Acridine-Based Antimalarials—From the Very First Synthetic Antimalarial to Recent Developments
Abstract
:1. Introduction
2. Quinacrine Derivatives
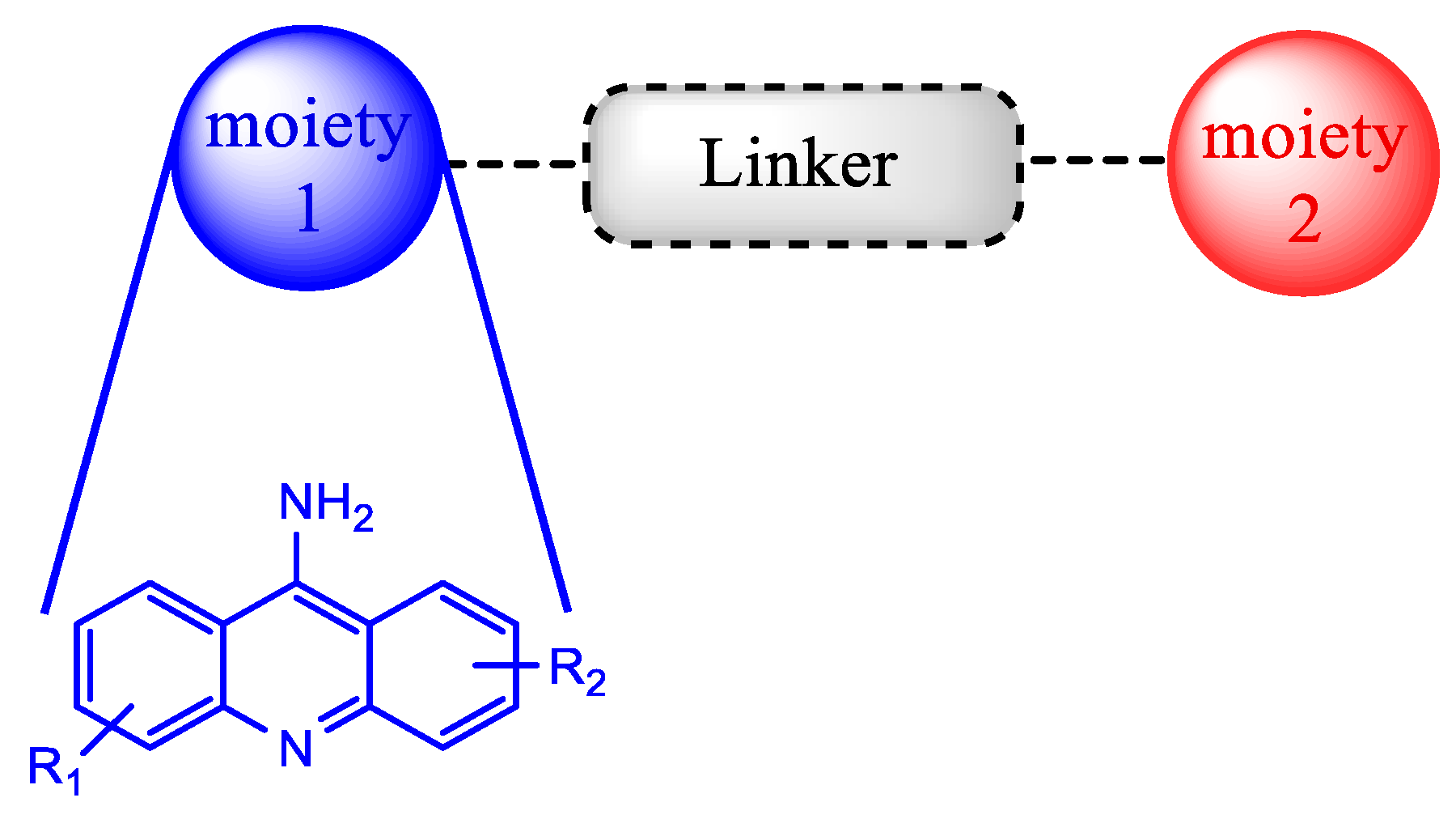
3. Hybrids Containing the 9-Aminoacridine Scaffold
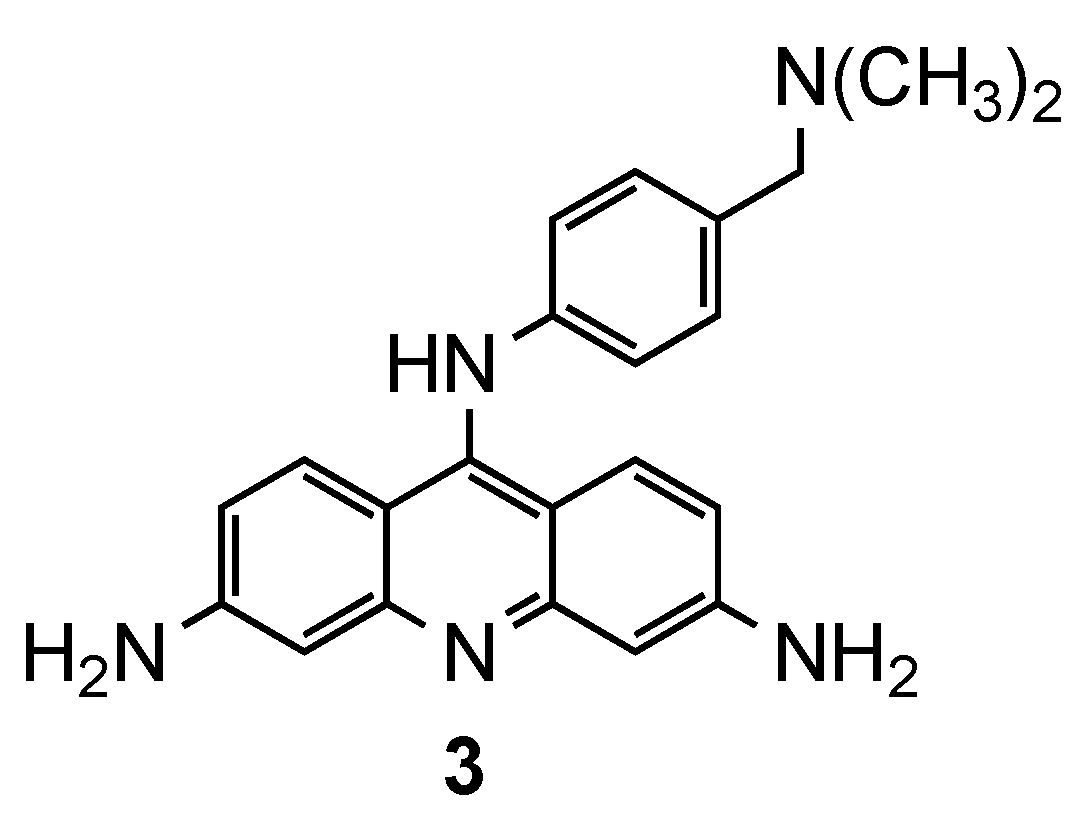
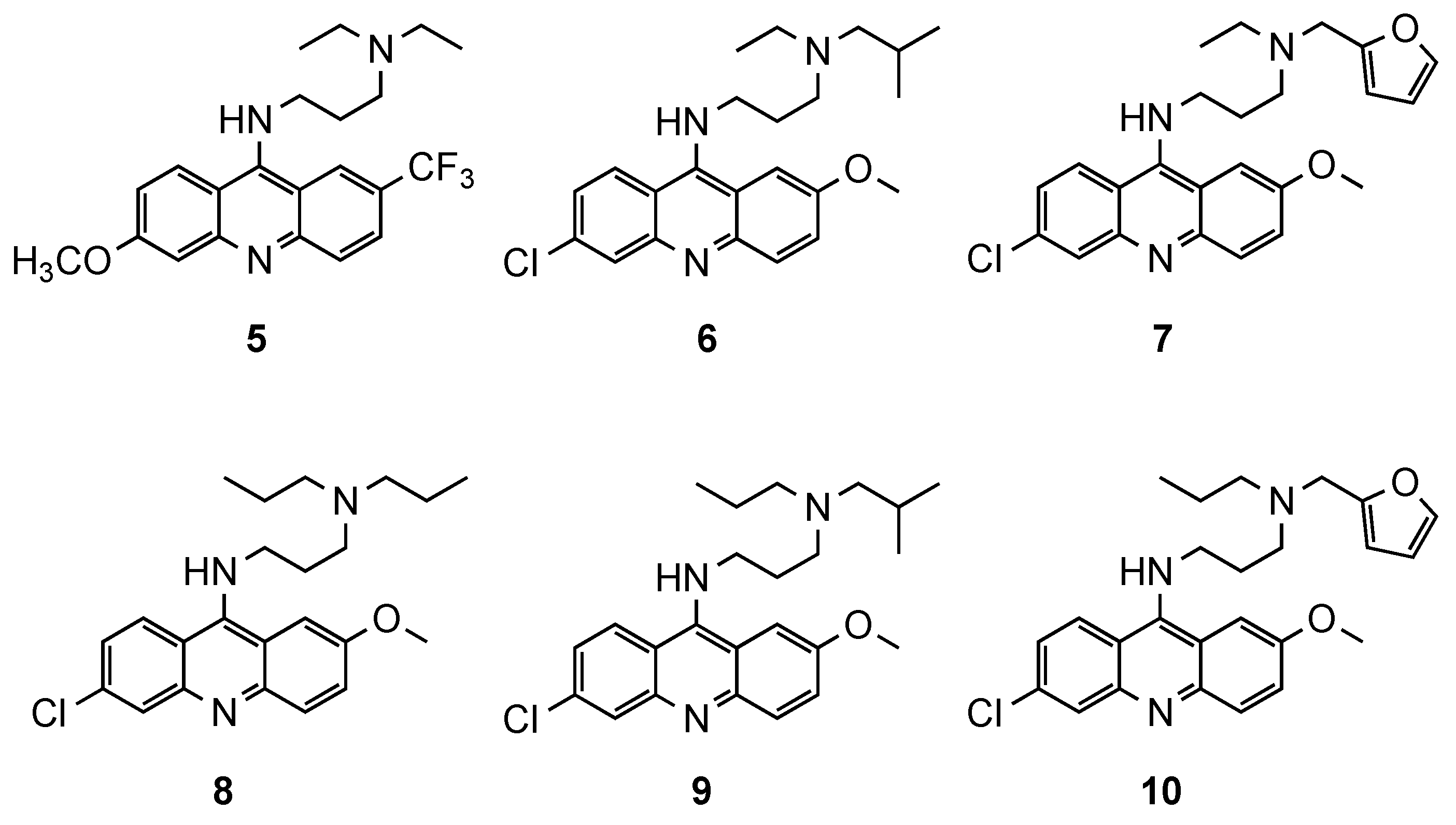
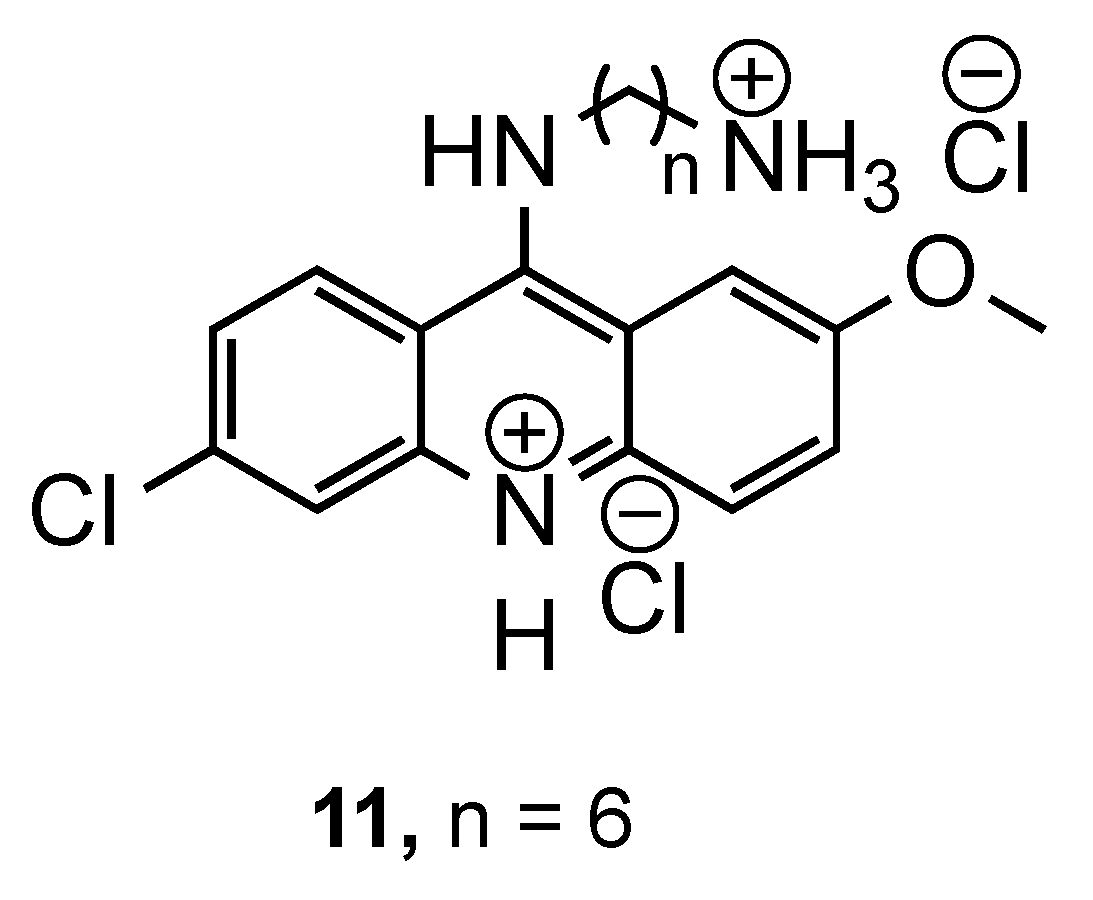
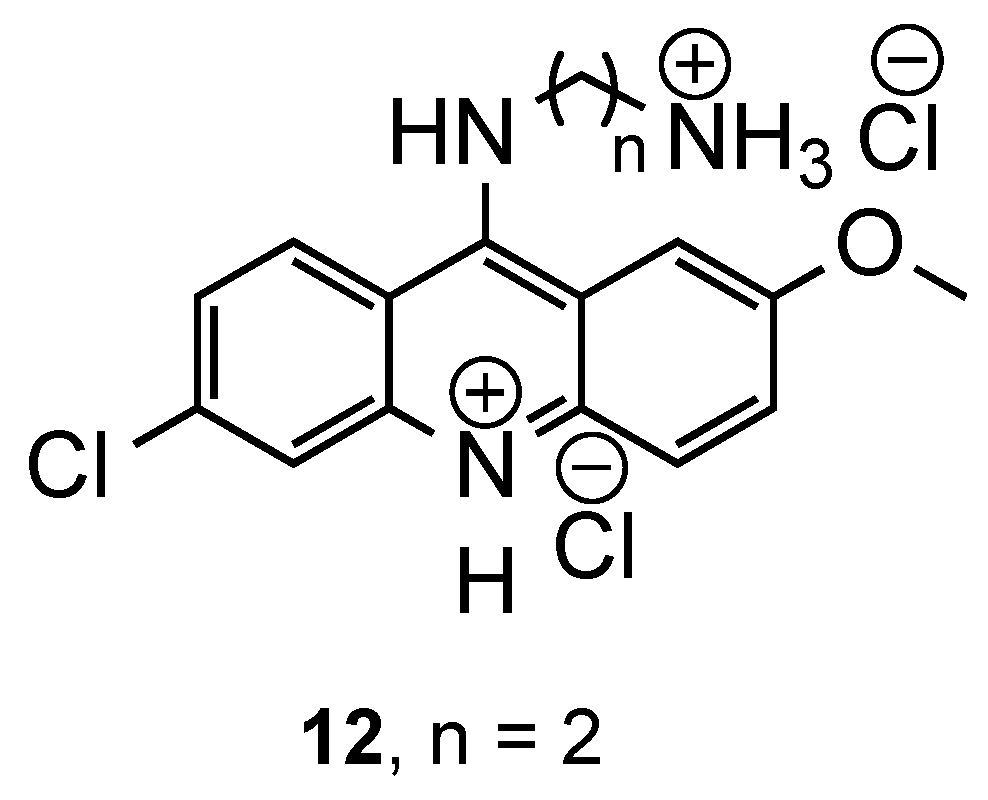
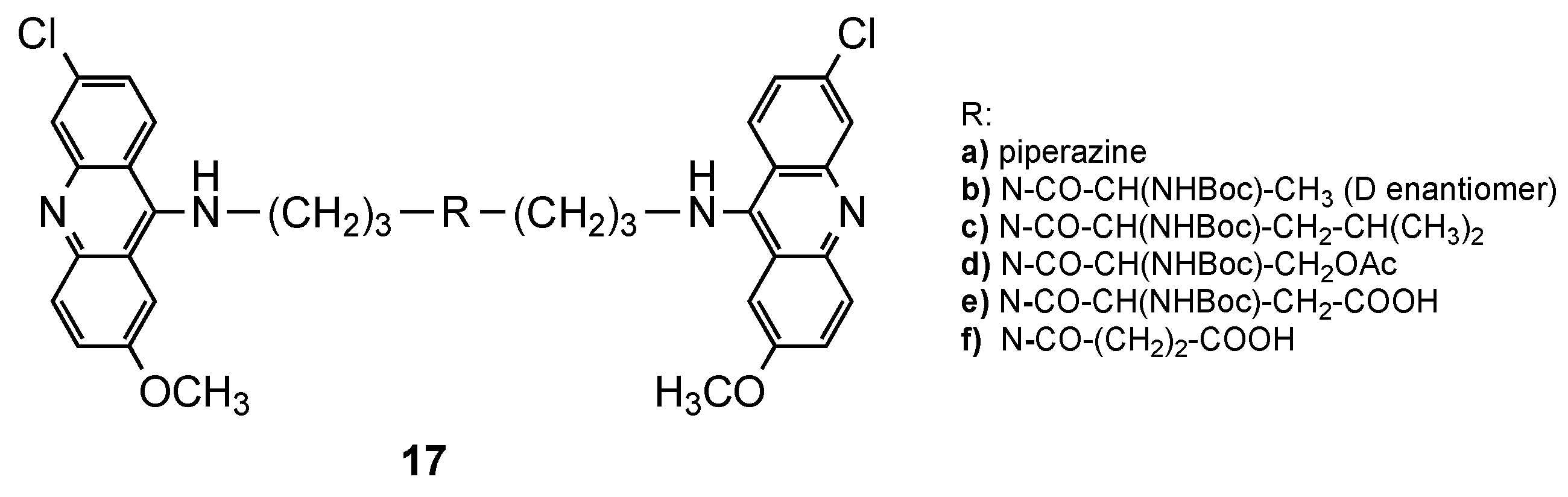
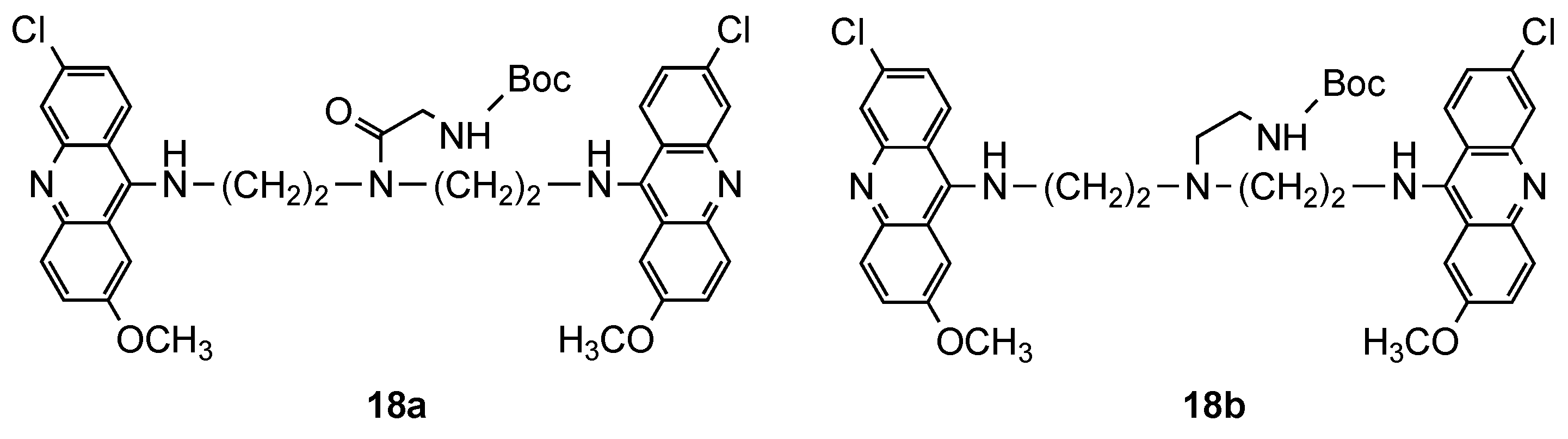
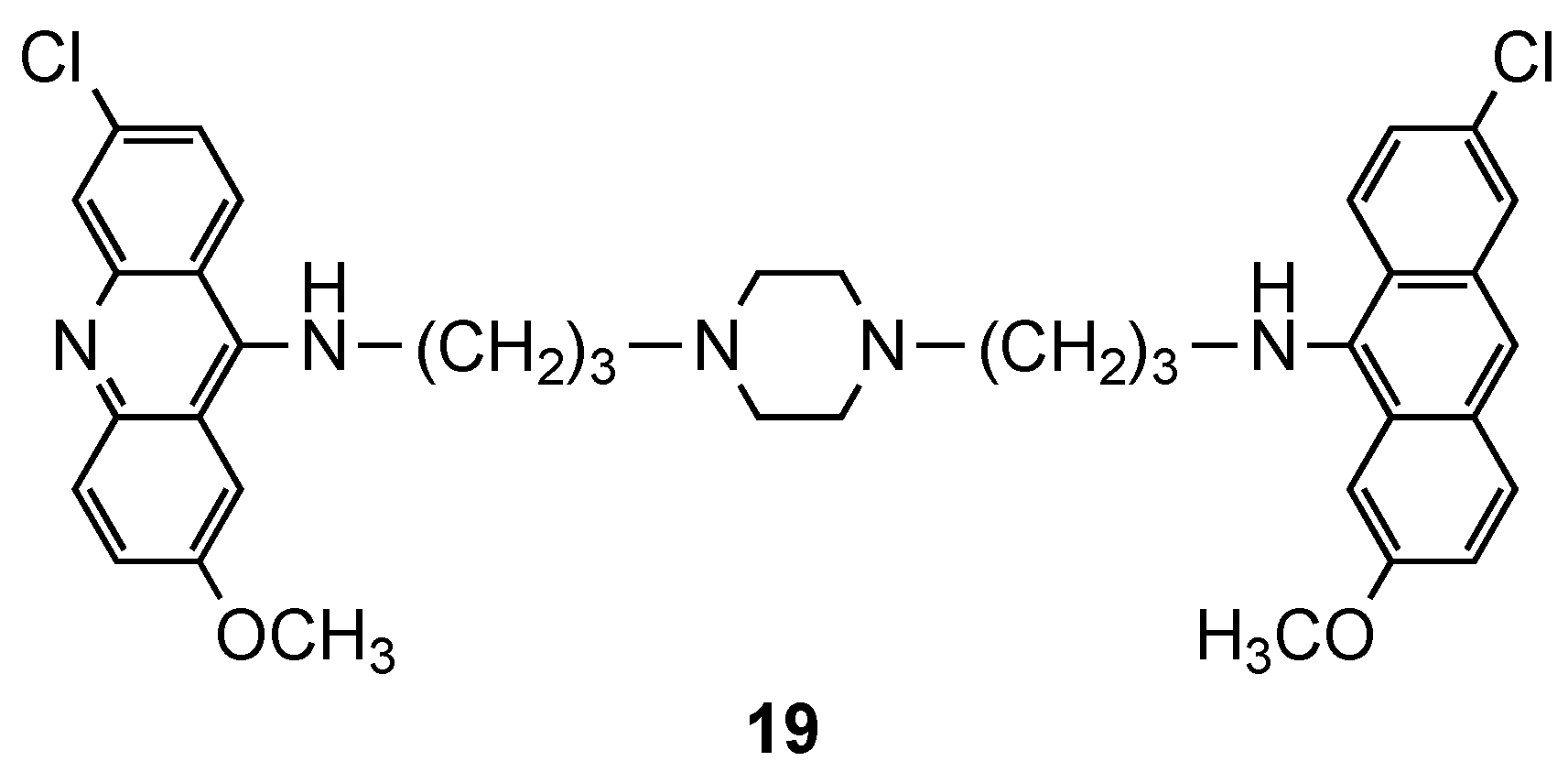
3.1. Bisacridine Hybrids
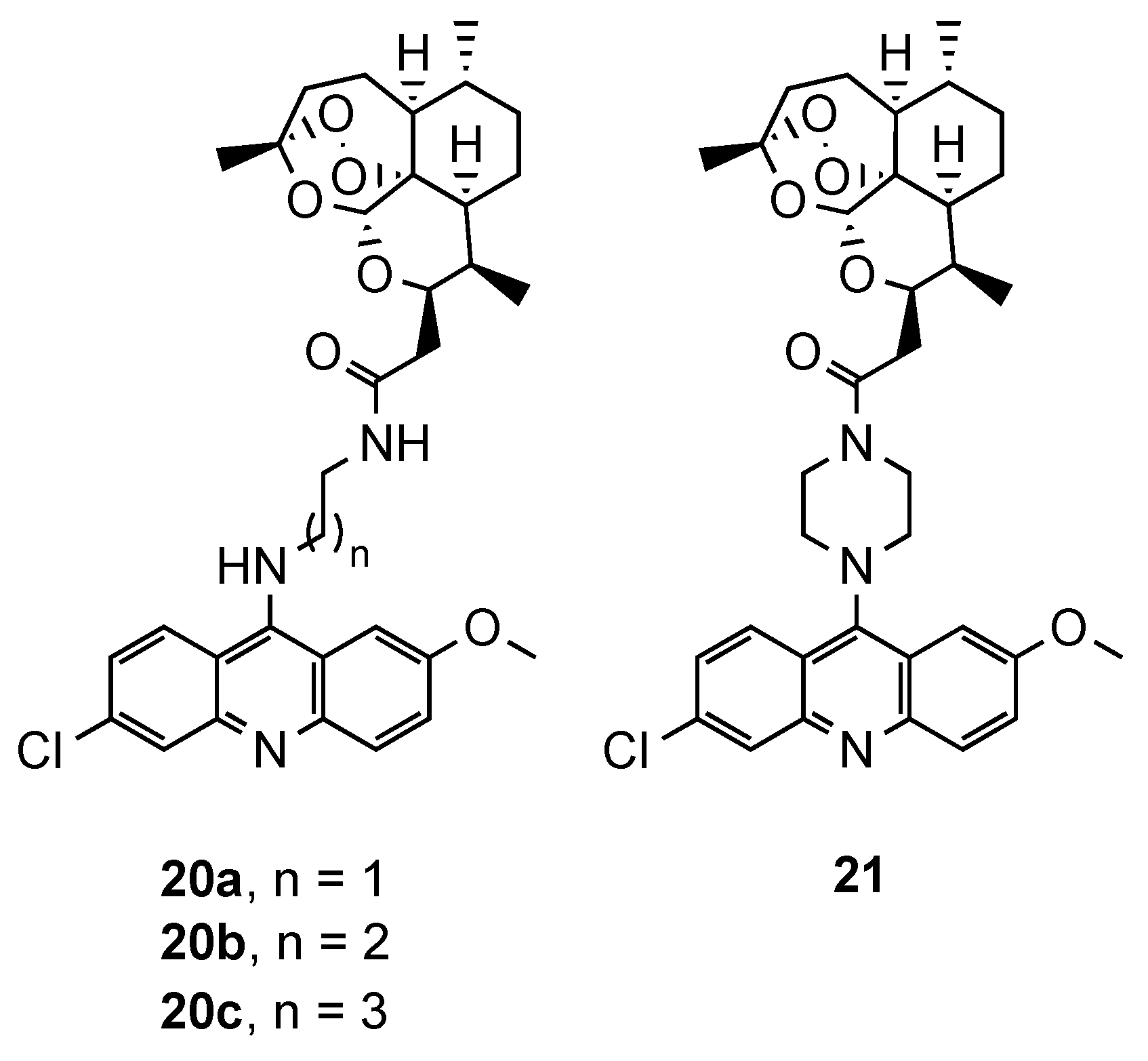
3.2. Acridine–Artemisinin Hybrids
3.3. Acridine–Aminoquinoline Hybrids
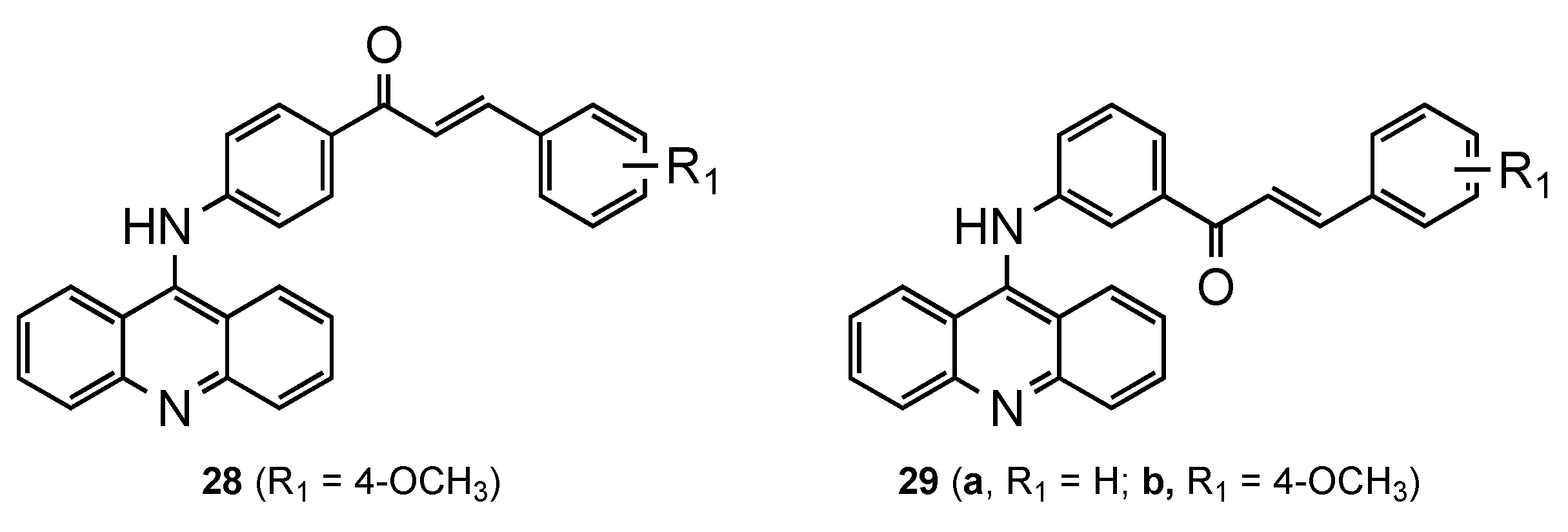
3.4. Acridine–Chalcone Hybrids
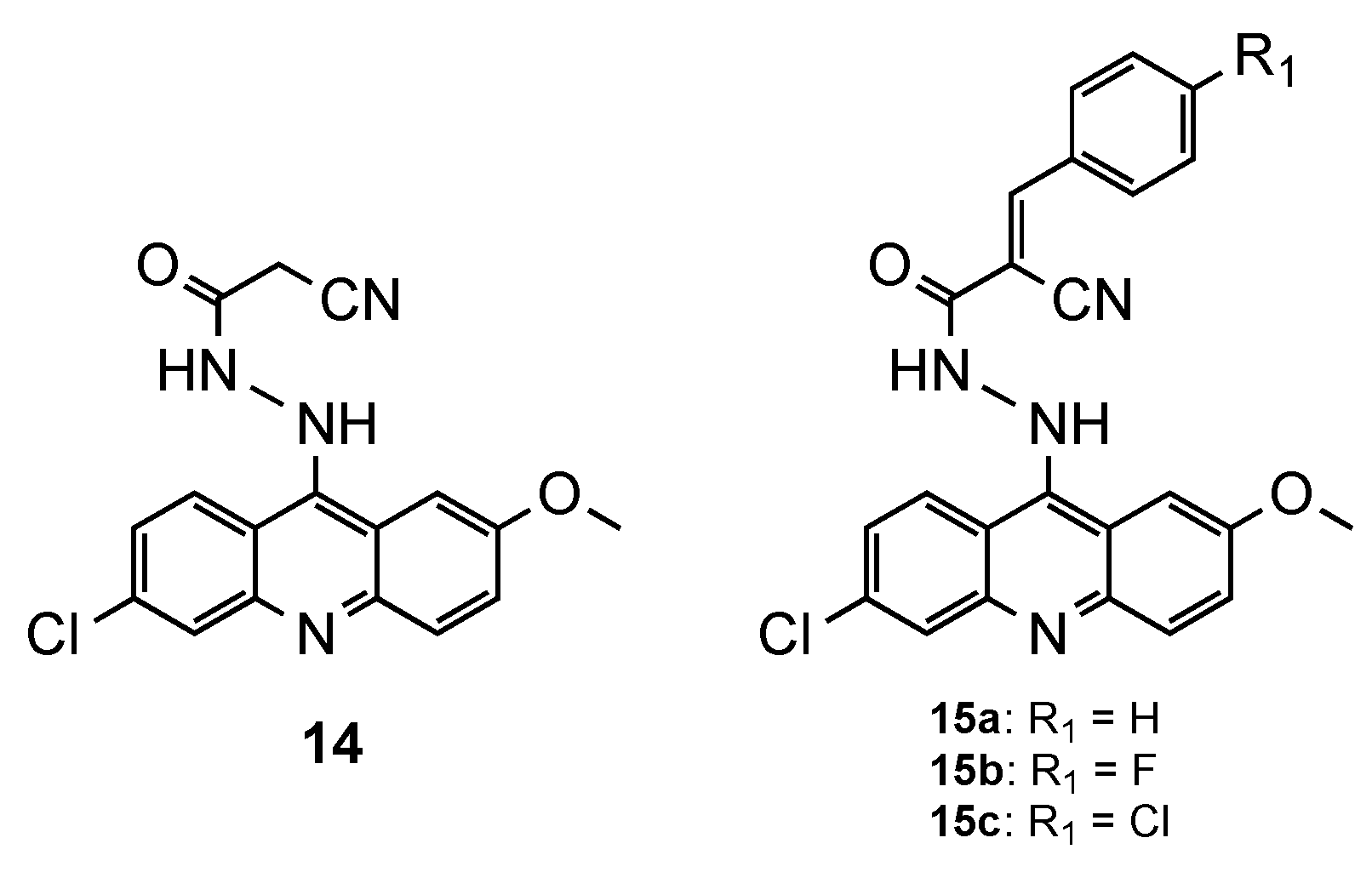
3.5. Acridine–Cinnamoyl Hybrids
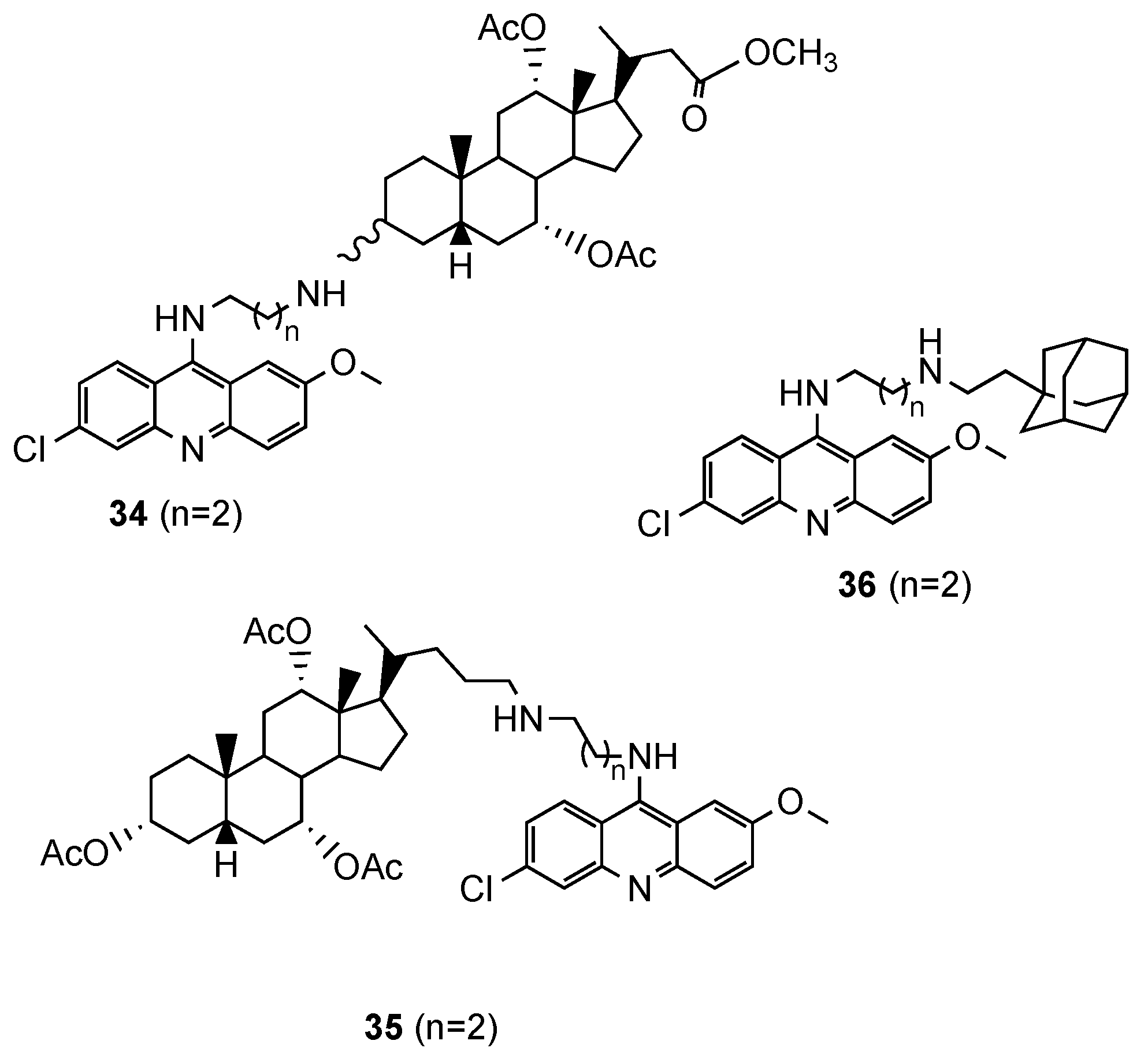
3.6. Other Acridine Hybrids
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Cox, F.E. History of the discovery of the malaria parasites and their vectors. Parasit Vectors 2010, 3, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- World Malaria Report 2019, World Health Organization. Available online: https://www.who.Int/publications/i/item/world-malaria-report-2019 (accessed on 10 December 2020).
- World Health Organization. Artemisinin Resistance and Artemisinin-Based Combination Therapy Efficacy: Status Report (2018). Available online: https://apps.Who.Int/iris/handle/10665/274362 (accessed on 10 December 2020.).
- Teixeira, C.; Vale, N.; Pérez, B.; Gomes, A.; Gomes, J.R.B.; Gomes, P. “Recycling” classical drugs for malaria. Chem. Rev. 2014, 114, 11164–11220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Delves, M.; Plouffe, D.; Scheurer, C.; Meister, S.; Wittlin, S.; Winzeler, E.A.; Sinden, R.E.; Leroy, D. The activities of current antimalarial drugs on the life cycle stages of plasmodium: A comparative study with human and rodent parasites. PLoS Med. 2012, 9, e1001169. [Google Scholar] [CrossRef] [Green Version]
- Gensicka-Kowalewska, M.; Cholewiński, G.; Dzierzbicka, K. Recent developments in the synthesis and biological activity of acridine/acridone analogues. RSC Adv. 2017, 7, 15776–15804. [Google Scholar] [CrossRef] [Green Version]
