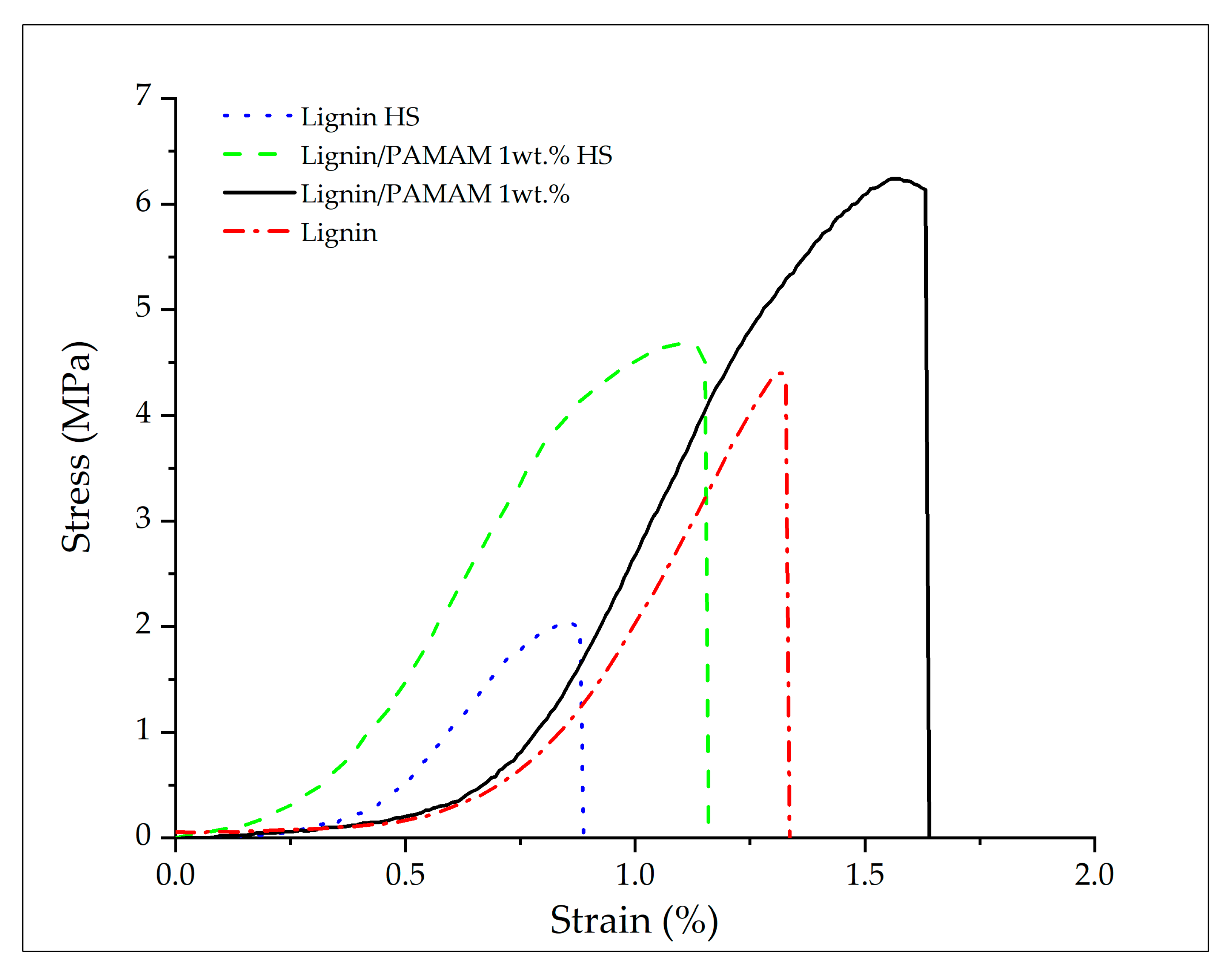
2.2. Mechanical Properties
2.3. Surface Charge
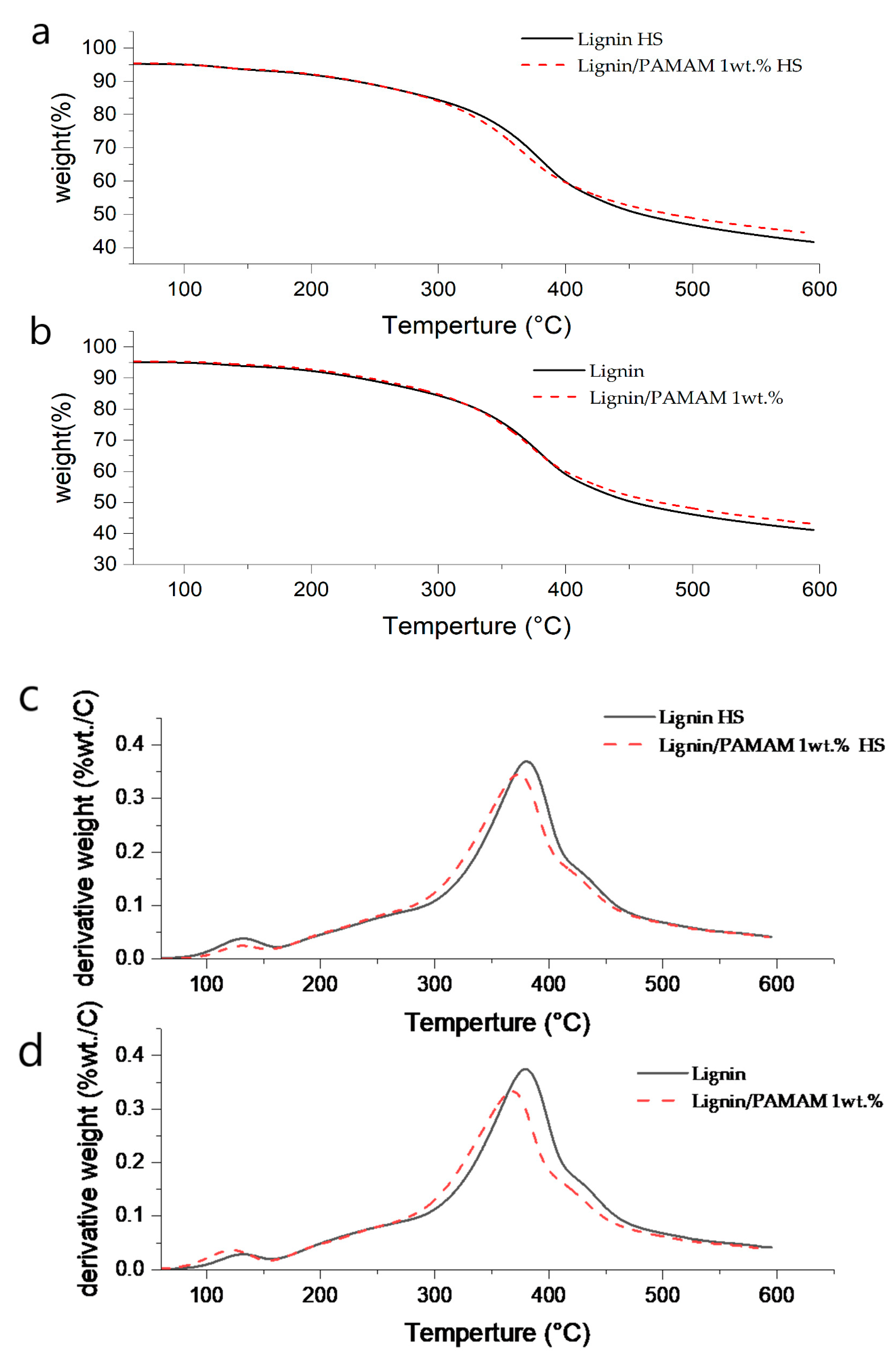
2.4. Thermogravimetric Analysis (TGA)
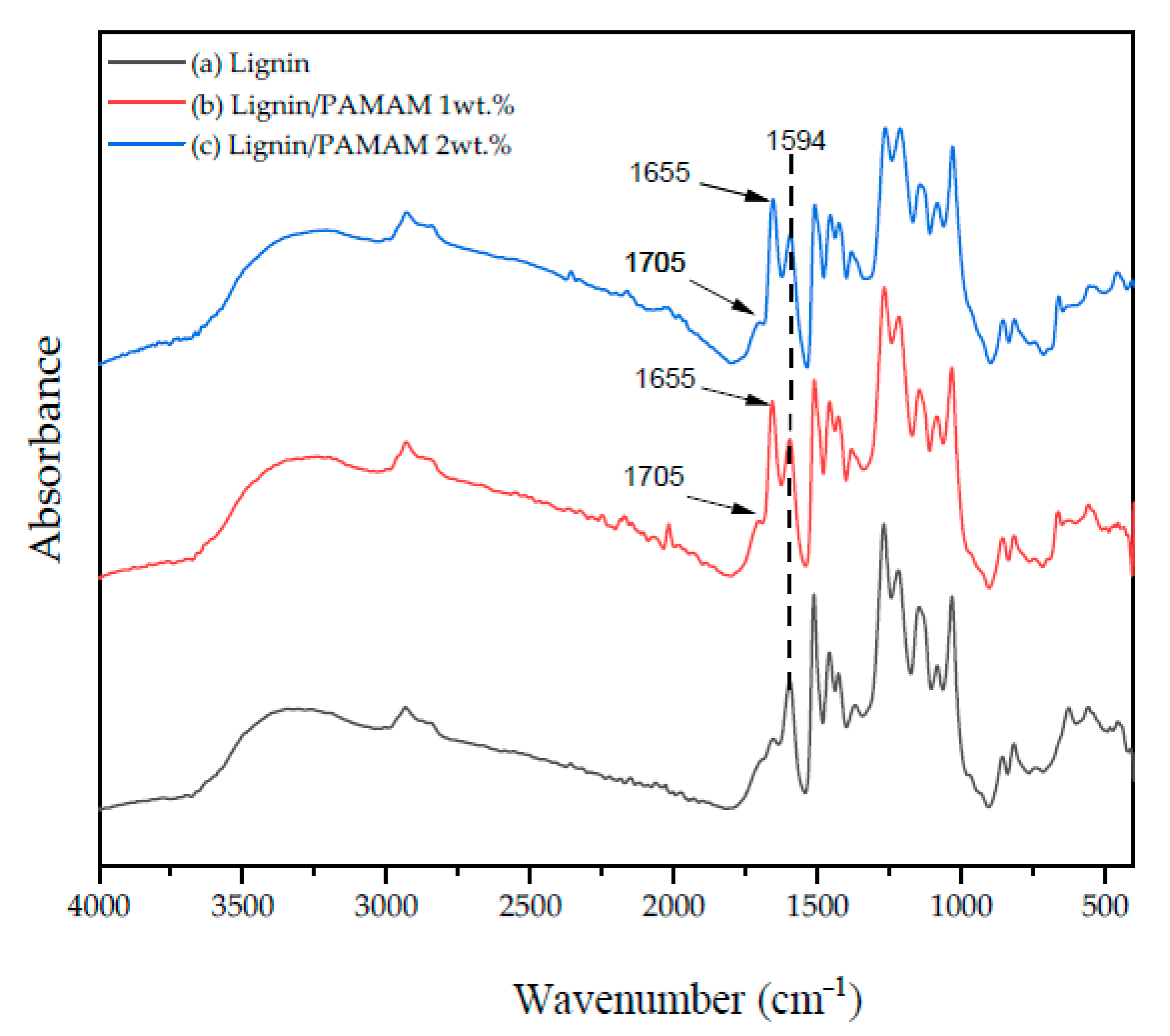
2.5. Fourier Transform Infrared Spectroscopy (FTIR)
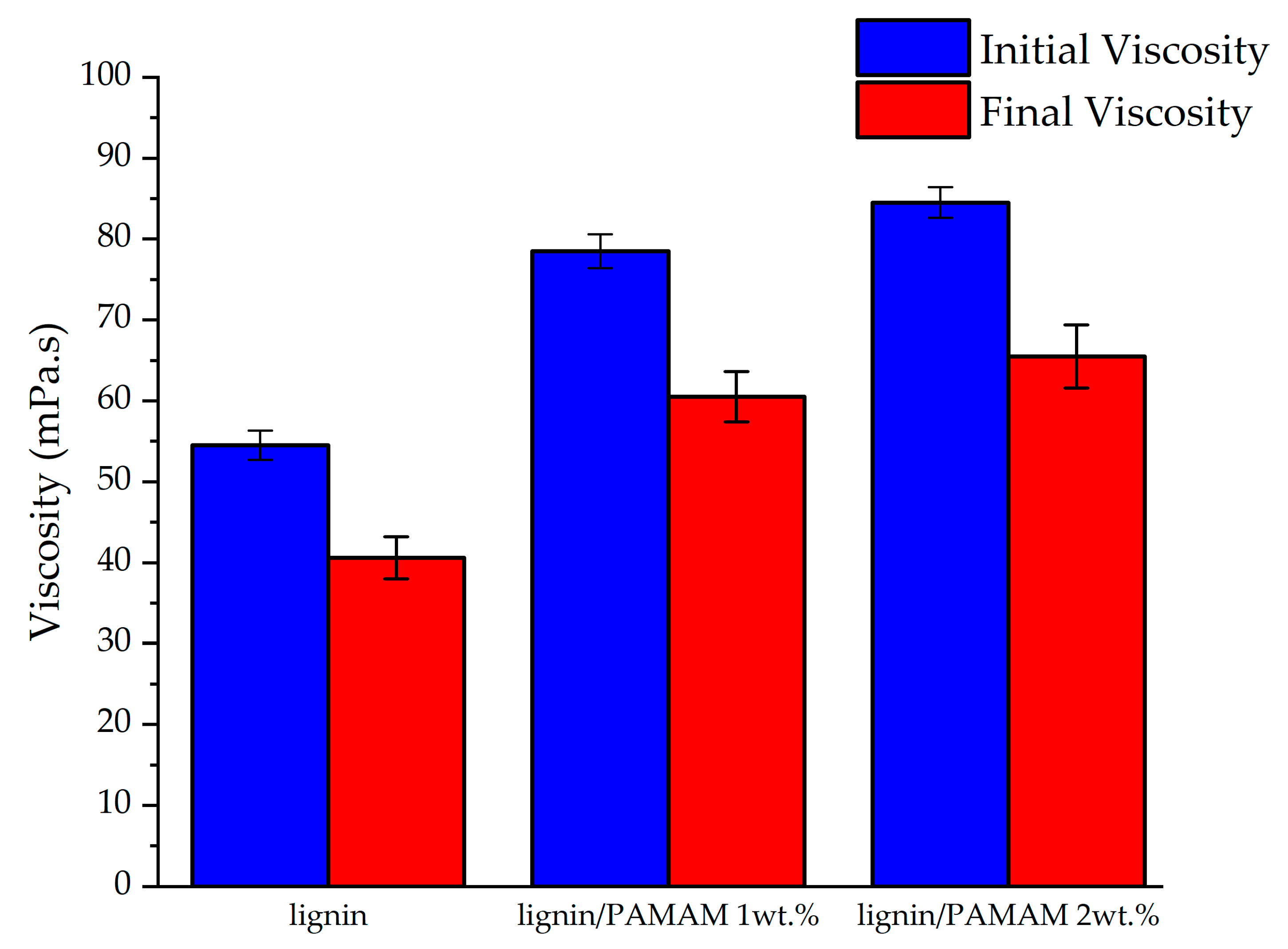
2.6. Viscosity
2.7. Reaction Mechanism between Lignin and Amine-Terminated Dendrimer
3. Materials and Methods
3.1. Materials
3.2. Preparation and Properties of Electrospinning Solutions
Heat-Setting and Stabilization Procedures
3.3. Characterization of Composite Membranes
3.3.1. Morphology Measurement
3.3.2. Mechanical Properties
3.3.3. Surface Charge
3.3.4. Thermogravimetric Analysis (TGA)
3.3.5. Fourier Transform Infrared Spectroscopy (FTIR)
3.3.6. Viscosity Measurement
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Faruk, O.; Sain, M. Lignin in Polymer Composites; William Andrew: Norwich, NY, USA, 2015. [Google Scholar]
- Pouteau, C.; Baumberger, S.; Cathala, B.; Dole, P. Lignin–polymer blends: Evaluation of compatibility by image analysis. Comptes Rendus Biol. 2004, 327, 935–943. [Google Scholar] [CrossRef] [PubMed]
- Boerjan, W.; Ralph, J.; Baucher, M. Lignin biosynthesis. Annu. Rev. Plant Biol. 2003, 54, 519–546. [Google Scholar] [CrossRef] [PubMed]
- Katahira, R.; Elder, T.J.; Beckham, G.T. A Brief Introduction to Lignin Structure. In Lignin Valorization: Emerging Approaches; Beckham, G.T., Ed.; The Royal Society of Chemistry: London, UK, 2018; pp. 1–20. [Google Scholar]
- Kumar, M.; Hietala, M.; Oksman, K. Lignin-based electrospun carbon nanofibers. Front. Mater. 2019, 6, 62. [Google Scholar] [CrossRef]
- La Cruz, D.; Banaag, F. Fate and Reactivity of Lignin in Municipal Solid Waste (MSW) Landfill. Ph.D. Thesis, Graduate Faculty of North Carolina State University, Raleigh, NC, USA, 2013. [Google Scholar]
- Dence, C.; Lin, S. Lignin determination. Methods Lignin Chem. 1992, 33, 61. [Google Scholar]
- Dos Santos, P.; Erdocia, S.X.; Gatto, D.A.; Labidi, J. Characterisation of Kraft lignin separated by gradient acid precipitation. Ind. Crops Prod. 2014, 55, 149–154. [Google Scholar] [CrossRef]
- Bertella, S.; Luterbacher, J.S. Lignin Functionalization for the Production of Novel Materials. Trends Chem. 2020. [Google Scholar] [CrossRef]
