Inorganic Nanoparticle-Loaded Exosomes for Biomedical Applications
Abstract
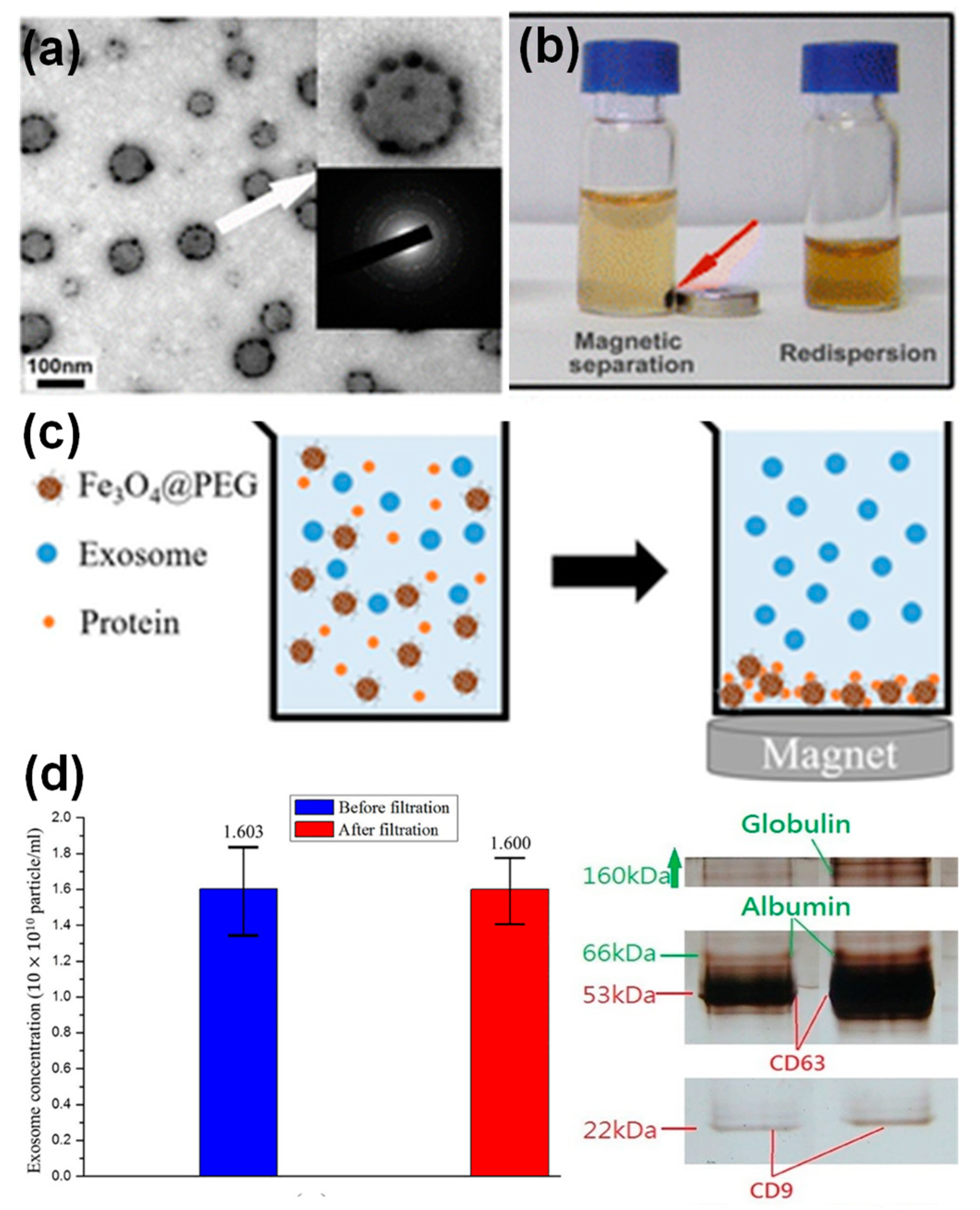
:1. Introduction
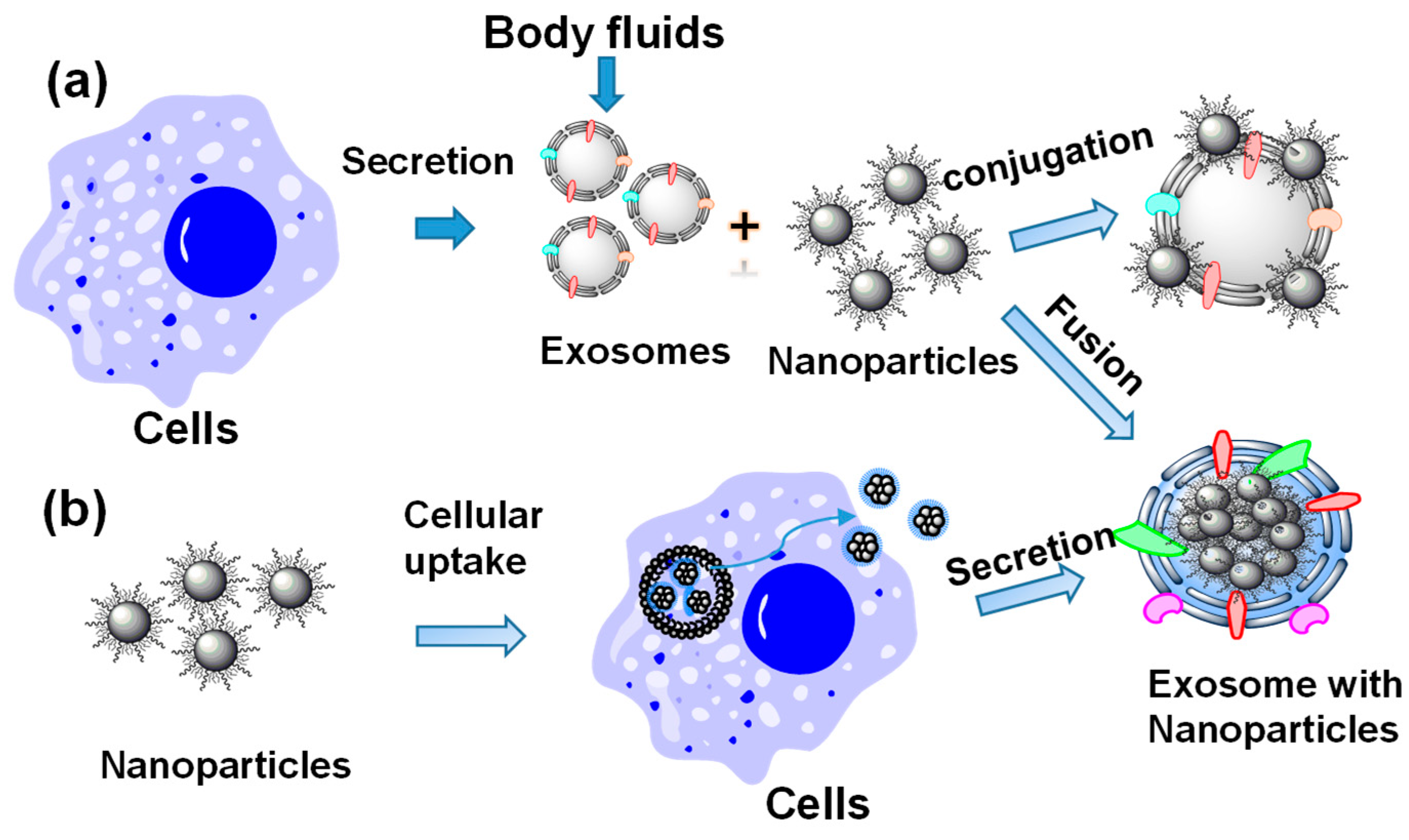
2. Preparation of Nanoparticle-Loaded Exosomes
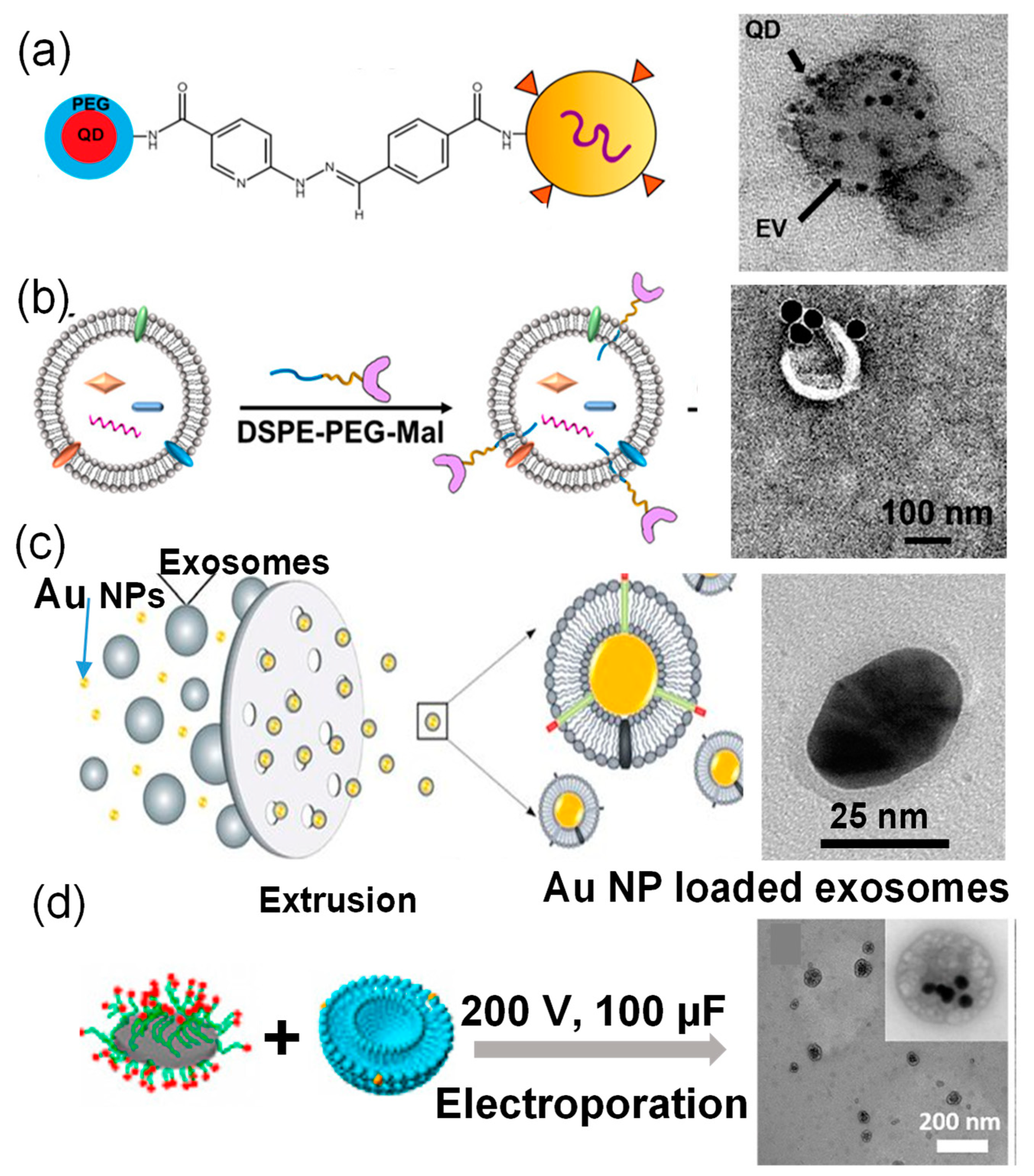
2.1. Preparation of Nanoparticle-Loaded Exosomes Post-Exosome Formation
2.2. Preparation of Nanoparticle-Loaded Exosomes during Exosome Formation through Biological Pathways
3. Applications of Inorganic Nanoparticle-Loaded Exosomes
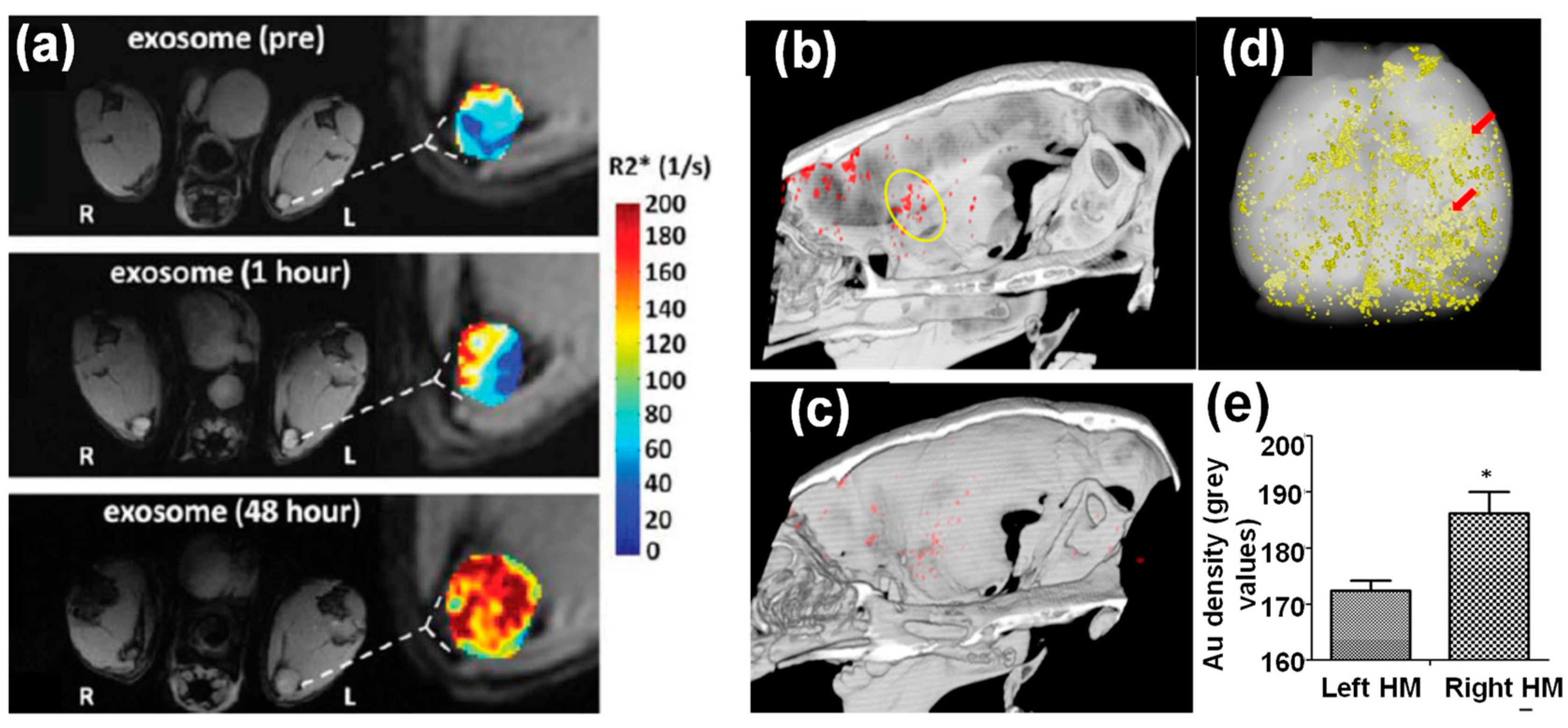
3.1. Inorganic Nanoparticle-Loaded Exosomes for Bioimaging
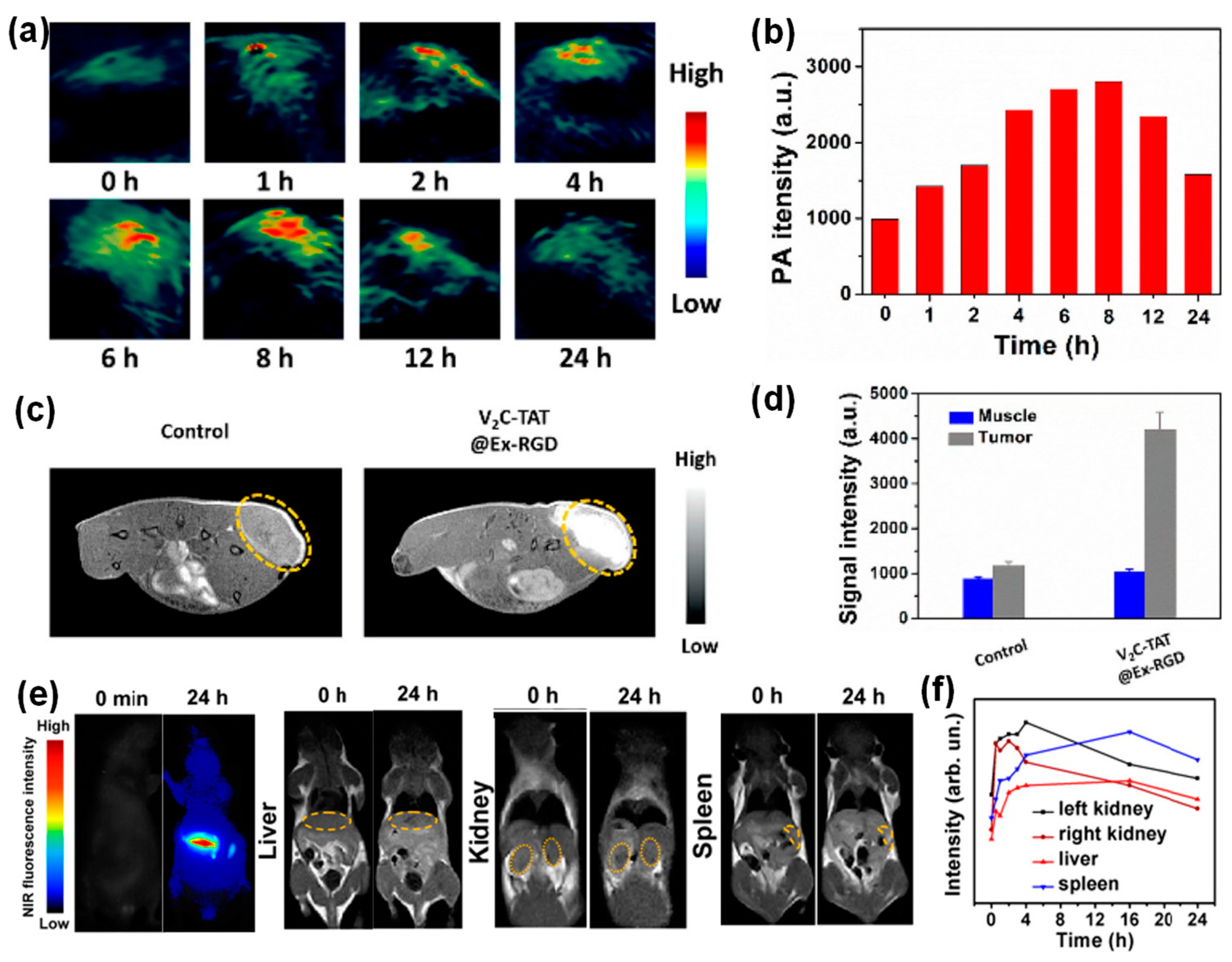
3.2. Inorganic Nanoparticle Loaded Exosomes for Theranostic Applications
3.3. Inorganic Nanoparticle-Loaded Exosomes for Other Applications
4. Summary and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Thery, C.; Zitvogel, L.; Amigorena, S. Exosomes: Composition, biogenesis and function. Nat. Rev. Immunol. 2002, 2, 569–579. [Google Scholar] [CrossRef]
- Hessvik, N.P.; Llorente, A. Current knowledge on exosome biogenesis and release. Cell. Mol. Life Sci. 2018, 75, 193–208. [Google Scholar] [CrossRef] [Green Version]
- Elliott, R.O.; He, M. Unlocking the Power of Exosomes for Crossing Biological Barriers in Drug Delivery. Pharmaceutics 2021, 13, 122. [Google Scholar] [CrossRef]
- He, C.; Zheng, S.; Luo, Y.; Wang, B. Exosome Theranostics: Biology and Translational Medicine. Theranostics 2018, 8, 237–255. [Google Scholar] [CrossRef]
- Lobb, R.J.; Lima, L.G.; Moller, A. Exosomes: Key mediators of metastasis and pre-metastatic niche formation. Semi. Cell Dev. Biol. 2017, 67, 3–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steinbichler, T.B.; Dudas, J.; Riechelmann, H.; Skvortsova, I.I. The role of exosomes in cancer metastasis. Semin. Cancer Biol. 2017, 44, 170–181. [Google Scholar] [CrossRef] [PubMed]
