On the Protein Fibrillation Pathway: Oligomer Intermediates Detection Using ATR-FTIR Spectroscopy
Abstract
:1. Introduction
2. Results and Discussion
2.1. Formation of Oligomers: ThT and ANS Fluorescence
2.2. Oligomer Morphology: Atomic Force Microscopy (AFM)
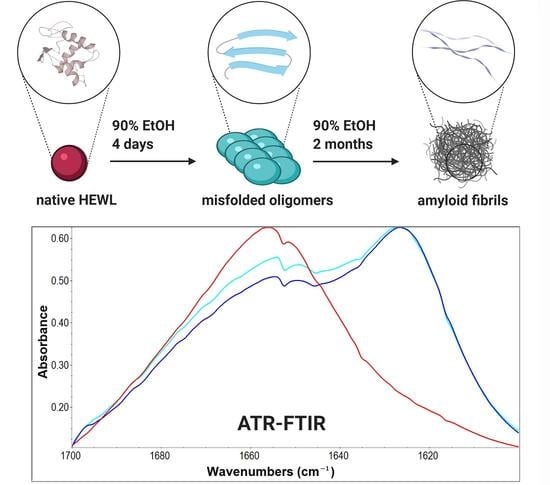
2.3. Qualitative Analysis of FTIR Spectra: Amide I Region
2.4. Qualitative Analysis of FTIR Spectra: Amide II Region
2.5. Qualitative Analysis of FTIR Spectra: Amide III Region
2.6. Quantitative Analysis of FTIR Spectra: Amide I Region Deconvolution
2.7. Quantitative Analysis of FTIR Spectra: Amide I/AMIDE II Ratio
3. Materials and Methods
3.1. Materials
3.2. HEWL Destabilization and Incubation
3.3. ThT Fluorescence
3.4. ANS Fluorescence
3.5. Atomic Force Microscopy
3.6. Fourier Transform Infrared Spectroscopy (FTIR)
3.7. Spectral Analysis: Second Derivatives and Spectral Correlation
3.8. Spectral Analysis: Deconvolution of the Amide I Region
3.9. Spectral Analysis: Amide I/Amide II Band Ratio
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Arosio, P.; Knowles, T.P.; Linse, S. On the lag phase in amyloid fibril formation. Phys. Chem. Chem Phys. PCCP 2015, 17, 7606–7618. [Google Scholar] [CrossRef] [Green Version]
- Breydo, L.; Uversky, V.N. Molecular Mechanisms of Protein Misfolding. In Bio-Nanoimaging: Protein Misfolding and Aggregation, 1st ed.; Vladimir Uversky, V.N., Lyubchenko, Y.L., Eds.; Academic Press: Cambridge, MA, USA, 2014; pp. 1–14. [Google Scholar]
- Knowles, T.P.; Vendruscolo, M.; Dobson, C.M. The amyloid state and its association with protein misfolding diseases. Nat. Rev. Mol. Cell Biol. 2014, 15, 384–396. [Google Scholar] [CrossRef]
- Urbic, T.; Najem, S.; Dias, C.L. Thermodynamic properties of amyloid fibrils in equilibrium. Biophys. Chem. 2017, 231, 155–160. [Google Scholar] [CrossRef]
- Kodali, R.; Wetzel, R. Polymorphism in the intermediates and products of amyloid assembly. Curr. Opin. Struct. Biol. 2007, 17, 48–57. [Google Scholar] [CrossRef]
- Buell, A.K.; Dobson, C.M.; Knowles, T.P. The physical chemistry of the amyloid phenomenon: Thermodynamics and kinetics of filamentous protein aggregation. Essays Biochem. 2014, 56, 11–39. [Google Scholar] [PubMed]
- Gharibyan, A.L.; Zamotin, V.; Yanamandra, K.; Moskaleva, O.S.; Margulis, B.A.; Kostanyan, I.A.; Morozova-Roche, L.A. Lysozyme amyloid oligomers and fibrils induce cellular death via different apoptotic/necrotic pathways. J. Mol. Biol. 2007, 365, 1337–1349. [Google Scholar] [CrossRef] [PubMed]
- Kayed, R.; Lasagna-Reeves, C.A. Molecular mechanisms of amyloid oligomers toxicity. J. Alzheimer's Dis. 2013, 33 (Suppl. S1), S67–S78. [Google Scholar] [CrossRef] [Green Version]
- Holley, M.; Eginton, C.; Schaefer, D.; Brown, L.R. Characterization of amyloidogenesis of hen egg lysozyme in concentrated ethanol solution. Biochem. Biophys. Res. Commun. 2008, 373, 164–168. [Google Scholar] [CrossRef] [PubMed]
- Meersman, F.; Atilgan, C.; Miles, A.J.; Bader, R.; Shang, W.; Matagne, A.; Wallace, B.A.; Koch, M.H.J. Consistent Picture of the Reversible Thermal Unfolding of Hen Egg-White Lysozyme from Experiment and Molecular Dynamics. Biophys. J. 2010, 99, 2255–2263. [Google Scholar] [CrossRef] [Green Version]