- Wang, J.P.; Matthews, M.L.; Williams, C.M.; Shi, R.; Yang, C.; Tunlaya-Anukit, S.; Chen, H.C.; Li, Q.; Liu, J.; Lin, C.Y.; et al. Improving wood properties for wood utilization through multi-omics integration in lignin biosynthesis. Nat. Commun. 2018, 9, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Guerra, A.; Gaspar, A.R.; Contreras, S.; Lucia, L.A.; Crestini, C.; Argyropoulos, D.S. On the propensity of lignin to associate: A size exclusion chromatography study with lignin derivatives isolated from different plant species. Phytochemistry 2007, 68, 2570–2583. [Google Scholar] [CrossRef]
- Ugartondo, V.; Mitjans, M.; Vinardell, M.P. Comparative antioxidant and cytotoxic effects of lignins from different sources. Bioresour. Technol. 2008, 99, 6683–6687. [Google Scholar] [CrossRef]
- Qian, Y.; Qiu, X.; Zhu, S. Lignin: A nature-inspired sun blocker for broad-spectrum sunscreens. Green Chem. 2015, 17, 320–324. [Google Scholar] [CrossRef]
- Glasser, W.G.; Northey, R.A.; Schultz, T.P. Lignin: Historical, Biological, and Materials Perspectives; ACS Publications: Washington, DC, USA, 1999. [Google Scholar]
- Beaucamp, A.; Wang, Y.; Culebras, M.; Collins, M.N. Carbon fibres from renewable resources: The role of the lignin molecular structure in its blendability with biobased poly (ethylene terephthalate). Green Chem. 2019, 21, 5063–5072. [Google Scholar] [CrossRef]
- De Wild, P.J.; Huijgen, W.J.; Gosselink, R.J. Lignin pyrolysis for profitable lignocellulosic biorefineries. Biofuels Bioprod. Biorefining 2014, 8, 645–657. [Google Scholar] [CrossRef]
- Björkman, A.J.S.P. Studies on finely divided wood. Part. 1. Extraction of lignin with neutral solvents. Sven. Papp. 1956, 59, 477–485. [Google Scholar]
- Boudet, A.M.; Kajita, S.; Grima-Pettenati, J.; Goffner, D. Lignins and lignocellulosics: A better control of synthesis for new and improved uses. Trends Plant Sci. 2003, 8, 576–581. [Google Scholar] [CrossRef] [PubMed]
- Kadla, J.F.; Kubo, S.; Venditti, R.A.; Gilbert, R.D.; Compere, A.L.; Griffith, W. Lignin-based carbon fibers for composite fiber applications. Carbon 2002, 40, 2913–2920. [Google Scholar] [CrossRef]
- Ago, M.; Okajima, K.; Jakes, J.E.; Park, S.; Rojas, O.J. Lignin-based electrospun nanofibers reinforced with cellulose nanocrystals. Biomacromolecules 2012, 13, 918–926. [Google Scholar] [CrossRef]
- Gul, V.; Lyubeshkina, E.; Shargorodskii, A. Mechanical properties of polypropylene modified by decomposition products of alkali sulfate lignin. Polym. Mech. 1965, 1, 1–4. [Google Scholar] [CrossRef]
- Košíková, B.; Kačuráková, M.; Demianova, V. Photooxidation of the composite lignin/polypropylene films. Chem. Pap. 1993, 47, 132–136. [Google Scholar]