- Ha, D.; Yang, N.; Nadithe, V. Exosomes as therapeutic drug carriers and delivery vehicles across biological membranes: Current perspectives and future challenges. Acta Pharm. Sin. 2016, 6, 287–296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luan, X.; Sansanaphongpricha, K.; Myers, I.; Chen, H.; Yuan, H.; Sun, D. Engineering exosomes as refined biological nanoplatforms for drug delivery. Acta Pharm. Sin. 2017, 38, 754–763. [Google Scholar] [CrossRef] [Green Version]
- Horibe, S.; Tanahashi, T.; Kawauchi, S.; Murakami, Y.; Rikitake, Y. Mechanism of recipient cell-dependent differences in exosome uptake. BMC Cancer 2018, 18, 47. [Google Scholar] [CrossRef] [Green Version]
- Conlan, R.S.; Pisano, S.; Oliveira, M.I.; Ferrari, M.; Pinto, I.M. Exosomes as Reconfigurable Therapeutic Systems. Trends Mol. Med. 2017, 23, 636–650. [Google Scholar] [CrossRef]
- Zhang, Z.; Dombroski, J.A.; King, M.R. Engineering of Exosomes to Target Cancer Metastasis. Cell. Mol. Bioeng. 2020, 13, 1–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, W.; Duan, Z. Roles of exosomes in liver metastases: Novel diagnosis and treatment choices. J. Cell. Physiol. 2019, 234, 21588–21600. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Wei, W.; Wang, X.; Li, C.; Bjorklund, M.; Ouyang, H.W. Stem cell derived exosomes: microRNA therapy for age-related musculoskeletal disorders. Biomaterials 2019, 224, 119492. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Ren, S.; Duscher, D.; Kang, Y.; Liu, Y.; Wang, C.; Yuan, M.; Guo, G.; Xiong, H.; Zhan, P.; et al. Exosomes from human adipose-derived stem cells promote sciatic nerve regeneration via optimizing Schwann cell function. J. Cell. Physiol. 2019, 234, 23097–23110. [Google Scholar] [CrossRef] [PubMed]