- Vaney, M.C.; Maignan, S.; Ries-Kautt, M.; Ducriux, A. High-resolution structure (1.33 A) of a HEW lysozyme tetragonal crystal grown in the APCF apparatus. Data and structural comparison with a crystal grown under microgravity from SpaceHab-01 mission. Acta Crystallographica. Sec. D Biol. Cryst. 1996, 52, 505–517. [Google Scholar] [CrossRef]
- Frare, E.; Polverino De Laureto, P.; Zurdo, J.; Dobson, C.M.; Fontana, A. A highly amyloidogenic region of hen lysozyme. J. Mol. Biol. 2004, 340, 1153–1165. [Google Scholar] [CrossRef]
- Arnaudov, L.N.; de Vries, R. Thermally induced fibrillar aggregation of hen egg white lysozyme. Biophys. J. 2005, 88, 515–526. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brudar, S.; Hribar-Lee, B. The Role of Buffers in Wild-Type HEWL Amyloid Fibril Formation Mechanism. Biomolecules 2019, 9, 65. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.; Ravi, V.K.; Swaminathan, R. Suppression of lysozyme aggregation at alkaline pH by tri-N-acetylchitotriose. Biochim. Biophys. Acta 2009, 1794, 913–920. [Google Scholar] [CrossRef]
- Sarkar, N.; Kumar, M.; Dubey, V.K. Rottlerin dissolves pre-formed protein amyloid: A study on hen egg white lysozyme. Biochim. Biophys. Acta 2011, 1810, 809–814. [Google Scholar] [CrossRef] [PubMed]
- Hameed, M.; Ahmad, B.; Khan, R.H.; Andrabi, K.I.; Fazili, K.M. Tertiary butanol induced amyloidogenesis of hen egg white lysozyme (HEWL) is facilitated by aggregation-prone alkali-induced molten globule like conformational state. Protein Pept. Lett. 2009, 16, 56–60. [Google Scholar] [CrossRef] [PubMed]
- Vernaglia, B.A.; Huang, J.; Clark, E.D. Guanidine hydrochloride can induce amyloid fibril formation from hen egg-white lysozyme. Biomacromolecules 2004, 5, 1362–1370. [Google Scholar] [CrossRef] [PubMed]
- Yonezawa, Y.; Tanaka, S.; Kubota, T.; Wakabayashi, K.; Yutani, K.; Fujiwara, S. An Insight into the pathway of the amyloid fibril formation of hen egg white lysozyme obtained from a small-angle X-ray and neutron scattering study. J. Mol. Biol 2002, 323, 237–251. [Google Scholar] [CrossRef]
- Chaari, A.; Fahy, C.; Chevillot-Biraud, A.; Rholam, M. Insights into Kinetics of Agitation-Induced Aggregation of Hen Lysozyme under Heat and Acidic Conditions from Various Spectroscopic Methods. PLoS ONE 2015, 10, e0142095. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frare, E.; Mossuto, M.F.; de Laureto, P.P.; Tolin, S.; Menzer, L.; Dumoulin, M.; Dobson, C.M.; Fontana, A. Characterization of oligomeric species on the aggregation pathway of human lysozyme. J. Mol. Biol 2009, 387, 17–27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zou, Y.; Hao, W.; Li, H.; Gao, Y.; Sun, Y.; Ma, G. New insight into amyloid fibril formation of hen egg white lysozyme using a two-step temperature-dependent FTIR approach. J. Phys. Chem. B 2014, 118, 9834–9843. [Google Scholar] [CrossRef] [PubMed]
