Thermal Energy Storage and Heat Transfer of Nano-Enhanced Phase Change Material (NePCM) in a Shell and Tube Thermal Energy Storage (TES) Unit with a Partial Layer of Eccentric Copper Foam
Abstract
:1. Introduction
2. Physical Model and Mathematical Formulation
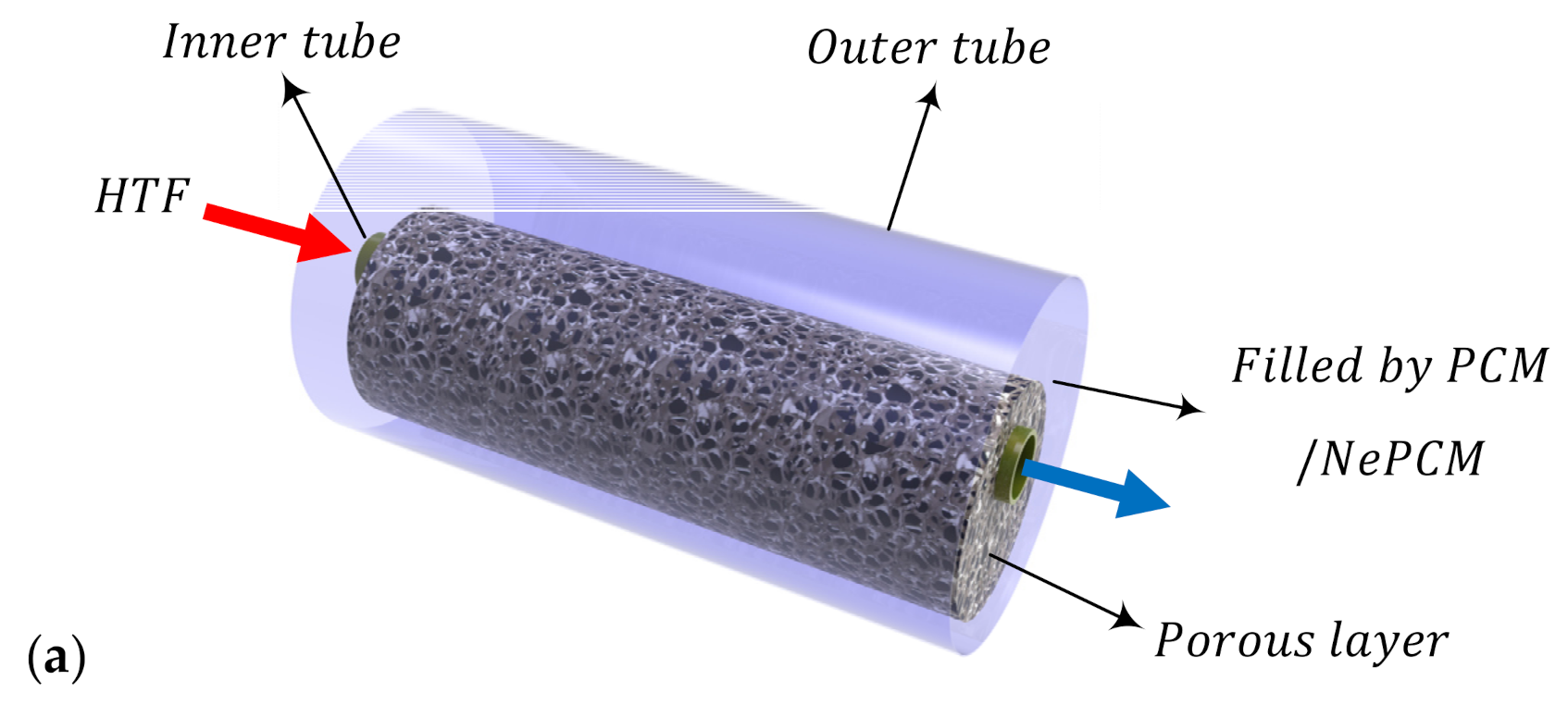
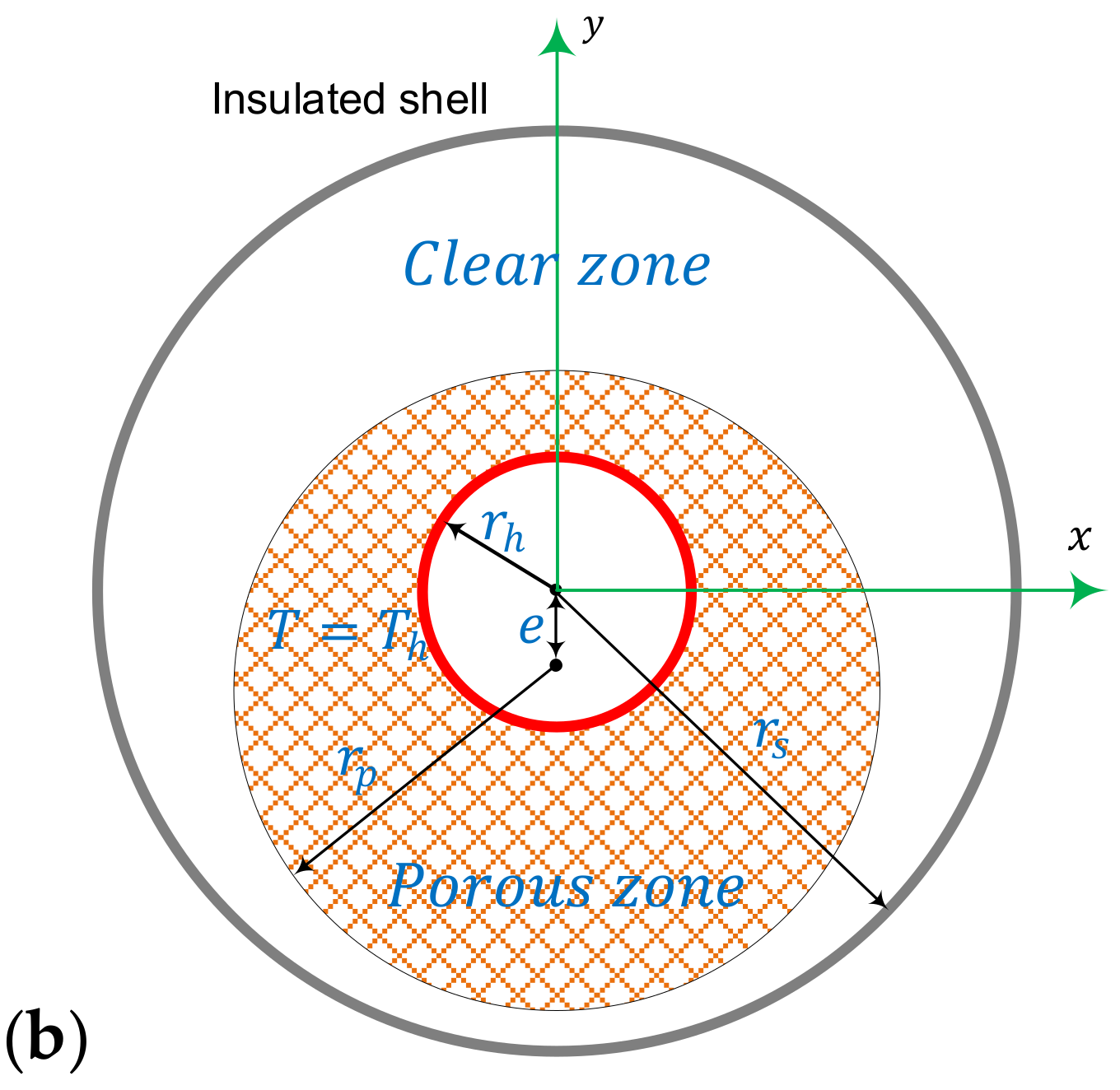
2.1. Model Description
2.2. Governing Equations
2.3. The Thermal and Physical Specifications of the PCM Enhanced with Nanoparticles
2.4. Boundary Conditions
- (a)
- at the interface of the porous and clear zones:
- (b)
- at the inner tube and shell:where Th = 316 K. The initial temperature was also considered as 304 K which is one degree below the fusion temperature.