- Singla, R.; Garner, K.H.; Samsam, M.; Cheng, Z.; Singla, D.K. Exosomes derived from cardiac parasympathetic ganglionic neurons inhibit apoptosis in hyperglycemic cardiomyoblasts. Mol. Cell. Biochem. 2019, 462, 1–10. [Google Scholar] [CrossRef]
- Betzer, O.; Barnoy, E.; Sadan, T.; Elbaz, I.; Braverman, C.; Liu, Z.; Popovtzer, R. Advances in imaging strategies for in vivo tracking of exosomes. Interdiscip. Rev. Nanomed. Nanobiotechnol. 2020, 12, e1594. [Google Scholar] [CrossRef]
- Thomas, R.; Park, I.-K.; Jeong, Y.Y. Magnetic Iron Oxide Nanoparticles for Multimodal Imaging and Therapy of Cancer. Int. J. Mol. Sci. 2013, 14, 15910–15930. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, Y.; Yang, T.; Sun, T. New insights into the synthesis, toxicity and applications of gold nanoparticles in CT imaging and treatment of cancer. Nanomedicine 2020, 15, 1127–1145. [Google Scholar] [CrossRef]
- Vauthier, C.; Tsapis, N.; Couvreur, P. Nanoparticles: Heating tumors to death? Nanomedicine 2011, 6, 99–109. [Google Scholar] [CrossRef]
- Chang, M.; Chang, Y.; Chao, P.; Yu, Q. Exosome purification based on PEG-coated Fe3O4 nanoparticles. PLoS ONE 2018, 13, e0199438. [Google Scholar] [CrossRef] [PubMed]
- Lobb, R.J.; Becker, M.; Wen, S.; Wong, C.S.F.; Wiegmans, A.P.; Leimgruber, A.; Moller, A. Optimized exosome isolation protocol for cell culture supernatant and human plasma. J. Extracell. Vesicles 2015, 4, 27031. [Google Scholar] [CrossRef]
- McKelvey, K.J.; Powell, K.L.; Ashton, A.W.; Morris, J.M.; McCracken, S.A. Exosomes: Mechanisms of Uptake. J. Circ. Biomark 2015, 4, 1–9. [Google Scholar] [CrossRef]
- Fu, S.; Wang, Y.; Xia, X.; Zheng, J. Exosome engineering: Current progress in cargo loading and targeted delivery. NanoImpact 2020, 20, 100261. [Google Scholar] [CrossRef]