- Prasher, P.; Sharma, M. Medicinal chemistry of acridine and its analogues. MedChemComm 2018, 9, 1589–1618. [Google Scholar] [CrossRef]
- Ehsanian, R.; Van Waes, C.; Feller, S.M. Beyond DNA binding–a review of the potential mechanisms mediating quinacrine’s therapeutic activities in parasitic infections, inflammation, and cancers. Cell Commun. Signal. 2011, 9, 13. [Google Scholar] [CrossRef] [Green Version]
- Joanny, F.; Held, J.; Mordmuller, B. In vitro activity of fluorescent dyes against asexual blood stages of plasmodium falciparum. Antimicrob. Agents Chemother. 2012, 56, 5982–5985. [Google Scholar] [CrossRef] [Green Version]
- Wainwright, M. Dyes in the development of drugs and pharmaceuticals. Dyes Pigments 2008, 76, 582–589. [Google Scholar] [CrossRef]
- Kitchen, L.W.; Vaughn, D.W.; Skillman, D.R. Role of us military research programs in the development of us food and drug administration--approved antimalarial drugs. Clin. Infect. Dis. 2006, 43, 67–71. [Google Scholar] [CrossRef] [PubMed]
- Chibale, K.; Haupt, H.; Kendrick, H.; Yardley, V.; Saravanamuthu, A.; Fairlamb, A.H.; Croft, S.L. Antiprotozoal and cytotoxicity evaluation of sulfonamide and urea analogues of quinacrine. Bioor. Med. Chem. Lett. 2001, 11, 2655–2657. [Google Scholar] [CrossRef]
- Auparakkitanon, S.; Noonpakdee, W.; Ralph, R.K.; Denny, W.A.; Wilairat, P. Antimalarial 9-anilinoacridine compounds directed at hematin. Antimicro. Agents Chemother. 2003, 47, 3708–3712. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sparatore, A.; Basilico, N.; Parapini, S.; Romeo, S.; Novelli, F.; Sparatore, F.; Taramelli, D. 4-aminoquinoline quinolizidinyl- and quinolizidinylalkyl-derivatives with antimalarial activity. Bioorg. Med. Chem. 2005, 13, 5338–5345. [Google Scholar] [CrossRef] [PubMed]
- Anderson, M.O.; Sherrill, J.; Madrid, P.B.; Liou, A.P.; Weisman, J.L.; DeRisi, J.L.; Guy, R.K. Parallel synthesis of 9-aminoacridines and their evaluation against chloroquine-resistant plasmodium falciparum. Bioorg. Med. Chem. 2006, 14, 334–343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guetzoyan, L.; Ramiandrasoa, F.; Dorizon, H.; Desprez, C.; Bridoux, A.; Rogier, C.; Pradines, B.; Perrée-Fauvet, M. In vitro efficiency of new acridyl derivatives against plasmodium falciparum. Bioorg. Med. Chem. 2007, 15, 3278–3289. [Google Scholar] [CrossRef]