- Raskovic, B.; Popovic, M.; Ostojic, S.; Andelkovic, B.; Tesevic, V.; Polovic, N. Fourier transform infrared spectroscopy provides an evidence of papain denaturation and aggregation during cold storage. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2015, 150, 238–246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruggeri, F.S.; Longo, G.; Faggiano, S.; Lipiec, E.; Pastore, A.; Dietler, G. Infrared nanospectroscopy characterization of oligomeric and fibrillar aggregates during amyloid formation. Nat. Comm. 2015, 6, 7831. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Milosevic, J.; Petric, J.; Jovcic, B.; Jankovic, B.; Polovic, N. Exploring the potential of infrared spectroscopy in qualitative and quantitative monitoring of ovalbumin amyloid fibrillation. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2020, 229, 117882. [Google Scholar] [CrossRef]
- Gade Malmos, K.; Blancas-Mejia, L.M.; Weber, B.; Buchner, J.; Ramirez-Alvarado, M.; Naiki, H.; Otzen, D. ThT 101: A primer on the use of thioflavin T to investigate amyloid formation. Amyloid 2017, 24, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Cao, A.; Hu, D.; Lai, L. Formation of amyloid fibrils from fully reduced hen egg white lysozyme. Protein Sci. 2004, 13, 319–324. [Google Scholar] [CrossRef] [PubMed]
- Goda, S.; Takano, K.; Yamagata, Y.; Nagata, R.; Akutsu, H.; Maki, S.; Namba, K.; Yutani, K. Amyloid protofilament formation of hen egg lysozyme in highly concentrated ethanol solution. Protein Sci. 2000, 9, 369–375. [Google Scholar] [CrossRef] [Green Version]
- Yanamandra, K.; Gruden, M.A.; Casaite, V.; Meskys, R.; Forsgren, L.; Morozova-Roche, L.A. α-Synuclein Reactive Antibodies as Diagnostic Biomarkers in Blood Sera of Parkinson’s Disease Patients. PLoS ONE 2011, 6, e18513. [Google Scholar] [CrossRef] [PubMed]
- Barth, A. Infrared spectroscopy of proteins. Biochim. Biophys. Acta 2007, 1767, 1073–1101. [Google Scholar] [CrossRef] [Green Version]
- Fandrich, M.; Dobson, C.M. The behaviour of polyamino acids reveals an inverse side chain effect in amyloid structure formation. Embo J. 2002, 21, 5682–5690. [Google Scholar] [CrossRef] [Green Version]
- Bouchard, M.; Zurdo, J.; Nettleton, E.J.; Dobson, C.M.; Robinson, C.V. Formation of insulin amyloid fibrils followed by FTIR simultaneously with CD and electron microscopy. Protein Sci. 2000, 9, 1960–1967. [Google Scholar] [CrossRef] [Green Version]
- Zandomeneghi, G.; Krebs, M.R.; McCammon, M.G.; Fandrich, M. FTIR reveals structural differences between native beta-sheet proteins and amyloid fibrils. Protein Sci. 2004, 13, 3314–3321. [Google Scholar] [CrossRef] [Green Version]
- Miti, T.; Mulaj, M.; Schmit, J.D.; Muschol, M. Stable, metastable, and kinetically trapped amyloid aggregate phases. Biomacromolecules 2015, 16, 326–335. [Google Scholar] [CrossRef] [PubMed]
- Du, K.; Sun, J.; Song, X.; Chen, H.; Feng, W.; Ji, P. Interaction of ionic liquid [bmin][CF3SO3] with lysozyme investigated by two-dimensional fourier transform infrared spectroscopy. ACS Sustain. Chem. Eng. 2014, 2, 1420–1428. [Google Scholar] [CrossRef]
- Seo, J.; Hoffmann, W.; Warnke, S.; Huang, X.; Gewinner, S.; Schollkopf, W.; Bowers, M.T.; von Helden, G.; Pagel, K. An infrared spectroscopy approach to follow beta-sheet formation in peptide amyloid assemblies. Nat. Chem. 2017, 9, 39–44. [Google Scholar] [CrossRef]
- Raskovic, B.; Babić, N.; Korać, J.; Polovic, N. Evidence of β-sheet structure induced kinetic stability of papain upon thermal and sodium dodecyl sulfate denaturation. J. Serb. Chem Soc. 2015, 80, 613–625. [Google Scholar] [CrossRef]