- Rodrigues, P.C.; Cantão, M.P.; Janissek, P.; Scarpa, P.C.; Mathias, A.L.; Ramos, L.P.; Gomes, M.A. Polyaniline/lignin blends: FTIR, MEV and electrochemical characterization. Eur. Polym. J. 2002, 38, 2213–2217. [Google Scholar] [CrossRef]
- Baumberger, S.; Lapierre, C.; Monties, B. Utilization of pine kraft lignin in starch composites: Impact of structural heterogeneity. J. Agric. Food Chem. 1998, 46, 2234–2240. [Google Scholar] [CrossRef]
- Wu, R.; Wang, S.; Leng, Y.; Li, Q. Preparation, structure, and properties of poly (ethyleneoxide)/lignin composites used for UV absorption. J. Appl. Polym. Sci. 2020, 137, 48593. [Google Scholar] [CrossRef]
- Bu, L.; Tang, Y.; Xing, Y.; Zhang, W.; Shang, X.; Jiang, J. Comparison of hydrophilic variation and bioethanol production of furfural residues after delignification pretreatment. Biosci. Biotechnol. Biochem. 2014, 78, 1435–1443. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, Y.; Doherty, W.O.; Halley, P.J. Developing lignin-based resin coatings and composites. Ind. Crops Prod. 2008, 27, 163–167. [Google Scholar] [CrossRef]
- Kirk, T.K.; Obst, J.R. Lignin determination. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1988; pp. 87–101. [Google Scholar]
- Cateto, C.A.; Barreiro, M.F.; Rodrigues, A.E.; Belgacem, M.N. Kinetic study of the formation of lignin-based polyurethanes in bulk. React. Funct. Polym. 2011, 71, 863–869. [Google Scholar] [CrossRef]
- Akbari, S.; Kozłowski, R.M. A review of application of amine-terminated dendritic materials in textile engineering. J. Text. Inst. 2019, 110, 460–467. [Google Scholar] [CrossRef]
- Seiler, M. Hyperbranched polymers: Phase behavior and new applications in the field of chemical engineering. Fluid Phase Equilibria 2006, 241, 155–174. [Google Scholar] [CrossRef]
- Ruiz-Rosas, R.; Bedia, J.; Lallave, M.; Loscertales, I.G.; Barrero, A.; Rodríguez-Mirasol, J.; Cordero, T. The production of submicron diameter carbon fibers by the electrospinning of lignin. Carbon 2010, 48, 696–705. [Google Scholar] [CrossRef]
- Dallmeyer, I.; Ko, F.; Kadla, J.F. Electrospinning of technical lignins for the production of fibrous networks. J. Wood Chem. Technol. 2010, 30, 315–329. [Google Scholar] [CrossRef]
- Poursorkhabi, V.; Mohanty, A.K.; Misra, M. Electrospinning of aqueous lignin/poly (ethylene oxide) complexes. J. Appl. Polym. Sci. 2015, 132. [Google Scholar] [CrossRef]
