Inactivation and Membrane Damage Mechanism of Slightly Acidic Electrolyzed Water on Pseudomonas deceptionensis CM2
Abstract
:1. Introduction
2. Results and Discussions
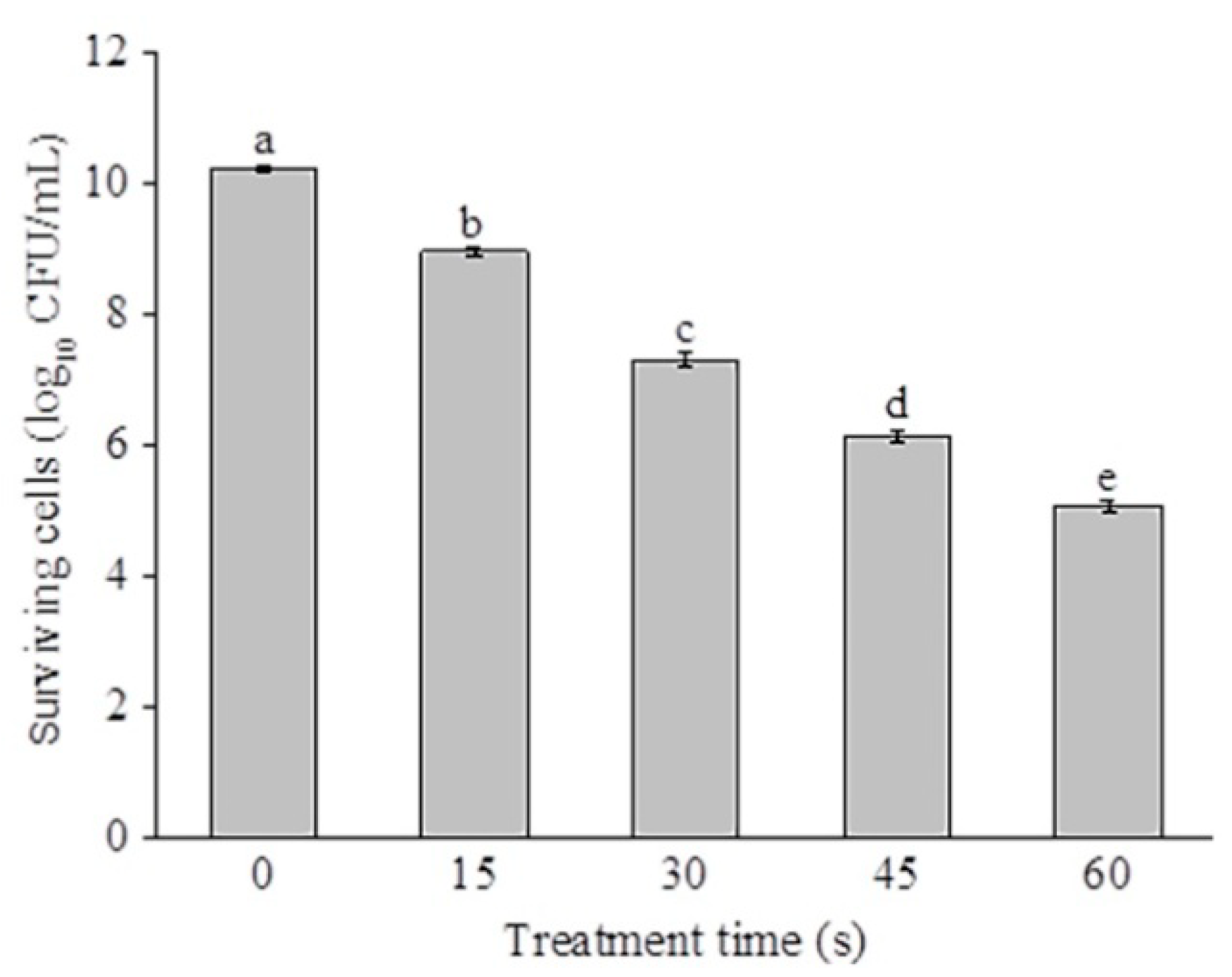
2.1. Disinfection Efficacy of SAEW against P. deceptionensis CM2
2.2. SAEW-Caused Morphological Changes of Bacterial Cells
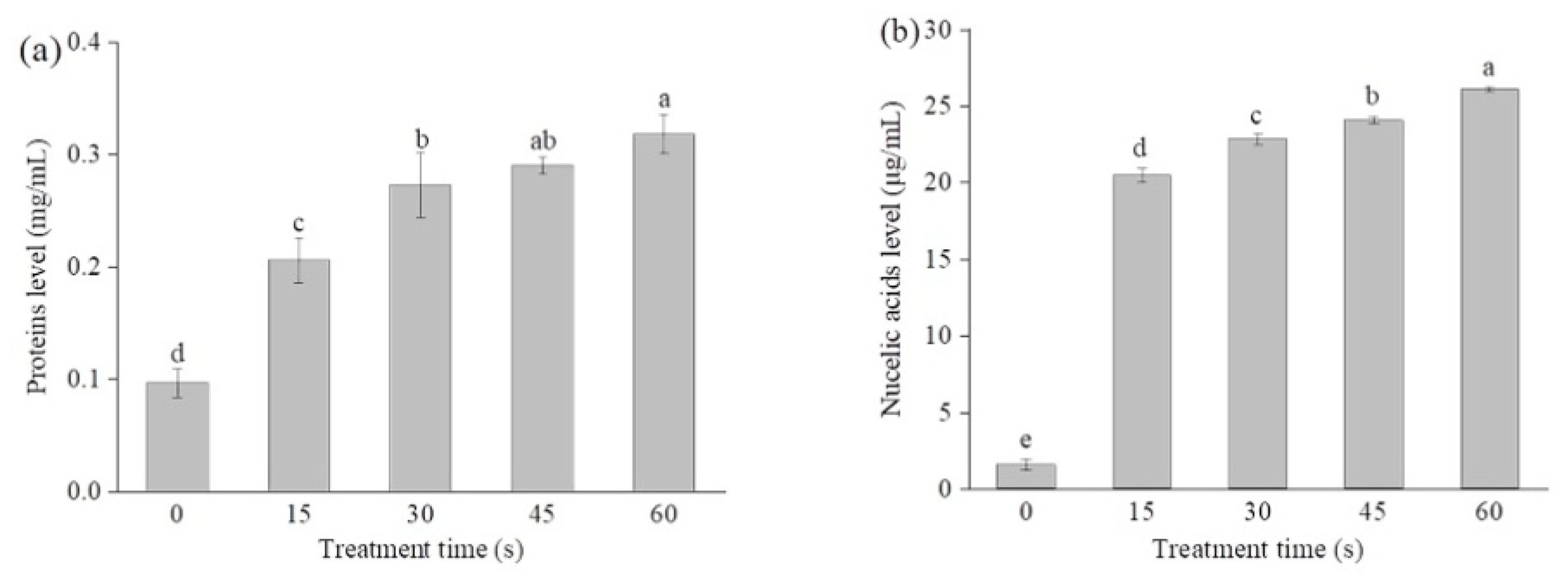
2.3. SAEW-Caused Alterations in the Cell Membrane Permeability
2.4. Changes in the Cytoplasmic Membrane
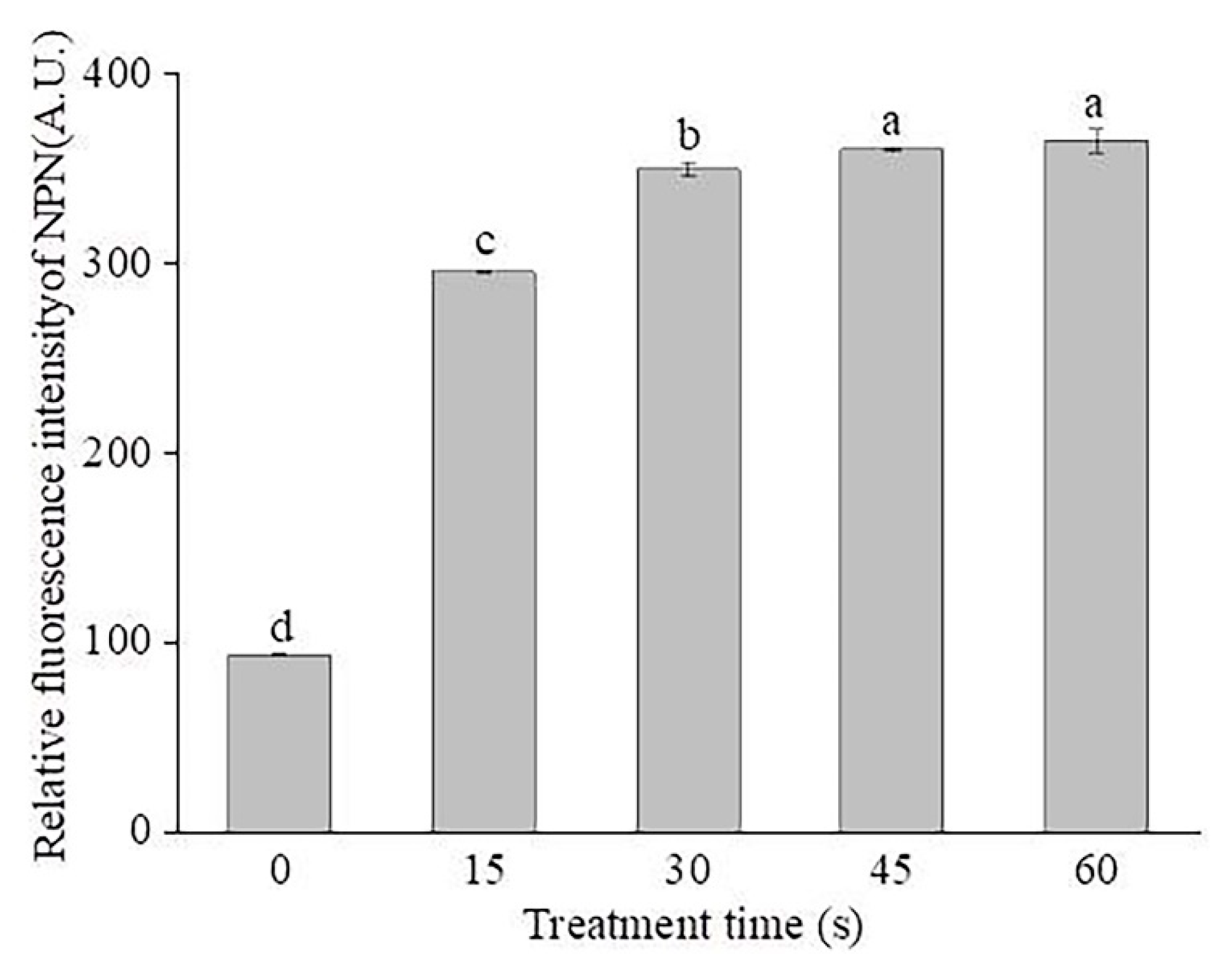
2.5. Changes in the Outer Membrane Permeabilization
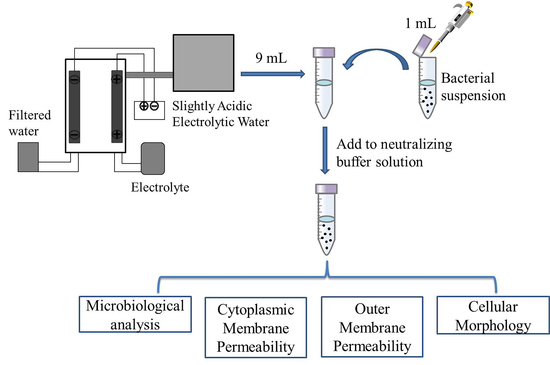
3. Materials and Methods
3.1. Bacterial Culture and Preparation of Inoculums
3.2. Preparation of SAEW
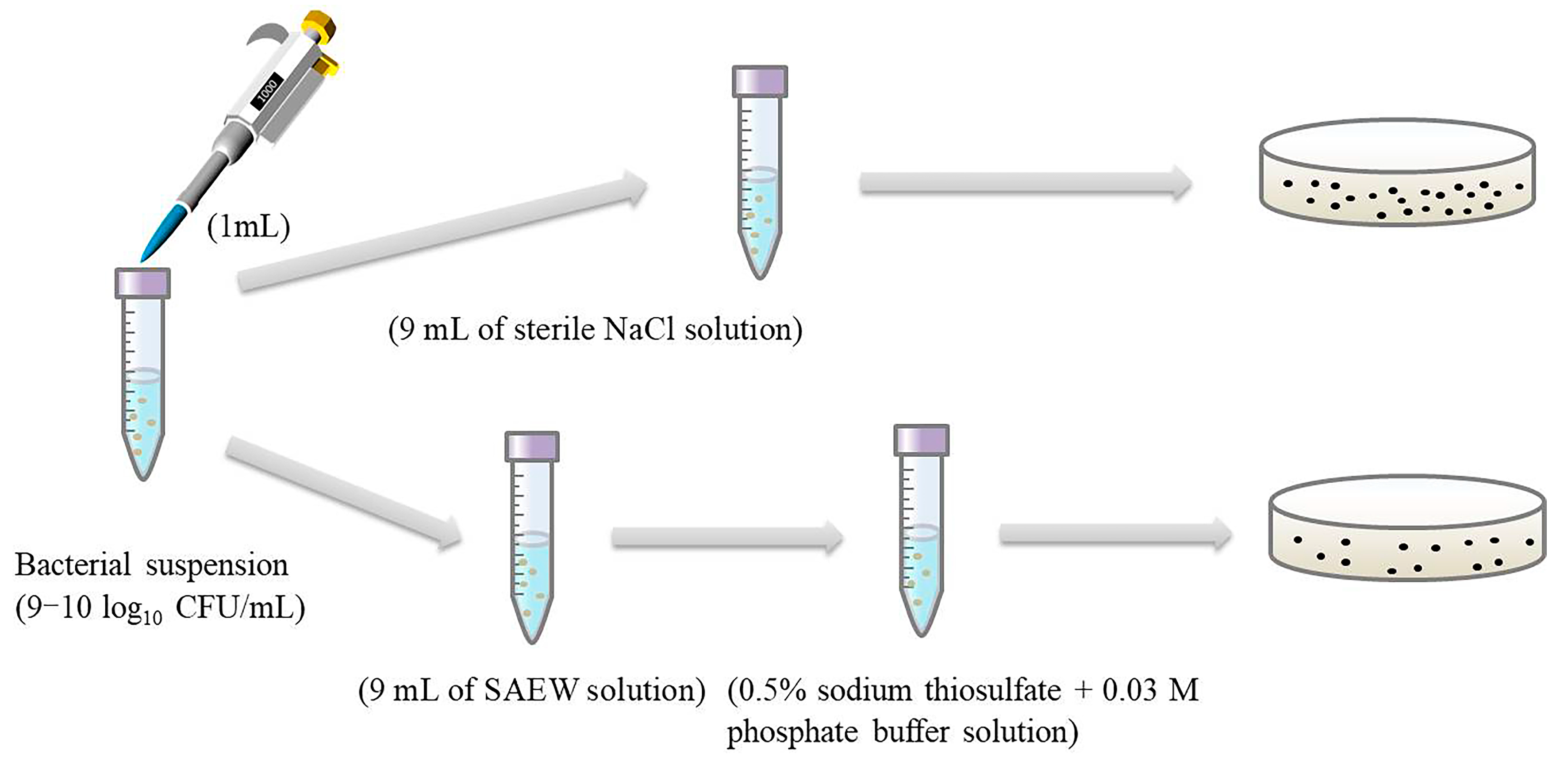
3.3. Antimicrobial Test of SAEW
3.4. Analysis of Cellular Morphology
3.5. Leakage of Extracellular Proteins and Nucleic Acids
3.6. Assay of Cytoplasmic Membrane Permeability
3.7. NPN Uptake Assay
3.8. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Date Availability Statement
Acknowledgments
Conflicts of Interest
References
- Odeyemi, O.A.; Alegbeleye, O.O.; Strateva, M.; Stratev, D. Understanding spoilage microbial community and spoilage mechanisms in foods of animal origin. Compr. Rev. Food Sci. Food Saf. 2020, 19, 311–331. [Google Scholar] [CrossRef] [Green Version]
- Gram, L.; Ravn, L.; Rasch, M.; Bruhn, J.B.; Christensen, A.B.; Givskov, M. Food spoilage-interactions between food spoilage bacteria. Int. J. Food Microbiol. 2002, 78, 79–97. [Google Scholar] [CrossRef]