- Bujis, J.; Norde, W.; Lichtenbelt, J.W.T. Changes in the secondary structure of adsorbed IgG and F(ab‘)2 studied by FTIR spectroscopy. Langmuir 1996, 12, 1605–1613. [Google Scholar] [CrossRef]
- Lee, T.H.; Lin, S.Y. Additives affecting thermal stability of salmon calcitonin in aqueous solution and structural similarity in lyophilized solid form. Process Biochem. 2011, 46, 2163–2169. [Google Scholar] [CrossRef]
- Cai, S.; Singh, B.R. Identification of beta-turn and random coil amide III infrared bands for secondary structure estimation of proteins. Biophys. Chem. 1999, 80, 7–20. [Google Scholar] [CrossRef]
- Cai, S.; Singh, B.R. A distinct utility of the amide III infrared band for secondary structure estimation of aqueous protein solutions using partial least squares methods. Biochemistry 2004, 43, 2541–2549. [Google Scholar] [CrossRef] [PubMed]
- Raskovic, B.; Vatic, S.; Andjelkovic, B.; Blagojevic, V.; Polovic, N. Optimizing storage conditions to prevent cold denaturation of trypsin for sequencing and to prolong its shelf life. Biochem. Eng. J. 2016, 105, 168–176. [Google Scholar] [CrossRef]
- Noinville, S.; Revault, M.; Baron, M.-H.; Tiss, A.; Yapoudjian, S.; Ivanova, M.; Verger, R. Conformational changes and orientation of Humicola lanuginosa lipase on a solid hydrophobic surface: An in situ interface Fourier transform infrared-attenuated total reflection study. Biophys. J. 2002, 82, 2709–2719. [Google Scholar] [CrossRef] [Green Version]
- Navarra, G.; Troia, F.; Militello, V.; Leone, M. Characterization of the nucleation process of lysozyme at physiological pH: Primary but not sole process. Biophys. Chem. 2013, 177–178, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Vedantham, G.; Sparks, H.G.; Sane, S.U.; Tzannis, S.; Przybycien, T.M. A holistic approach for protein secondary structure estimation from infrared spectra in H2O solutions. Anal. Biochem. 2000, 285, 33–49. [Google Scholar] [CrossRef]










| HEWL Sample | β-Sheet | Aggregation β-Sheet | α-Helix | Random Coil + Turn | Error |
|---|---|---|---|---|---|
| native, H2O | 10.3 | 8.6 | 41.7 | 39.4 | 1 |
| 0 h, 90% ethanol | 10.5 | 29.7 | 21.8 | 38 | 0.8 |
| 24 h, 90% ethanol | 10.1 | 34.7 | 22.8 | 32.4 | 0.6 |
| 96 h, 90% ethanol | 10.2 | 35.1 | 21.8 | 32.9 | 1.1 |
| 60 days, 90% ethanol | 10.3 | 38.2 | 19.6 | 31.9 | 0.8 |
| X-ray diffraction | 18.7 | / | 42.6 | 38.7 | / |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Milošević, J.; Prodanović, R.; Polović, N. On the Protein Fibrillation Pathway: Oligomer Intermediates Detection Using ATR-FTIR Spectroscopy. Molecules 2021, 26, 970. https://doi.org/10.3390/molecules26040970
Milošević J, Prodanović R, Polović N. On the Protein Fibrillation Pathway: Oligomer Intermediates Detection Using ATR-FTIR Spectroscopy. Molecules. 2021; 26(4):970. https://doi.org/10.3390/molecules26040970
Chicago/Turabian StyleMilošević, Jelica, Radivoje Prodanović, and Natalija Polović. 2021. "On the Protein Fibrillation Pathway: Oligomer Intermediates Detection Using ATR-FTIR Spectroscopy" Molecules 26, no. 4: 970. https://doi.org/10.3390/molecules26040970