- Seo, D.K.; Jeun, J.P.; Kim, H.B.; Kang, P.H. Preparation and characterization of the carbon nanofiber mat produced from electrospun PAN/lignin precursors by electron beam irradiation. Rev. Adv. Mater. Sci. 2011, 28, 31–34. [Google Scholar]
- Dallmeyer, I.; Lin, L.T.; Li, Y.; Ko, F.; Kadla, J.F. Preparation and characterization of interconnected, kraft lignin-based carbon fibrous materials by electrospinning. Macromol. Mater. Eng. 2014, 299, 540–551. [Google Scholar] [CrossRef]
- Bahi, A.; Shao, J.; Mohseni, M.; Ko, F.K. Membranes based on electrospun lignin-zeolite composite nanofibers. Sep. Purif. Technol. 2017, 187, 207–213. [Google Scholar] [CrossRef]
- Kim, S.; Dale, B.E. Global potential bioethanol production from wasted crops and crop residues. Biomass Bioenergy 2004, 26, 361–375. [Google Scholar] [CrossRef]
- Pourmoazzen, Z.; Sadeghifar, H.; Yang, G.; Lucia, L. Cholesterol-modified lignin: A new avenue for green nanoparticles, meltable materials, and drug delivery. Colloids Surf. B Biointerfaces 2020, 186, 110685. [Google Scholar] [CrossRef] [PubMed]
- Lora, J.H.; Glasser, W.G. Recent industrial applications of lignin: A sustainable alternative to nonrenewable materials. J. Polym. Environ. 2002, 10, 39–48. [Google Scholar] [CrossRef]
- Ramakrishna, S. An Introduction to Electrospinning and Nanofibers; World Scientific: Toh Tuck Link, Singapore, 2005. [Google Scholar]
- Ebers, L.S.; Auvergne, R.; Boutevin, B.; Laborie, M.P. Impact of PEO structure and formulation on the properties of a Lignin/PEO blend. Ind. Crops Prod. 2020, 143, 111883. [Google Scholar] [CrossRef]
- Yang, M.; Zhang, X.; Guan, S.; Dou, Y.; Gao, X. Preparation of lignin containing cellulose nanofibers and its application in PVA nanocomposite films. Int. J. Biol. Macromol. 2020. [Google Scholar] [CrossRef]
- Cui, M.; Nguyen, N.A.; Bonnesen, P.V.; Uhrig, D.; Keum, J.K.; Naskar, A.K. Rigid oligomer from lignin in designing of tough, self-healing elastomers. ACS Macro Lett. 2018, 7, 1328–1332. [Google Scholar] [CrossRef]
- Brosse, N.; El Hage, R.; Chaouch, M.; Pétrissans, M.; Dumarçay, S.; Gérardin, P. Investigation of the chemical modifications of beech wood lignin during heat treatment. Polym. Degrad. Stab. 2010, 95, 1721–1726. [Google Scholar] [CrossRef]
- Kim, J.Y.; Hwang, H.; Oh, S.; Kim, Y.S.; Kim, U.J.; Choi, J.W. Investigation of structural modification and thermal characteristics of lignin after heat treatment. Int. J. Biol. Macromol. 2014, 66, 57–65. [Google Scholar] [CrossRef]