- Iulietto, M.F.; Sechi, P.; Borgoni, E.; Cenci-Goga, B.T. Meat Spoilage: A critical review of a neglected alteration due to ropy slime producing bacteria. Ital. J. Anim. Sci. 2015, 14, 4011. [Google Scholar] [CrossRef]
- Wang, G.Y.; Wang, H.H.; Han, Y.W.; Xing, T.; Ye, K.P.; Xu, X.L.; Zhou, G.H. Evaluation of the spoilage potential of bacteria isolated from chilled chicken in vitro and in situ. Food Microbiol. 2017, 63, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Correa, A.P.F.; Doroit, D.J.; Velho, R.V.; Brandelli, A. Hydrolytic potential of a psychrotrophic Pseudomonas isolated from refrigerated raw milk. Braz. J. Microbiol. 2011, 42, 1479–1484. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Franzetti, L.; Scarpellini, M. Characterisation of Pseudomonas spp. isolated from foods. Ann. Microbiol. 2007, 57, 39–47. [Google Scholar] [CrossRef]
- Jamnrak, A.R.; Vukušić, T.; Donsi, F.; Paniwnyk, L.; Djekic, I. Three pillars of novel nonthermal food technologies: Food safety, quality, and environment. J. Food Qual. 2018, 2018, 8619707. [Google Scholar]
- D’Evoli, L.; Lombardi-Boccia, G.; Lucarini, M. Influence of heat treatments on carotenoid content of cherry tomatoes. Foods 2013, 2, 352–363. [Google Scholar] [CrossRef] [Green Version]
- Mei, J.; Ma, X.; Xie, J. Review on natural preservatives for extending fish shelf life. Foods 2019, 10, 490. [Google Scholar] [CrossRef] [Green Version]
- Gadelha, J.R.; Allende, A.; López-Gálvez, F.; Fernández, P.; Gil, M.I.; Egea, J.A. Chemical risks associated with ready-to-eat vegetables: Quantitative analysis to estimate formation and/or accumulation of disinfection byproducts during washing. EFSA J. 2019, 17, e170913. [Google Scholar]