- Théry, C.; Clayton, A.; Amigorena, S.; Raposo, G.A. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr. Protoc. Cell Biol. 2006, 30, 3–22. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Vojtech, L.; Ye, Z.; Hladik, F.; Nance, E. Quantum Dot Labeling and Visualization of Extracellular Vesicles. ACS Appl. Nano Mater. 2020, 3, 7211–7222. [Google Scholar] [CrossRef]
- Di, H.; Zeng, E.; Zhang, P.; Liu, X.; Zhang, C.; Yang, J.; Liu, D. General Approach to Engineering Extracellular Vesicles for Biomedical Analysis. Anal. Chem. 2019, 91, 12752–12759. [Google Scholar] [CrossRef]
- Sancho-Albero, M.; Encabo-Berzosa, M.D.; Beltran-Visiedo, M.; Fernandez-Messina, L.; Sebastian, V.; Sanchez-Madrid, F.; Arruebo, M.; Santamaria, J.; Martin-Duque, P. Efficient encapsulation of theranostic nanoparticles in cell-derived exosomes: Leveraging the exosomal biogenesis pathway to obtain hollow gold nanoparticle-hybrids. Nanoscale 2019, 11, 18825–18836. [Google Scholar] [CrossRef]
- Khongkow, M.; Yata, T.; Boonrungsiman, S.; Ruktanonchai, U.R.; Graham, D.; Namdeel, K. Surface modification of gold nanoparticles with neuron-targeted exosome for enhanced blood-brain barrier penetration. Sci. Rep. 2019, 9, 8278. [Google Scholar] [CrossRef]
- Hood, J.L.; Scott, M.J.; Wickline, S.A. Maximizing exosome colloidal stability following electroporation. Anal. Biochem. 2014, 448, 41–49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, Y.; Wu, T.; Zhang, K.; Meng, X.; Dai, W.; Wang, D.; Dong, H.; Zhang, X. Engineered Exosome-Mediated Near-Infrared-II Region V2C Quantum Dot Delivery for Nucleus-Target Low-Temperature Photothermal Therapy. ACS Nano 2019, 13, 1499–1510. [Google Scholar] [CrossRef]