- Guetzoyan, L.; Yu, X.M.; Ramiandrasoa, F.; Pethe, S.; Rogier, C.; Pradines, B.; Cresteil, T.; Perrée-Fauvet, M.; Mahy, J.P. Antimalarial acridines: Synthesis, in vitro activity against p. Falciparum and interaction with hematin. Bioorg. Med. Chem. 2009, 17, 8032–8039. [Google Scholar] [CrossRef]
- Yu, X.M.; Ramiandrasoa, F.; Guetzoyan, L.; Pradines, B.; Quintino, E.; Gadelle, D.; Forterre, P.; Cresteil, T.; Mahy, J.P.; Pethe, S. Synthesis and biological evaluation of acridine derivatives as antimalarial agents. ChemMedChem 2012, 7, 587–605. [Google Scholar] [CrossRef]
- de M. Silva, M.; Macedo, T.S.; Teixeira, H.M.P.; Moreira, D.R.M.; Soares, M.B.P.; da C. Pereira, A.L.; de L. Serafim, V.; Mendonça-Júnior, F.J.B.; do Carmo A., M.; de Moura, R.O.; et al. Correlation between DNA/hsa-interactions and antimalarial activity of acridine derivatives: Proposing a possible mechanism of action. J. Photochem. Photobiol B 2018, 189, 165–175. [Google Scholar] [CrossRef]
- Nafisi, S.; Panahyab, A.; Bagheri Sadeghi, G. Interactions between β-carboline alkaloids and bovine serum albumin: Investigation by spectroscopic approach. J. Lumin. 2012, 132, 2361–2366. [Google Scholar] [CrossRef]
- Fonte, M.; Tassi, N.; Fontinha, D.; Bouzon-Arnaiz, I.; Ferraz, R.; Araujo, M.J.; Fernandez-Busquets, X.; Prudencio, M.; Gomes, P.; Teixeira, C. 4,9-diaminoacridines and 4-aminoacridines as dual-stage antiplasmodial hits. ChemMedChem 2020. [Google Scholar] [CrossRef]
- Fonte, M.; Fagundes, N.; Gomes, A.; Ferraz, R.; Prudêncio, C.; Araújo, M.J.; Gomes, P.; Teixeira, C. Development of a synthetic route towards n4,n9-disubstituted 4,9-diaminoacridines: On the way to multi-stage antimalarials. Tetrahedron Lett. 2019, 60, 1166–1169. [Google Scholar] [CrossRef]