- Gedde, U. Polymer Physics; Springer Science & Business Media: Berlin, Germany, 1995. [Google Scholar]
- Ali Mohammadpoor, S.; Akbari, S.; Sadrjahani, M.; Nourpanah, P. Fabrication of electrospun ibuprofen-loaded poly (vinyl alcohol)/hyper-branched poly (ethylenimine) fibers and their release behaviors. J. Biomater. Sci. Polym. Ed. 2020, 31, 261–275. [Google Scholar] [CrossRef] [PubMed]
- Norberg, I. Carbon Fibres from Kraft Lignin; KTH Royal Institute of Technology: Stockholm, Sweden, 2012. [Google Scholar]
- Jämsä, S.; Viitaniemi, P. Heat treatment of wood–Better durability without chemicals. In Proceedings of Special Seminar Held in Antibes, France; The European Commission Research Directorate: Brussels, Belgium, 2001. [Google Scholar]
- Yildiz, S.; Gezer, E.D.; Yildiz, U.C. Mechanical and chemical behavior of spruce wood modified by heat. Build. Environ. 2006, 41, 1762–1766. [Google Scholar] [CrossRef]
- Cho, M.; Karaaslan, M.A.; Renneckar, S.; Ko, F. Enhancement of the mechanical properties of electrospun lignin-based nanofibers by heat treatment. J. Mater. Sci. 2017, 52, 9602–9614. [Google Scholar] [CrossRef]
- Klapiszewski, Ł.; Nowacka, M.; Milczarek, G.; Jesionowski, T. Physicochemical and electrokinetic properties of silica/lignin biocomposites. Carbohydr. Polym. 2013, 94, 345–355. [Google Scholar] [CrossRef] [PubMed]
- Kuzniatsova, T.; Kim, Y.; Shqau, K.; Dutta, P.K.; Verweij, H. Zeta potential measurements of zeolite Y: Application in homogeneous deposition of particle coatings. Microporous Mesoporous Mater. 2007, 103, 102–107. [Google Scholar] [CrossRef]
- Bernsmann, F.; Frisch, B.; Ringwald, C.; Ball, V. Protein adsorption on dopamine–melanin films: Role of electrostatic interactions inferred from ζ-potential measurements versus chemisorption. J. Colloid Interface Sci. 2010, 344, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Faleva, A.V.; Kozhevnikov, A.Y.; Pokryshkin, S.A.; Falev, D.I.; Shestakov, S.L.; Popova, J.A. Structural characteristics of different softwood lignins according to 1D and 2D NMR spectroscopy. J. Wood Chem. Technol. 2020, 40, 178–189. [Google Scholar] [CrossRef]
- Kai, D.; Jiang, S.; Low, Z.W.; Loh, X.J. Engineering highly stretchable lignin-based electrospun nanofibers for potential biomedical applications. J. Mater. Chem. B 2015, 3, 6194–6204. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, S.; Zheng, Y.; Luo, Z.; Cen, K. Mechanism study of wood lignin pyrolysis by using TG–FTIR analysis. J. Anal. Appl. Pyrolysis 2008, 82, 170–177. [Google Scholar] [CrossRef]