- Zhao, Y.M.; de Alba, M.; Sun, D.W.; Tiwari, B. Principles and recent applications of novel non-thermal processing technologies for the fish industry-a review. Crit. Rev. Food Sci. Nutr. 2019, 59, 728–742. [Google Scholar] [CrossRef]
- Rahman, S.M.E.; Khan, I.; Oh, D.H. Electrolyzed water as a novel sanitizer in the food industry: Current trends and future perspectives. Compr. Rev. Food Sci. Food Saf. 2016, 15, 471–490. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Z.H.; Zhou, B.; Li, X.T.; Li, S.G. Effect of alkaline electrolyzed water on physicochemical and structural properties of apricot protein isolate. Food Sci. Biotechnol. 2018, 28, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Ongeng, D.; Devlieghere, F.; Debevere, J.; Coosemans, J.; Ryckeboer, J. The efficacy of electrolysed oxidising water for inactivating spoilage microorganisms in process water and on minimally processed vegetables. Int. J. Food Microbiol. 2006, 109, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Gómez-López, V.M.; Ragaert, P.; Ryckeboer, J.; Jeyachchandran, V.; Debevere, J.; Devlieghere, F. Shelf-life of minimally processed cabbage treated with neutral electrolysed oxidising water and stored under equilibrium modified atmosphere. Int. J. Food Microbiol. 2007, 117, 91–98. [Google Scholar] [CrossRef]
- Xiang, Q.S.; Kang, C.D.; Niu, L.Y.; Zhao, D.B.; Li, K.; Bai, Y.H. Antibacterial activity and a membrane damage mechanism of plasma-activated water against Pseudomonas deceptionensis CM2. LWT-Food Sci. Technol. 2018, 96, 395–401. [Google Scholar] [CrossRef]