- Meunier, B. Hybrid molecules with a dual mode of action: Dream or reality? Acc. Chem. Res. 2008, 41, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, D.; Gupta, R.D.; Awasthi, S.K. Are antimalarial hybrid molecules a close reality or a distant dream? Antimicrob. Agents Chemother. 2017, 61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- King, H.D.; Wilson, W.D.; Gabbay, E.J. Interactions of some novel amide-linked bis(acridines) with deoxyribonucleic acid. Biochemistry 1982, 21, 4982–4989. [Google Scholar] [CrossRef] [PubMed]
- Girault, S.; Grellier, P.; Berecibar, A.; Maes, L.; Mouray, E.; Lemière, P.; Debreu, M.A.; Davioud-Charvet, E.; Sergheraert, C. Antimalarial, antitrypanosomal, and antileishmanial activities and cytotoxicity of bis(9-amino-6-chloro-2-methoxyacridines): Influence of the linker. J. Med. Chem. 2000, 43, 2646–2654. [Google Scholar] [CrossRef]
- Girault, S.; Delarue, S.; Grellier, P.; Berecibar, A.; Maes, L.; Quirijnen, L.; Lemiere, P.; Debreu-Fontaine, M.A.; Sergheraert, C. Antimalarial in-vivo activity of bis(9-amino-6-chloro-2-methoxyacridines). J. Pharm. Pharmacol. 2001, 53, 935–938. [Google Scholar] [CrossRef]
- Caffrey, C.R.; Steverding, D.; Swenerton, R.K.; Kelly, B.; Walshe, D.; Debnath, A.; Zhou, Y.-M.; Doyle, P.S.; Fafarman, A.T.; Zorn, J.A.; et al. Bis-acridines as lead antiparasitic agents: Structure-activity analysis of a discrete compound library in vitro. Antimicrob. Agents Chemother. 2007, 51, 2164–2172. [Google Scholar] [CrossRef] [Green Version]
- Crespo-Ortiz, M.P.; Wei, M.Q. Antitumor activity of artemisinin and its derivatives: From a well-known antimalarial agent to a potential anticancer drug. J. Biomed. Biotechnol. 2012, 2012, 247597. [Google Scholar] [CrossRef] [PubMed]
- Li, Y. Qinghaosu (artemisinin): Chemistry and pharmacology. Acta Pharmacol. Sin. 2012, 33, 1141–1146. [Google Scholar] [CrossRef] [Green Version]
- O’Neill, P.M.; Posner, G.H. A medicinal chemistry perspective on artemisinin and related endoperoxides. J. Med. Chem. 2004, 47, 2945–2964. [Google Scholar] [CrossRef]
- Araújo, N.C.P.; Barton, V.; Jones, M.; Stocks, P.A.; Ward, S.A.; Davies, J.; Bray, P.G.; Shone, A.E.; Cristiano, M.L.S.; O’Neill, P.M. Semi-synthetic and synthetic 1,2,4-trioxaquines and 1,2,4-trioxolaquines: Synthesis, preliminary sar and comparison with acridine endoperoxide conjugates. Bioorg. Med. Chem. Lett. 2009, 19, 2038–2043. [Google Scholar] [CrossRef]