- Zhao, J.; Chen, G.; Gu, Y.; Cui, R.; Zhang, Z.; Yu, Z.; Tang, B.; Zhao, Y.; Pang, D. Ultrasmall Magnetically Engineered Ag2Se Quantum. Dots for Instant Efficient Labeling and Whole-Body High-Resolution Multimodal Real-Time Tracking of Cell-Derived Microvesicles. J. Am. Chem. Soc. 2016, 138, 1893–1903. [Google Scholar] [CrossRef]
- Betzer, O.; Perets, N.; Angel, A.; Motiei, M.; Sadan, T.; Yadid, G.; Offen, D.; Popovtzer, R. In vivo Neuroimaging of Exosomes Using Gold Nanoparticles. ACS Nano 2017, 11, 10883–10893. [Google Scholar] [CrossRef] [PubMed]
- Tanziela, T.; Shaikh, S.; Jiang, H.; Lu, Z.; Wang, X. Efficient encapsulation of biocompatible nanoparticles in exosomes for cancer theranostics. Nano Today 2020, 35, 100964. [Google Scholar] [CrossRef]
- Jung, K.O.; Jo, H.; Yu, J.; Gambhir, S.S.; Pratx, G. Development and MPI tracking of novel hypoxia-targeted theranostic exosomes. Biomaterials 2018, 177, 139–148. [Google Scholar] [CrossRef]
- Lee, J.R.; Park, B.W.; Kim, J.; Choo, Y.W.; Kim, H.Y.; Yoon, J.K.; Kim, H.; Hwang, J.W.; Kang, M.; Kwon, S.P.; et al. Nanovesicles derived from iron oxide nanoparticles-incorporated mesenchymal stem cells for cardiac repair. Sci. Adv. 2020, 6, eaaz0952. [Google Scholar] [CrossRef]
- Lara, P.; Palma-Florez, S.; Salas-Huenuleo, E.; Polakovicova, I.; Guerrero, S.; Lobos-Gonzalez, L.; Campos, A.; Munoz, L.; Jorquera-Cordero, C.; Varas-Godoy, M.; et al. Gold nanoparticle based double-labeling of melanoma extracellular vesicles to determine the specificity of uptake by cells and preferential accumulation in small metastatic lung tumors. J. Nanobiotechol. 2020, 18, 20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yong, T.; Zhang, X.; Bie, N.; Zhang, H.; Zhang, X.; Li, F.; Hakeem, A.; Hu, J.; Gan, L.; Santos, H.A.; et al. Tumor exosome-based nanoparticles are efficient drug carriers for chemotherapy. Nat. Commun. 2019, 10, 3838. [Google Scholar] [CrossRef] [Green Version]