- Zeng, X.P.; Tang, W.W.; Ye, G.Q.; Ouyang, T.; Tian, L.; Ni, Y.M.; Li, P. Studies on disinfection mechanism of electrolyzed oxidizing water on E. coli and Staphylococcus aureus. J. Food Sci. 2010, 75, M253–M260. [Google Scholar] [CrossRef]
- Fenner, D.; Burge, B.; Kayser, H.; Wittenbrink, M. The anti-microbial activity of electrolysed oxidizing water against microorganisms relevant in veterinary medicine. J. Vet. Med. Ser. B 2006, 53, 133–137. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.B.; Guo, J.Y.; Rahman, S.M.E.; Ahn, J.; Oh, D.H. Synergistic effect of electrolyzed water and citric acid against Bacillus cereus cells and spores on cereal grains. J. Food Sci. 2009, 74, M185–M189. [Google Scholar] [CrossRef]
- Kang, C.D.; Xiang, Q.S.; Zhao, D.B.; Wang, W.J.; Niu, L.Y.; Bai, Y.H. Inactivation of Pseudomonas deceptionensis CM2 on chicken breasts using plasma-activated water. J. Food Sci. Technol. 2019, 56, 4938–4945. [Google Scholar] [CrossRef]
- Len, S.V.; Hung, Y.C.; Erickson, M.; Kim, C. Ultraviolet spectrophotometric characterization and bactericidal properties of electrolyzed oxidizing water as influenced by amperage and pH. J. Food Protect. 2000, 63, 1534–1537. [Google Scholar] [CrossRef]
- Koseki, S.; Yoshida, K.; Isobe, S.; Itoh, K. Efficacy of acidic electrolyzed water for microbial decontamination of cucumbers and strawberries. J. Food Protect. 2004, 67, 1247–1251. [Google Scholar] [CrossRef]
- Zhang, C.L.; Lu, Z.H.; Li, Y.Y.; Shang, Y.C.; Zhang, G.; Cao, W. Reduction of Escherichia coli O157:H7 and Salmonella enteritidis on mung bean seeds and sprouts by slightly acidic electrolyzed water. Food Control 2011, 22, 792–796. [Google Scholar] [CrossRef]