- Jones, M.; Mercer, A.E.; Stocks, P.A.; La Pensée, L.J.I.; Cosstick, R.; Park, B.K.; Kennedy, M.E.; Piantanida, I.; Ward, S.A.; Davies, J.; et al. Antitumour and antimalarial activity of artemisinin–acridine hybrids. Bioorg. Med. Chem. Lett. 2009, 19, 2033–2037. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joubert, J.P.; Smit, F.J.; du Plessis, L.; Smith, P.J.; N’Da, D.D. Synthesis and in vitro biological evaluation of aminoacridines and artemisinin-acridine hybrids. Eur. J. Pharm. Sci. 2014, 56, 16–27. [Google Scholar] [CrossRef] [PubMed]
- Raynes, K.; Foley, M.; Tilley, L.; Deady, L.W. Novel bisquinoline antimalarials: Synthesis, antimalarial activity, and inhibition of haem polymerisation. Biochem. Pharmacol. 1996, 52, 551–559. [Google Scholar] [CrossRef]
- Vennerstrom, J.L.; Ager, A.L.; Dorn, A.; Andersen, S.L.; Gerena, L.; Ridley, R.G.; Milhous, W.K. Bisquinolines. 2. Antimalarial n,n-bis(7-chloroquinolin-4-yl)heteroalkanediamines. J. Med. Chem. 1998, 41, 4360–4364. [Google Scholar] [CrossRef] [PubMed]
- Liebman, K.M.; Burgess, S.J.; Gunsaru, B.; Kelly, J.X.; Li, Y.; Morrill, W.; Liebman, M.C.; Peyton, D.H. Unsymmetrical bisquinolines with high potency against p. Falciparum malaria. Molecules 2020, 25, 2251. [Google Scholar] [CrossRef]
- Kumar, A.; Srivastava, K.; Kumar, S.R.; Puri, S.K.; Chauhan, P.M. Synthesis of new 4-aminoquinolines and quinoline-acridine hybrids as antimalarial agents. Bioorg. Med. Chem. Lett. 2010, 20, 7059–7063. [Google Scholar] [CrossRef]
- Gutteridge, C.E.; Vo, J.V.; Tillett, C.B.; Vigilante, J.A.; Dettmer, J.R.; Patterson, S.L.; Werbovetz, K.A.; Capers, J.; Nichols, D.A.; Bhattacharjee, A.K.; et al. Antileishmanial and antimalarial chalcones: Synthesis, efficacy and cytotoxicity of pyridinyl and naphthalenyl analogs. J. Med. Chem. 2007, 3, 115–119. [Google Scholar] [CrossRef]
- Li, R.; Kenyon, G.L.; Cohen, F.E.; Chen, X.; Gong, B.; Dominguez, J.N.; Davidson, E.; Kurzban, G.; Miller, R.E.; Nuzum, E.O.; et al. In vitro antimalarial activity of chalcones and their derivatives. J. Med. Chem. 1995, 38, 5031–5037. [Google Scholar] [CrossRef]