- Tayyaba-Rehman, F.U.; Shaikh, S.; Tanziela-Semcheddine, F.; Du, T.; Jiang, H.; Wang, X. In situ self-assembled Ag-Fe3O4 nanoclusters in exosomes for cancer diagnosis. J. Mater. Chem. 2020, 8, 2845–2855. [Google Scholar] [CrossRef] [PubMed]
- Yi, Y.; Lee, J.H.; Kim, S.Y.; Pack, C.G.; Ha, D.H.; Park, S.R.; Youn, J.; Cho, B.S. Advances in Analysis of Biodistribution of Exosomes by Molecular Imaging. Int. J. Mol. Sci. 2020, 21, 665. [Google Scholar] [CrossRef] [Green Version]
- Salunkhe, S.; Dheeraj, B.M.; Chitkara, D.; Mittal, A. Surface functionalization of exosomes for target-specific delivery and in vivo imaging & tracking: Strategies and significance. J. Control. Release 2020, 326, 599–614. [Google Scholar]
- Bull, E.; Madani, S.Y.; Sheth, R.; Seifalian, A.; Green, M. Stem Cell tracking using iron oxide nanoparticles. Int. J. Nanomed. 2014, 9, 1641–1653. [Google Scholar]
- Hu, L.; Wickline, S.A.; Hood, J.L. Magnetic resonance imaging of melanoma exosomes in lymph nodes. Magn. Reson. Med. 2015, 74, 266–271. [Google Scholar] [CrossRef] [PubMed]
- Busato, A.; Bonafede, R.; Bontempi, P.; Scambi, I.; Schiaffino, L.; Benati, D.; Malatesta, M.; Sbarbati, A.; Marzola, P.; Mariotti, R. Magnetic resonance imaging of ultrasmall superparamagnetic iron oxide-labeled exosomes from stem cells: A new method to obtain labeled exosomes. Int. J. Nanomed. 2016, 11, 2481–2490. [Google Scholar]
- Bao, Y.; Wen, T.; Samia, A.; Khandhar, A.; Krishnan, K.M. Magnetic nanoparticles material engineering and emerging applications in lithography and biomedicine. J. Mater. Sci. 2015, 50, 1–44. [Google Scholar] [CrossRef] [Green Version]
- Perets, N.; Betzer, O.; Shapira, R.; Brenstein, S.; Angel, A.; Sadan, T.; Ashery, U.; Popovtzer, R.; Offen, D. Golden Exosomes Selectively Target Brain Pathologies in Neurodegenerative and Neurodevelopmental Disorders. Nano Lett. 2019, 19, 3422–3431. [Google Scholar] [CrossRef] [PubMed]