- Ma, A.; Zhou, L.; Chang, J.J.N. Conversion of lignin-nanofibers to CNFs. Nano 2015, 10, 1550092. [Google Scholar] [CrossRef]
- Shen, D.K.; Gu, S.; Luo, K.H.; Wang, S.R.; Fang, M.X. The pyrolytic degradation of wood-derived lignin from pulping process. Bioresour. Technol. 2010, 101, 6136–6146. [Google Scholar] [CrossRef] [PubMed]
- Fenner, R.A.; Lephardt, J.O. Examination of the thermal decomposition of kraft pine lignin by Fourier transform infrared evolved gas analysis. J. Agric. Food Chem. 1981, 29, 846–849. [Google Scholar] [CrossRef]
- Zheng, P.; Gao, L.; Sun, X.; Mei, S.H. The thermolysis behaviours of the first generation dendritic polyamidoamine. Iran. Polym. J. 2009, 18, 157–264. [Google Scholar]
- Kanani-Jazi, M.H.; Akbari, S.; Kish, M.H. Efficient removal of Cr (VI) from aqueous solution by halloysite/poly (amidoamine) dendritic nano-hybrid materials: Kinetic, isotherm and thermodynamic studies. Adv. Powder Technol. 2020, 31, 4018–4030. [Google Scholar] [CrossRef]
- Fengel, D.; Wegener, G. Wood: Chemistry, Ultrastructure, Reactions; Walter de Gruyter: Berlin, Germany, 2011. [Google Scholar]
- Pasquali, C.L.; Herrera, H. Pyrolysis of lignin and IR analysis of residues. Thermochim. Acta 1997, 293, 39–46. [Google Scholar] [CrossRef]
- Mohseni, M.; Akbari, S.; Pajootan, E.; Mazaheri, F. Amine-terminated dendritic polymers as a multifunctional chelating agent for heavy metal ion removals. Environ. Sci. Pollut. Res. 2019, 26, 12689–12697. [Google Scholar] [CrossRef]
- Dong, D.; Fricke, A.L. Intrinsic viscosity and the molecular weight of kraft lignin. Polymer 1995, 36, 2075–2078. [Google Scholar] [CrossRef]
- Siochi, E.J.; Ward, T.C.; Haney, M.A.; Mahn, B. The absolute molecular weight distribution of hydroxypropylated lignins. Macromolecules 1990, 23, 1420–1429. [Google Scholar] [CrossRef]
- Crestini, C.; Lange, H.; Sette, M.; Argyropoulos, D.S. On the structure of softwood kraft lignin. Green Chem. 2017, 19, 4104–4121. [Google Scholar] [CrossRef]
- Matsushita, Y.; Yasuda, S. Reactivity of a condensed–type lignin model compound in the Mannich reaction and preparation of cationic surfactant from sulfuric acid lignin. J. Wood Sci. 2003, 49, 166–171. [Google Scholar] [CrossRef]
- Coats, A.; Redfern, J. Thermogravimetric analysis. A review. Analyst 1963, 88, 906–924. [Google Scholar] [CrossRef]