- Tomar, V.; Bhattacharjee, G.; Kamaluddin; Rajakumar, S.; Srivastava, K.; Puri, S.K. Synthesis of new chalcone derivatives containing acridinyl moiety with potential antimalarial activity. Eur. J. Med. Chem. 2010, 45, 745–751. [Google Scholar] [CrossRef]
- Prajapati, S.P.; Kaushik, N.K.; Zaveri, M.; Mohanakrishanan, D.; Kawathekar, N.; Sahal, D. Synthesis, characterization and antimalarial evaluation of new β-benzoylstyrene derivatives of acridine. Arab. J. Chem. 2017, 10, S274–S280. [Google Scholar] [CrossRef] [Green Version]
- Pérez, B.; Teixeira, C.; Albuquerque, I.S.; Gut, J.; Rosenthal, P.J.; Prudêncio, M.; Gomes, P. Primacins, n-cinnamoyl-primaquine conjugates, with improved liver-stage antimalarial activity. MedChemComm 2012, 3, 1170–1172. [Google Scholar] [CrossRef] [Green Version]
- Pérez, B.; Teixeira, C.; Gut, J.; Rosenthal, P.J.; Gomes, J.R.; Gomes, P. Cinnamic acid/chloroquinoline conjugates as potent agents against chloroquine-resistant plasmodium falciparum. ChemMedChem 2012, 7, 1537–1540. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pérez, B.C.; Teixeira, C.; Albuquerque, I.S.; Gut, J.; Rosenthal, P.J.; Gomes, J.R.B.; Prudêncio, M.; Gomes, P. N-cinnamoylated chloroquine analogues as dual-stage antimalarial leads. J. Med. Chem. 2013, 56, 556–567. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pérez, B.; Teixeira, C.; Gomes, A.S.; Albuquerque, I.S.; Gut, J.; Rosenthal, P.J.; Prudêncio, M.; Gomes, P. In vitro efficiency of 9-(n-cinnamoylbutyl)aminoacridines against blood- and liver-stage malaria parasites. Bioorg. Med. Chem. Lett. 2013, 23, 610–613. [Google Scholar] [CrossRef] [Green Version]
- Gomes, A.; Pérez, B.; Albuquerque, I.; Machado, M.; Prudêncio, M.; Nogueira, F.; Teixeira, C.; Gomes, P. N-cinnamoylation of antimalarial classics: Quinacrine analogues with decreased toxicity and dual-stage activity. ChemMedChem 2014, 9, 305–310. [Google Scholar] [CrossRef] [Green Version]
- Gemma, S.; Campiani, G.; Butini, S.; Kukreja, G.; Coccone, S.S.; Joshi, B.P.; Persico, M.; Nacci, V.; Fiorini, I.; Novellino, E.; et al. Clotrimazole scaffold as an innovative pharmacophore towards potent antimalarial agents: Design, synthesis, and biological and structure–activity relationship studies. J. Med. Chem. 2008, 51, 1278–1294. [Google Scholar] [CrossRef] [Green Version]