- Bose, R.J.C.; Kumar, S.U.; Zeng, Y.T.; Afjei, R.; Robinson, E.; Lau, K.; Bermudez, A.; Habte, F.; Pitteri, S.J.; Sinclair, R.; et al. Tumor Cell-Derived Extracellular Vesicle-Coated Nanocarriers: An Efficient Theranostic Platform for the Cancer-Specific Delivery of Anti-miR-21 and Imaging Agents. ACS Nano 2018, 12, 10817–10832. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Luo, C.; Yang, M.; Li, J.; Ma, P.; Zhang, X. Intracellular uptake of and sensing with SERS-active hybrid exosomes: Insight into a role of metal nanoparticles. Nanomedicine 2020, 15, 913–926. [Google Scholar] [CrossRef]
- Sancho-Albero, M.; Navascués, N.; Mendoza, G.; Sebastián, V.; Arruebo, M.; Martín-Duque, P.; Santamaría, J. Exosome Origin Determines Cell Targeting and the Transfer of Therapeutic Nanoparticles Towards Target Cells. J. Nanobiotechnol. 2019, 17, 16. [Google Scholar] [CrossRef]
- Altanerova, U.; Babincova, M.; Babinec, P.; Benejova, K.; Jakubechova, J.; Altanerova, V.; Zduriencikova, M.; Repiska, V.; Altaner, C. Human mesenchymal stem cell-derived iron oxide exosomes allow targeted ablation of tumor cells via magnetic hyperthermia. Int. J. Nanomed. 2017, 12, 7923–7936. [Google Scholar] [CrossRef] [Green Version]
- Mulens-Arias, V.; Nicolas-Boluda, A.; Silva, A.K.A.; Gazeau, F. Theranostic Iron Oxide Nanoparticle Cargo Defines Extracellular Vesicle-Dependent Modulation of Macrophage Activation and Migratory Behavior. Adv. Biosyst. 2018, 2, 1800079. [Google Scholar] [CrossRef]
- Qi, H.; Liu, C.; Long, L.; Ren, Y.; Zhang, S.; Chang, X.; Qian, X.; Jia, H.; Zhao, J.; Sun, J.; et al. Blood Exosomes Endowed with Magnetic and Targeting Properties for Cancer Therapy. ACS Nano 2016, 10, 3323–3333. [Google Scholar] [CrossRef] [PubMed]