- Chen, Y.; Zou, C.; Mastalerz, M.; Hu, S.; Gasaway, C.; Tao, X. Applications of micro-fourier transform infrared spectroscopy (FTIR) in the geological sciences—A review. Int. J. Mol. Sci. 2015, 16, 30223–30250. [Google Scholar] [CrossRef] [PubMed]
- von Aulock, F.W.; Kennedy, B.M.; Schipper, C.I.; Castro, J.M.; Martin, D.E.; Oze, C.; Watkins, J.M.; Wallace, P.J.; Puskar, L.; Bégué, F.; et al. Advances in Fourier transform infrared spectroscopy of natural glasses: From sample preparation to data analysis. Lithos 2014, 206, 52–64. [Google Scholar] [CrossRef]
- Rossman, G.R. Analytical methods for measuring water in nominally anhydrous minerals. Rev. Mineral. Geochem. 2006, 62, 1–28. [Google Scholar] [CrossRef]
- Gao, J.; Liang, Z.; Liang, J.; Wang, W.; Lü, J.; Qin, Y. Spectrum reconstruction of a spatially modulated fourier transform. Spectrometer based on stepped mirrors. Appl. Spectrosc. 2017, 71, 1348–1356. [Google Scholar] [CrossRef] [PubMed]
- Giummarella, N.; Lindgren, C.; Lindström, M.; Henriksson, G. Lignin prepared by ultrafiltration of black liquor: Investigation of solubility, viscosity, and ash content. BioResources 2016, 11, 3494–3510. [Google Scholar] [CrossRef] [Green Version]

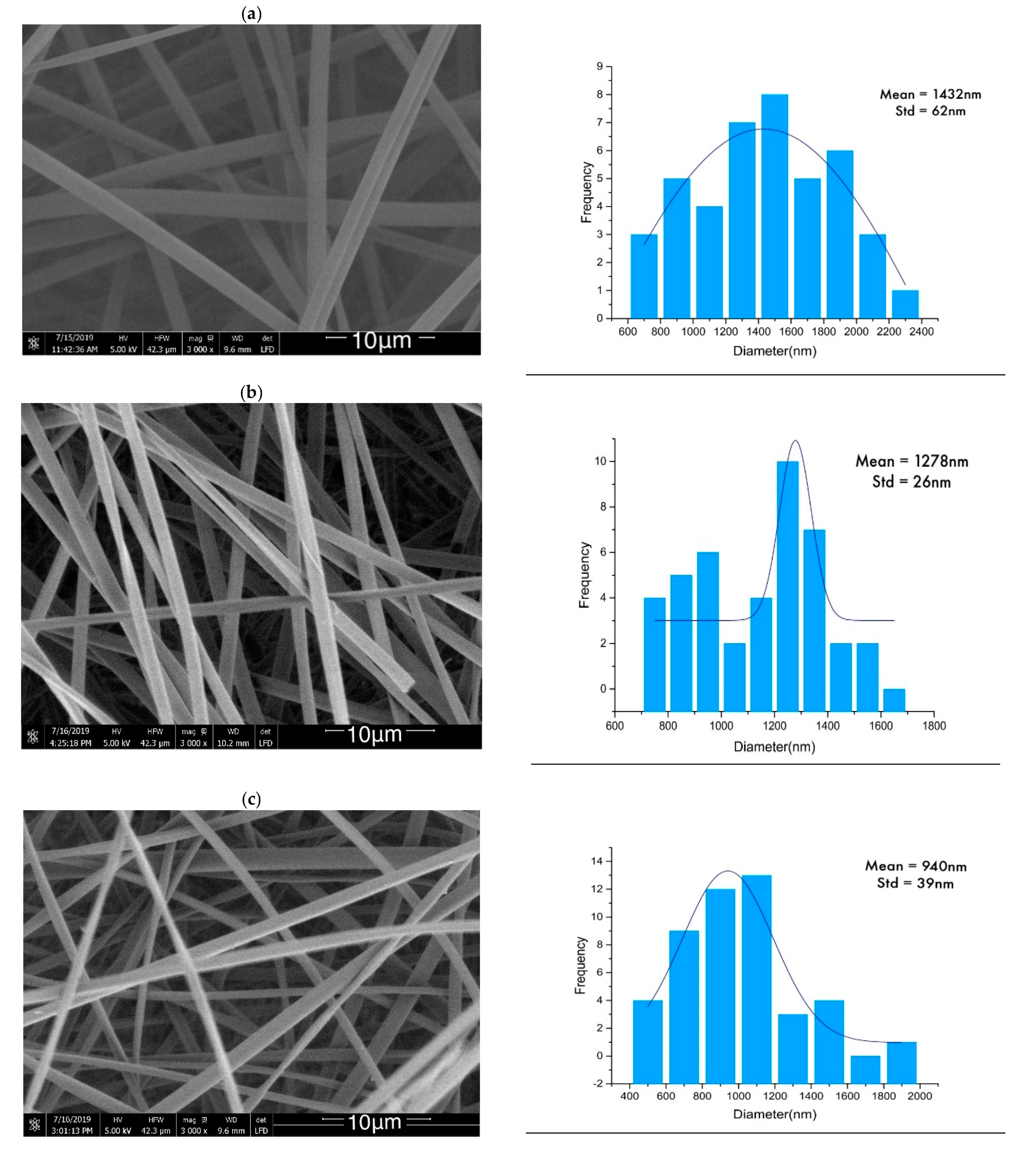
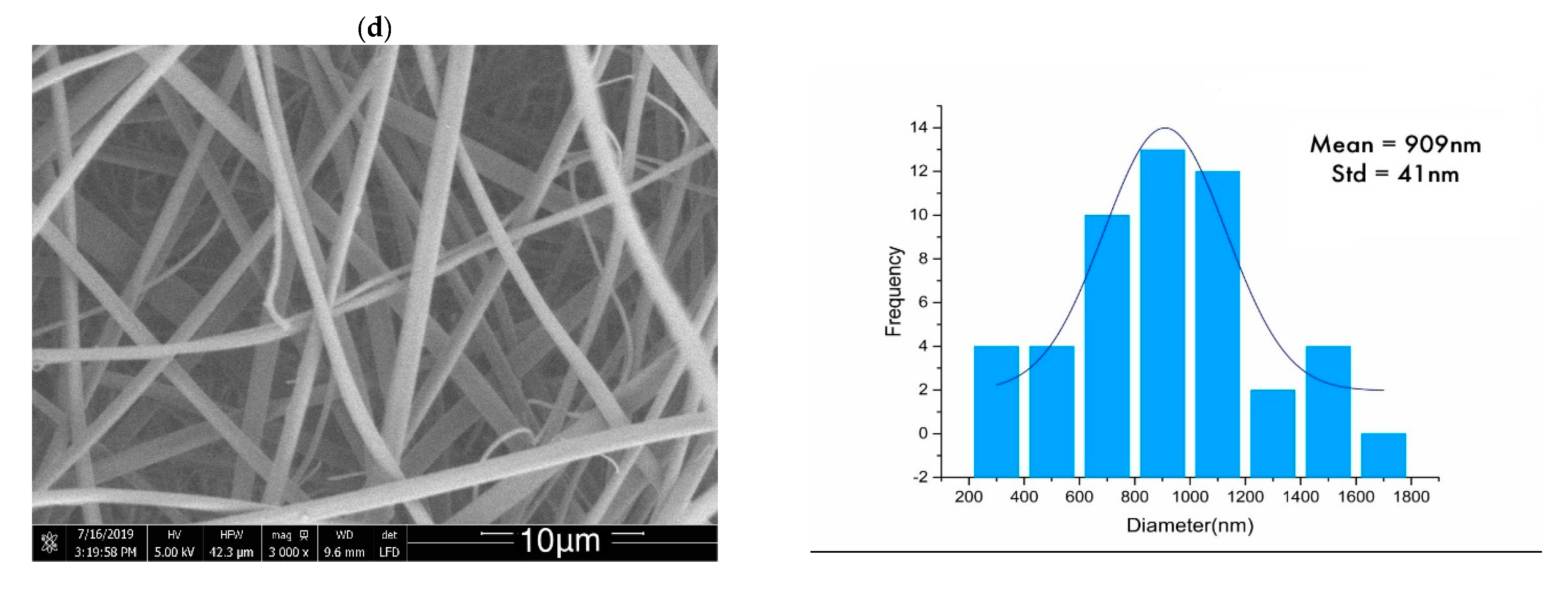
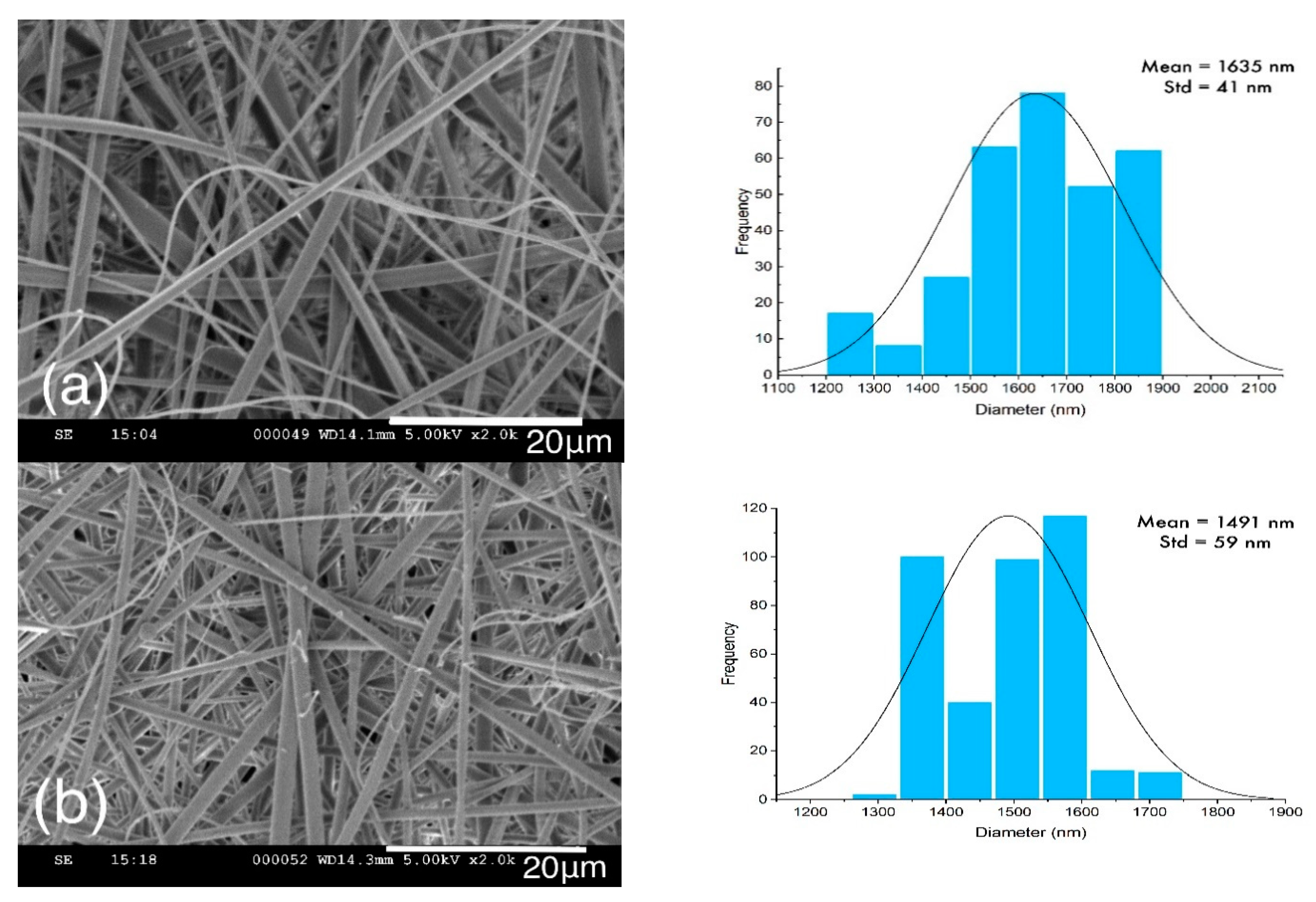

| Sample | Untreated (nm) | Heat-Set (nm) | Stabilized (nm) |
|---|---|---|---|
| Lignin | 1432 ± 62 | 940 ± 39 | 622 ± 21 |
| Lignin/PAMAM | 1278 ± 26 | 909 ± 41 | 866 ± 29 |
| Properties | Untreated Lignin Nanofiber Mats | Heat-Set Lignin Nanofiber Mats | ||
|---|---|---|---|---|
| Lignin | Lignin/PAMAM | Lignin | Lignin/PAMAM | |
| Tensile Stress (MPa) | 4.52 ± 0.97 | 6.30 ± 1.70 | 2.23 ± 0.41 | 4.47 ± 0.56 |
| Strain at break (%) | 1.32 ± 0.12 | 1.57 ± 0.27 | 0.85 ± 0.08 | 1.10 ± 0.19 |
| Elastic modulus (E) (MPa) | 342.42 ± 8.01 | 401.27 ± 1.73 | 262.35 ± 6.68 | 442.57 ± 4.74 |
| Sample | Heat-Set (HS) Condition | Tensile Stress (MPa) | Strain at Break (%) | Elastic Modulus (GPa) | Ref. | |||
|---|---|---|---|---|---|---|---|---|
| Before HS | After HS | Before HS | After HS | Before HS | After HS | |||
| Lignin/PEO | Carbonization | 32.01 ± 9 | 74.1 ± 14.6 | 0.9 ± 0.3 | 3.01 ± 1.1 | 4.8 ± 0.6 | 4.1 ± 1.4 | [36] |
| Lignin/PEO | Thermo-stabilization | 17.2 ± 3.4 | 31.4 ± 6.4 | 2.3 ± 0.5 | 3.9 ± 1.4 | 0.8 ± 0.06 | 1.3 ± 0.09 | [36] |
| Lignin Carbon fiber 25 wt% | Carbonization | 5.13 ± 0.64 | 45.3 ± 9.93 | 1.73 ± 0.45 | 0.7 ± 0.05 | 0.5 ± 0.07 | 6.23 ± 1.01 | [52] |