- Huang, Y.R.; Hung, Y.C.; Hsu, S.Y.; Huang, Y.; Hwang, D.F. Application of electrolyzed water in the food industry. Food Control 2008, 19, 329–345. [Google Scholar] [CrossRef]
- Ding, T.; Xuan, X.T.; Li, J.; Chen, S.G.; Liu, D.H.; Ye, X.Q.; Shi, J.; Xue, S.J. Disinfection efficacy and mechanism of slightly acidic electrolyzed water on Staphylococcus aureus in pure culture. Food Control 2016, 60, 505–510. [Google Scholar] [CrossRef]
- Ersoy, Z.G.; Dinc, O.; Cinar, B.; Gedik, S.T.; Dimoglo, A. Comparative evaluation of disinfection mechanism of sodium hypochlorite, chlorine dioxide and electroactivated water on Enterococcus faecalis. LWT-Food Sci. Technol. 2018, 102, 205–213. [Google Scholar] [CrossRef]
- Casares, D.; Escribá, P.V.; Rosselló, C.A. Membrane lipid composition: Effect on membrane and organelle structure, function and compartmentalization and therapeutic avenues. Int. J. Mol. Sci. 2019, 20, 2167. [Google Scholar] [CrossRef] [Green Version]
- Rowlett, V.W.; Mallampalli, V.K.P.S.; Karlstaedt, A.; Dowhan, W.; Taegtmeyer, H.; Margolin, W.; Vitrac, H. Impact of membrane phospholipid alterations in Escherichia coli on cellular function and bacterial stress adaptation. J. Bacteriol. 2017, 199, e00849. [Google Scholar] [CrossRef] [Green Version]
- Berney, M.; Hammes, F.; Bosshard, F.; Weilenmann, H.U.; Egli, T. Assessment and interpretation of bacterial viability by using the LIVE/DEAD BacLight kit in combination with flow cytometry. Appl. Environ. Microbiol. 