- Ashby, J.; Pan, S.; Zhong, W. Size and Surface Functionalization of Iron Oxide Nanoparticles Influence the Composition and Dynamic Nature of Their Protein Corona. ACS Appl. Mater. Interf. 2014, 6, 15412–15419. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Nanoparticles | Size (nm) | Exosome Origin | Preparation Method | Hybrid Structure | Ref. |
|---|---|---|---|---|---|
| QDs | 10–20 nm | Body fluids | Surface Conjugation | Nanoparticles on exosome surfaces | [25] |
| Au | 40 nm | MCF-7 cells | Surface Conjugation | Nanoparticles on exosome surfaces | [26] |
| Hollow Au | 40 nm | B16-F10 cells | Electroporation (950 µF) | Multiple nanoparticle encapsulation | [27] |
| Au | 20–30 nm | HEK293T cells | Extrusion | Single nanoparticle encapsulation | [28] |
| Iron Oxide | 5 nm | B16-F10 cells | Electroporation with 50 mM trehalose | Multiple nanoparticle encapsulation | [29] |
| V2C QDs | 16 nm | MCF-7 cells | Electroporation (200 V, 100 µF) | Multiple nanoparticle encapsulation | [30] |
| Ag2Se@Mn | 2 nm | MCF-7, CAL27, and A549 cells | Electroporation (250 V, 350 µF) | Multiple nanoparticle encapsulation | [31] |
| Au | 5 and 20 nm | Human MSCs | Passive incubation (37 °C, 3 h) | N/A | [32] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barjesteh, T.; Mansur, S.; Bao, Y. Inorganic Nanoparticle-Loaded Exosomes for Biomedical Applications. Molecules 2021, 26, 1135. https://doi.org/10.3390/molecules26041135
Barjesteh T, Mansur S, Bao Y. Inorganic Nanoparticle-Loaded Exosomes for Biomedical Applications. Molecules. 2021; 26(4):1135. https://doi.org/10.3390/molecules26041135
Chicago/Turabian StyleBarjesteh, Taraneh, Shomit Mansur, and Yuping Bao. 2021. "Inorganic Nanoparticle-Loaded Exosomes for Biomedical Applications" Molecules 26, no. 4: 1135. https://doi.org/10.3390/molecules26041135