- Gemma, S.; Campiani, G.; Butini, S.; Kukreja, G.; Joshi, B.P.; Persico, M.; Catalanotti, B.; Novellino, E.; Fattorusso, E.; Nacci, V.; et al. Design and synthesis of potent antimalarial agents based on clotrimazole scaffold: Exploring an innovative pharmacophore. J. Med. Chem. 2007, 50, 595–598. [Google Scholar] [CrossRef]
- Gemma, S.; Campiani, G.; Butini, S.; Joshi, B.P.; Kukreja, G.; Coccone, S.S.; Bernetti, M.; Persico, M.; Nacci, V.; Fiorini, I.; et al. Combining 4-aminoquinoline- and clotrimazole-based pharmacophores toward innovative and potent hybrid antimalarials. J. Med. Chem. 2009, 52, 502–513. [Google Scholar] [CrossRef] [Green Version]
- Kumar, A.; Srivastava, K.; Raja Kumar, S.; Puri, S.K.; Chauhan, P.M.S. Synthesis of 9-anilinoacridine triazines as new class of hybrid antimalarial agents. Bioorg. Med. Chem. Lett. 2009, 19, 6996–6999. [Google Scholar] [CrossRef]
- Solaja, B.A.; Opsenica, D.; Smith, K.S.; Milhous, W.K.; Terzić, N.; Opsenica, I.; Burnett, J.C.; Nuss, J.; Gussio, R.; Bavari, S. Novel 4-aminoquinolines active against chloroquine-resistant and sensitive p. Falciparum strains that also inhibit botulinum serotype a. J. Med. Chem. 2008, 51, 4388–4391. [Google Scholar] [CrossRef]
- Tot, M.; Opsenica, D.; Mitric, M.; Burnett, J.; Gomba, L.; Bavari, S.; Solaja, B. New 9-aminoacridine derivatives as inhibitors of botulinum neurotoxins and P. falciparum malaria. J. Serb. Chem. Soc. 2013, 78, 1847–1864. [Google Scholar] [CrossRef]
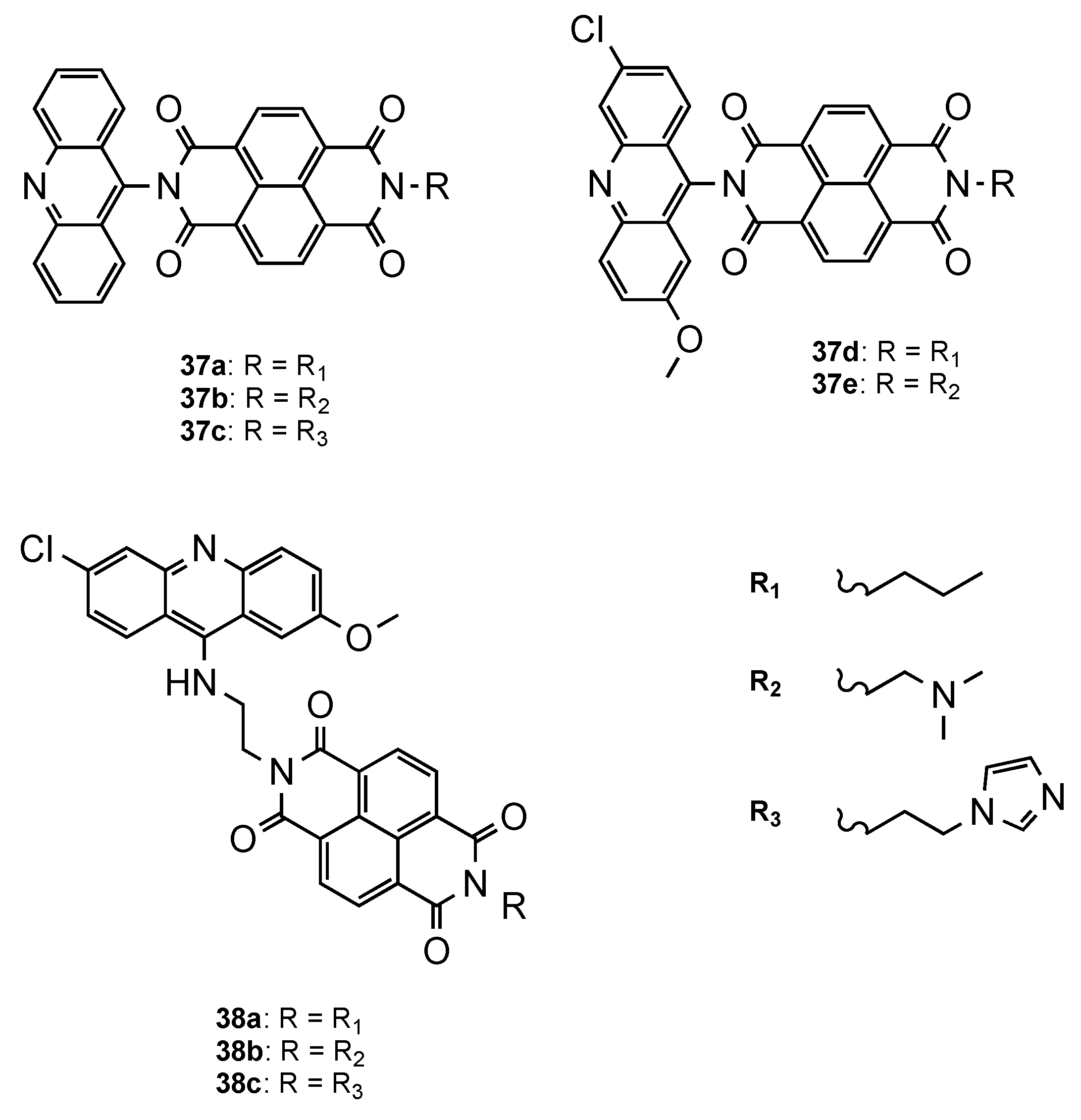
- Dana, S.; Keshri, S.K.; Shukla, J.; Vikramdeo, K.S.; Mondal, N.; Mukhopadhyay, P.; Dhar, S.K. Design, synthesis and evaluation of bifunctional acridinine−naphthalenediimide redox-active conjugates as antimalarials. ACS Omega 2016, 1, 318–333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pandey, S.K.; Biswas, S.; Gunjan, S.; Chauhan, B.S.; Singh, S.K.; Srivastava, K.; Singh, S.; Batra, S.; Tripathi, R. Pyrrolidine-acridine hybrid in artemisinin-based combination: A pharmacodynamic study. Parasitology 2016, 143, 1421–1432. [Google Scholar] [CrossRef] [PubMed]