2007, 73, 3283–3290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ye, Z.Y.; Wang, S.; Chen, T.; Gao, W.S.; Zhu, S.M.; He, J.S.; Han, Z.Y. Inactivation mechanism of Escherichia coli induced by slightly acidic electrolyzed water. Sci. Rep. 2017, 7, 6279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeng, X.P.; Ye, G.Q.; Tang, W.W.; Ouyang, T.; Tian, L.; Ni, Y.M.; Li, P. Fungicidal efficiency of electrolyzed oxidizing water on Candida albicans and its biochemical mechanism. J. Biosci. Bioeng. 2011, 112, 86–91. [Google Scholar] [CrossRef] [PubMed]
- Breijyeh, Z.; Jubeh, B.; Karaman, R. Resistance of gram-negative bacteria to current antibacterial agents and approaches to resolve it. Molecules 2020, 25, 1340. [Google Scholar] [CrossRef] [Green Version]
- Gravel, J.; Paradis-Bleau, C.; Schmitzer, A.R. Adaptation of a bacterial membrane permeabilization assay for quantitative evaluation of benzalkonium chloride as a membrane-disrupting agent. Medchemcomm 2017, 8, 1408–1413. [Google Scholar] [CrossRef]
- Guo, Y.R.; Liu, Y.; Zhang, Z.H.; Chen, M.H.; Zhang, D.X.; Tian, C.L.; Liu, M.C.; Jiang, G.T. The antibacterial activity and mechanism of action of luteolin against trueperella pyogenes. Infect. Drug Resist. 2020, 2020, 1697–1711. [Google Scholar] [CrossRef]
- Liao, L.B.; Chen, W.M.; Xiao, X.M. The generation and inactivation mechanism of oxidation-reduction potential of electrolyzed oxidizing water. J. Food Eng. 2007, 78, 1326–1332. [Google Scholar] [CrossRef]
- Park, H.; Hung, Y.C.; Chung, D.H. Effects of chlorine and pH on efficacy of electrolyzed water for inactivating Escherichia coli O157:H7 and Listeria monocytogenes. Int. J. Food Microbiol. 2004, 91, 13–18. [Google Scholar] [CrossRef]
- Orejel, J.R.C.; Cano-Buendia, J.A. Applications of electrolyzed water as a sanitizer in the food and animal-by products industry. Processes 2020, 8, 534. [Google Scholar] [CrossRef]