| Lignin Carbon fiber 27 wt% | Carbonization | 8.35 ± 1.15 | 53.1 ± 22 | 1.5 ± 0.2 | 0.5 ± 0.07 | 0.7 ± 0.05 | 6.8 ± 1.01 | [52] |
| Lignin carbon fiber 25 wt% | Stabilization | 8.35 ± 1.15 | 23.86 ± 4.55 | 1.73 ± 0.45 | 3.12 ± 0.61 | 0.5 ± 0.13 | 0.9 ± 0.13 | [52] |
| Lignin carbon fiber 25 wt% | Stabilization | 8.35 ± 1.15 | 25.5 ± 4.55 | 1.5 ± 0.3 | 1.75 ± 0.3 | 0.7 ± 0.05 | 1.9 ± 0.22 | [52] |
| Component | Tonset a (°C) | Td b (°C) | Residue Weight at Td % | Tf c (°C) | Residue Weight at Tf % | α1 d | α2 e |
|---|---|---|---|---|---|---|---|
| Lignin | 47 | 380 | 65.81 | 596 | 41.12 | 0.374 | 0.029 |
| Lignin/PAMAM | 50 | 367 | 69.66 | 594 | 43.2 | 0.345 | 0.038 |
| Lignin (HS) | 52 | 381 | 66.31 | 596 | 41.69 | 0.369 | 0.024 |
| Lignin/PAMAM (HS) | 51 | 373 | 66.38 | 589 | 45.54 | 0.332 | 0.036 |
| Wavenumber (cm−1) | Functional Group | Vibration | Compounds | Refs. |
|---|---|---|---|---|
| 645–690 | C=O | Dactyl-zone | [60,65] | |
| 974–1058 | C–O | Stretching | R–OH | [60,64] |
| 1000–1300 | C–O | Stretching | [58,65] | |
| 1093–1188 | C–C | Skeleton | [60] | |
| 1310–1365 | C–CH3 | Bending | Alkyls | [60,65] |
| 1300–1400 | O–H | Bending | [61,63] | |
| 1513 | C=C–OH | Stretching | [65] | |
| 1613 | C=C | Stretching | Aromatics | [65] |
| 1645–1750 | O–H | Bending | H2O | [60] |
| 2020–2220 | C–O | Stretching | CO | [60] |
| 2210–2390 | C=O | Stretching | CO2 | [60,65] |
| 2750–2990 | C–H2 | Asymmetric Stretching | [58,60] | |
| 2904–2979 | C–H | Stretching | [64] | |
| 2990–3010 | C–H | Stretching | [58,64] | |
| 3020–3190 | C–H | Stretching | CH4 | [61,64] |
| 3500–3600 | O–H | Stretching | [58,64] | |
| 3823–3870 | O–H | Stretching | [60,64] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akbari, S.; Bahi, A.; Farahani, A.; Milani, A.S.; Ko, F. Fabrication and Characterization of Lignin/Dendrimer Electrospun Blended Fiber Mats. Molecules 2021, 26, 518. https://doi.org/10.3390/molecules26030518
Akbari S, Bahi A, Farahani A, Milani AS, Ko F. Fabrication and Characterization of Lignin/Dendrimer Electrospun Blended Fiber Mats. Molecules. 2021; 26(3):518. https://doi.org/10.3390/molecules26030518
Chicago/Turabian StyleAkbari, Somaye, Addie Bahi, Ali Farahani, Abbas S. Milani, and Frank Ko. 2021. "Fabrication and Characterization of Lignin/Dendrimer Electrospun Blended Fiber Mats" Molecules 26, no. 3: 518. https://doi.org/10.3390/molecules26030518