- Dana, S.; Valissery, P.; Kumar, S.; Gurung, S.K.; Mondal, N.; Dhar, S.K.; Mukhopadhyay, P. Synthesis of novel ciprofloxacin-based hybrid molecules toward potent antimalarial activity. ACS Med. Chem. Lett. 2020, 11, 1450–1456. [Google Scholar] [CrossRef]
- Divo, A.A.; Sartorelli, A.C.; Patton, C.L.; Bia, F.J. Activity of fluoroquinolone antibiotics against plasmodium falciparum in vitro. Antimicrob. Agents Chemother. 1988, 32, 1182–1186. [Google Scholar] [CrossRef] [Green Version]
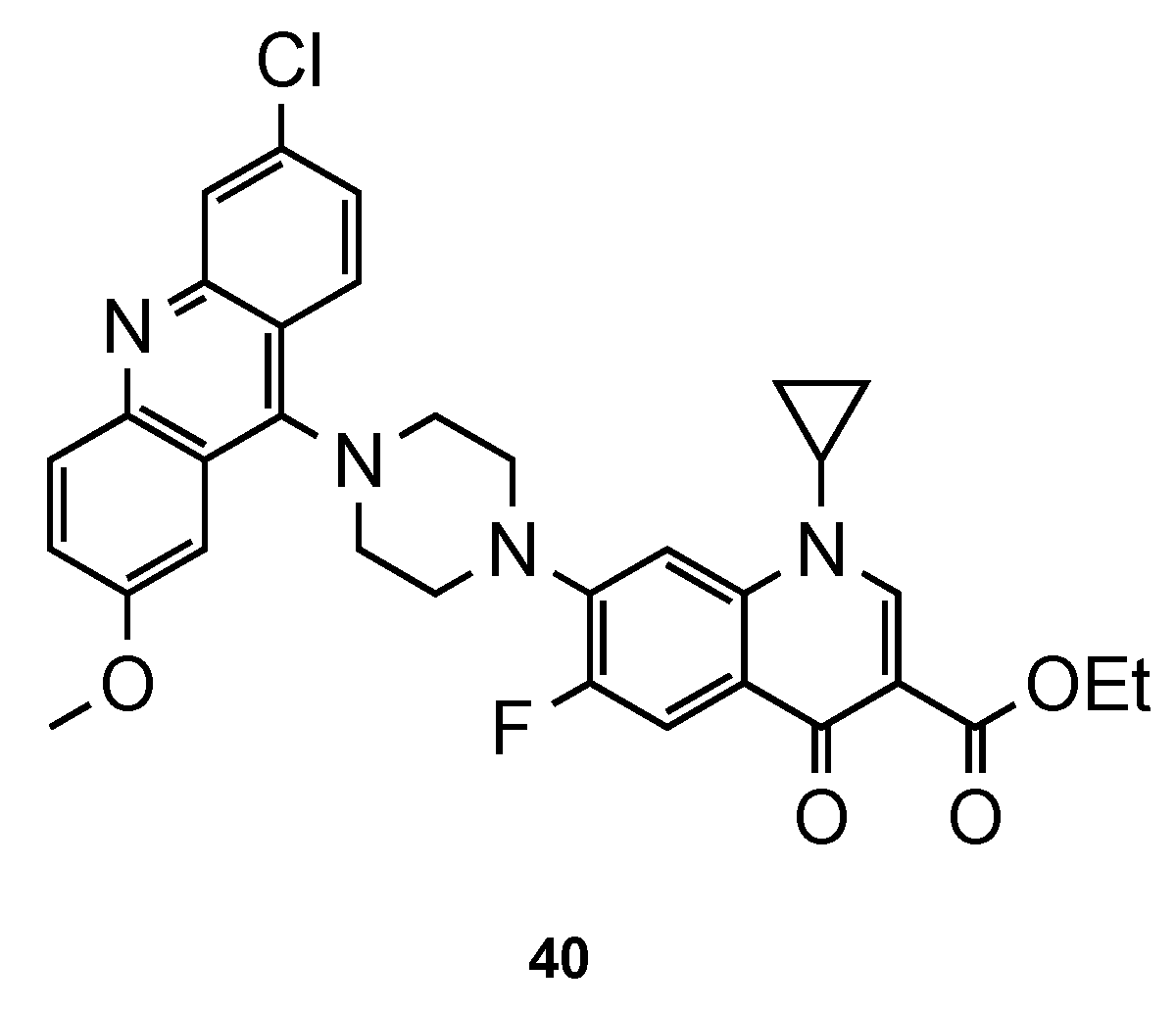
- Dubar, F.; Anquetin, G.; Pradines, B.; Dive, D.; Khalife, J.; Biot, C. Enhancement of the antimalarial activity of ciprofloxacin using a double prodrug/bioorganometallic approach. J. Med. Chem. 2009, 52, 7954–7957. [Google Scholar] [CrossRef]
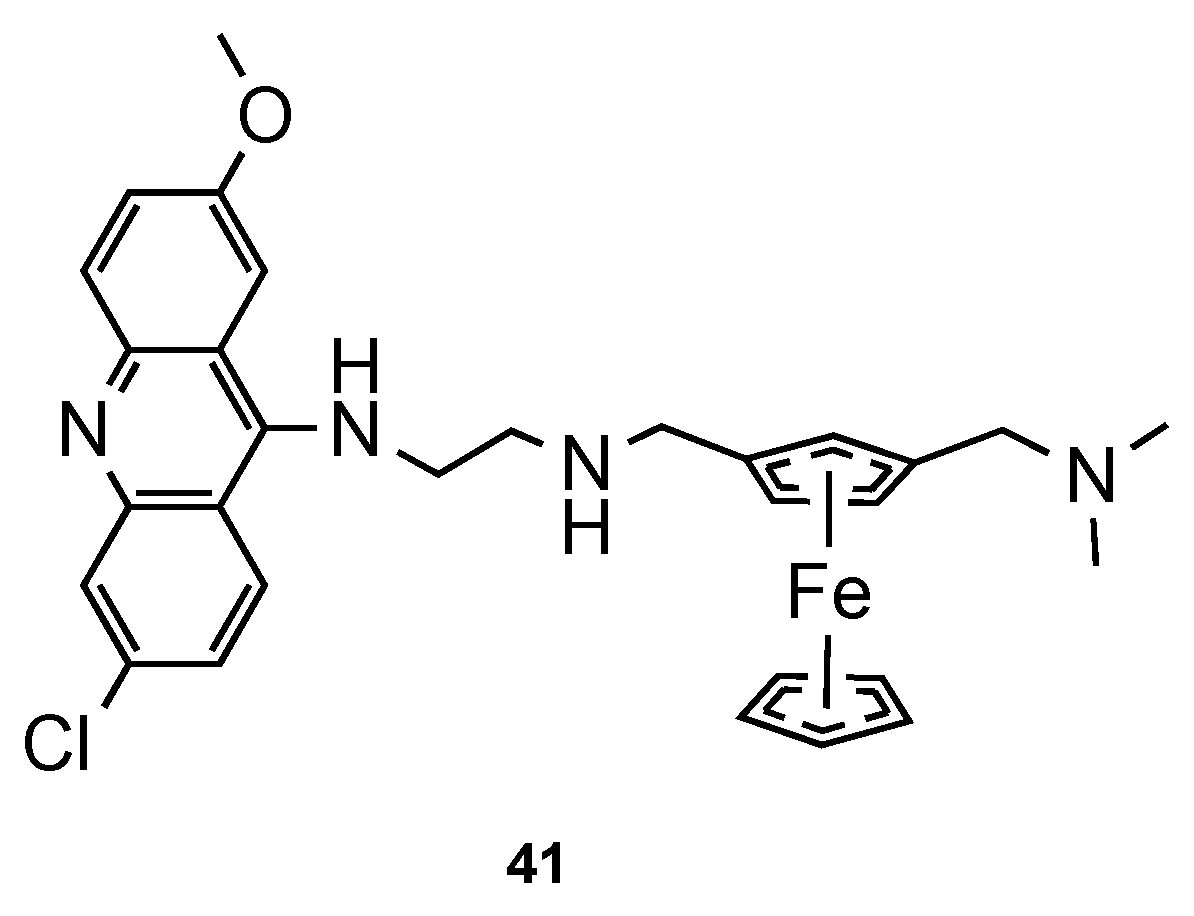
- Blackie, M.A.L.; Beagley, P.; Croft, S.L.; Kendrick, H.; Moss, J.R.; Chibale, K. Metallocene-based antimalarials: An exploration into the influence of the ferrocenyl moiety on in vitro antimalarial activity in chloroquine-sensitive and chloroquine-resistant strains of plasmodium falciparum. Bioorg. Med. Chem. 2007, 15, 6510–6516. [Google Scholar] [CrossRef]
- Peter, S.; Aderibigbe, B.A. Ferrocene-based compounds with antimalaria/anticancer activity. Molecules 2019, 24, 3604. [Google Scholar] [CrossRef] [Green Version]












Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fonte, M.; Tassi, N.; Gomes, P.; Teixeira, C. Acridine-Based Antimalarials—From the Very First Synthetic Antimalarial to Recent Developments. Molecules 2021, 26, 600. https://doi.org/10.3390/molecules26030600
Fonte M, Tassi N, Gomes P, Teixeira C. Acridine-Based Antimalarials—From the Very First Synthetic Antimalarial to Recent Developments. Molecules. 2021; 26(3):600. https://doi.org/10.3390/molecules26030600
Chicago/Turabian StyleFonte, Mélanie, Natália Tassi, Paula Gomes, and Cátia Teixeira. 2021. "Acridine-Based Antimalarials—From the Very First Synthetic Antimalarial to Recent Developments" Molecules 26, no. 3: 600. https://doi.org/10.3390/molecules26030600