- Cao, W.; Zhu, Z.W.; Shi, Z.X.; Wang, C.Y.; Li, B.M. Efficiency of slightly acidic electrolyzed water for inactivation of Salmonella enteritidis and its contaminated shell eggs. Int. J. Food Microbiol. 2009, 130, 88–93. [Google Scholar] [CrossRef]
- Xiang, Q.; Liu, X.; Li, J.; Liu, S.; Zhang, H.; Bai, Y. Effects of dielectric barrier discharge plasma on the inactivation of Zygosaccharomyces rouxii and quality of apple juice. Food Chem. 2018, 254, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Boda, S.K.; Broda, J.; Schiefer, F.; Weber-Heynemann, J.; Hoss, M.; Simon, U.; Basu, B.; Jahnen-Dechent, W. Cytotoxicity of ultrasmall gold nanoparticles on planktonic and biofilm encapsulated gram-positive Staphylococci. Small 2015, 11, 3183–3193. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Zhang, M.; Meng, X.; He, X.; Zhao, W.; Liu, Y.; He, Y. Inactivation and Membrane Damage Mechanism of Slightly Acidic Electrolyzed Water on Pseudomonas deceptionensis CM2. Molecules 2021, 26, 1012. https://doi.org/10.3390/molecules26041012
Liu X, Zhang M, Meng X, He X, Zhao W, Liu Y, He Y. Inactivation and Membrane Damage Mechanism of Slightly Acidic Electrolyzed Water on Pseudomonas deceptionensis CM2. Molecules. 2021; 26(4):1012. https://doi.org/10.3390/molecules26041012
Chicago/Turabian StyleLiu, Xiao, Mingli Zhang, Xi Meng, Xiangli He, Weidong Zhao, Yongji Liu, and Yu He. 2021. "Inactivation and Membrane Damage Mechanism of Slightly Acidic Electrolyzed Water on Pseudomonas deceptionensis CM2" Molecules 26, no. 4: 1012. https://doi.org/10.3390/molecules26041012