2.5. Characteristic Parameters
3. Numerical Method
3.1. Solution Approach
3.2. Mesh Sensitivity Analysis
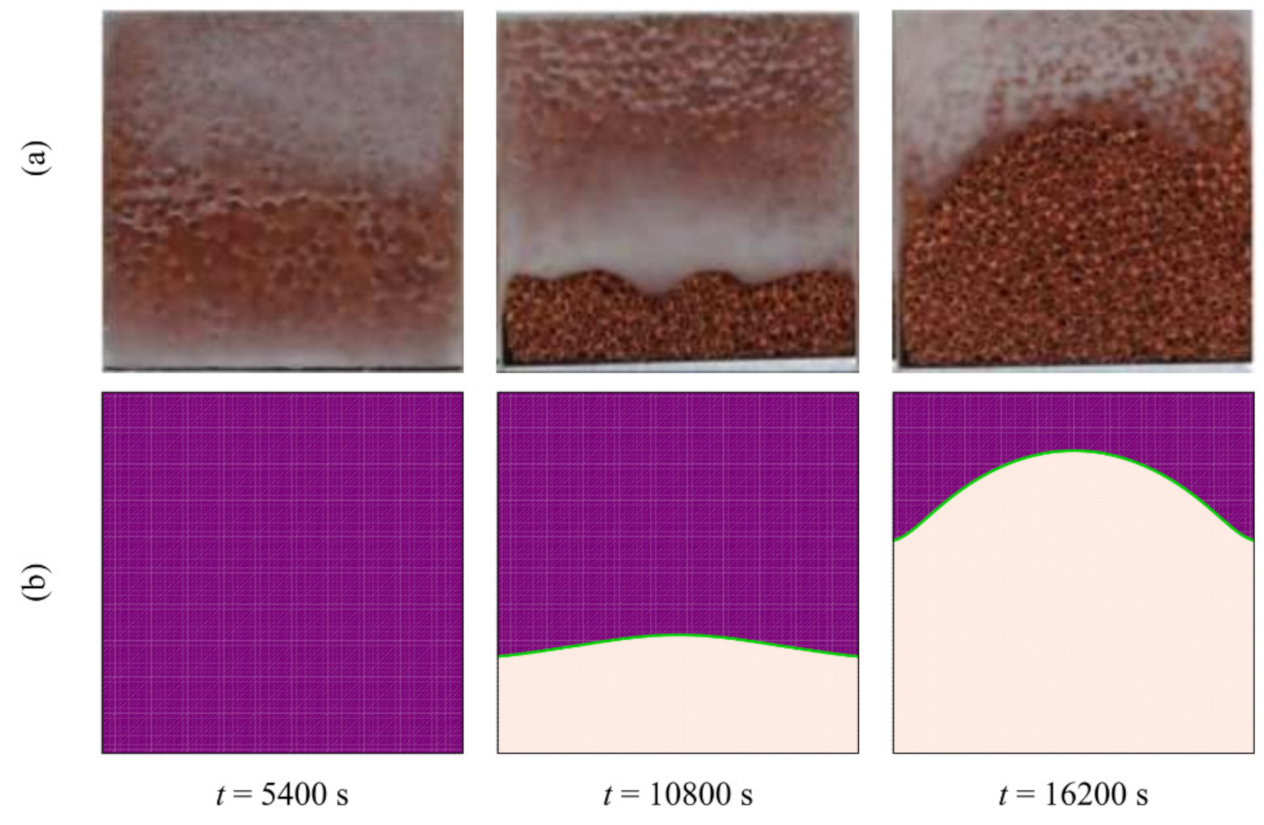
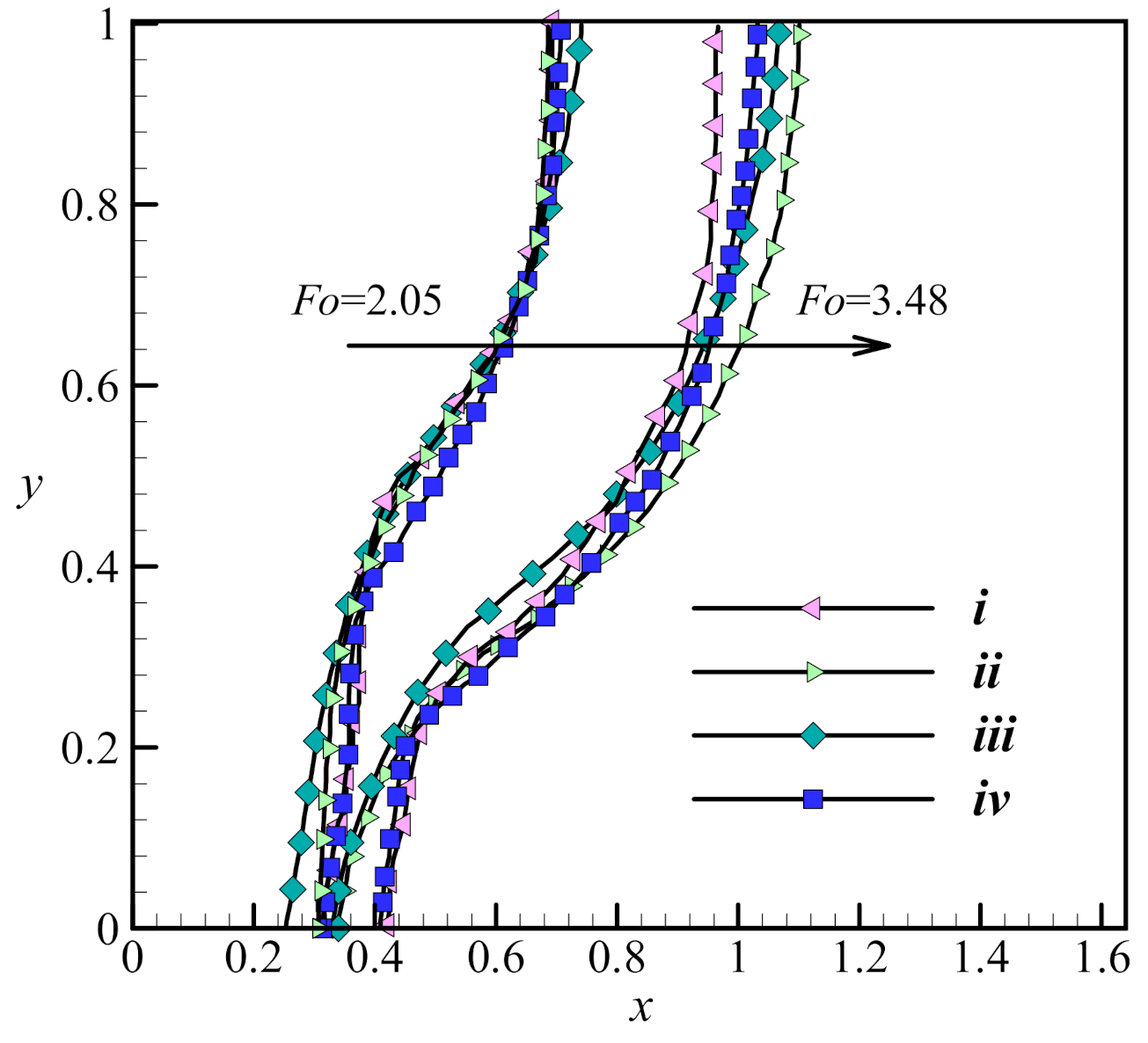
3.3. Validation with the Literature
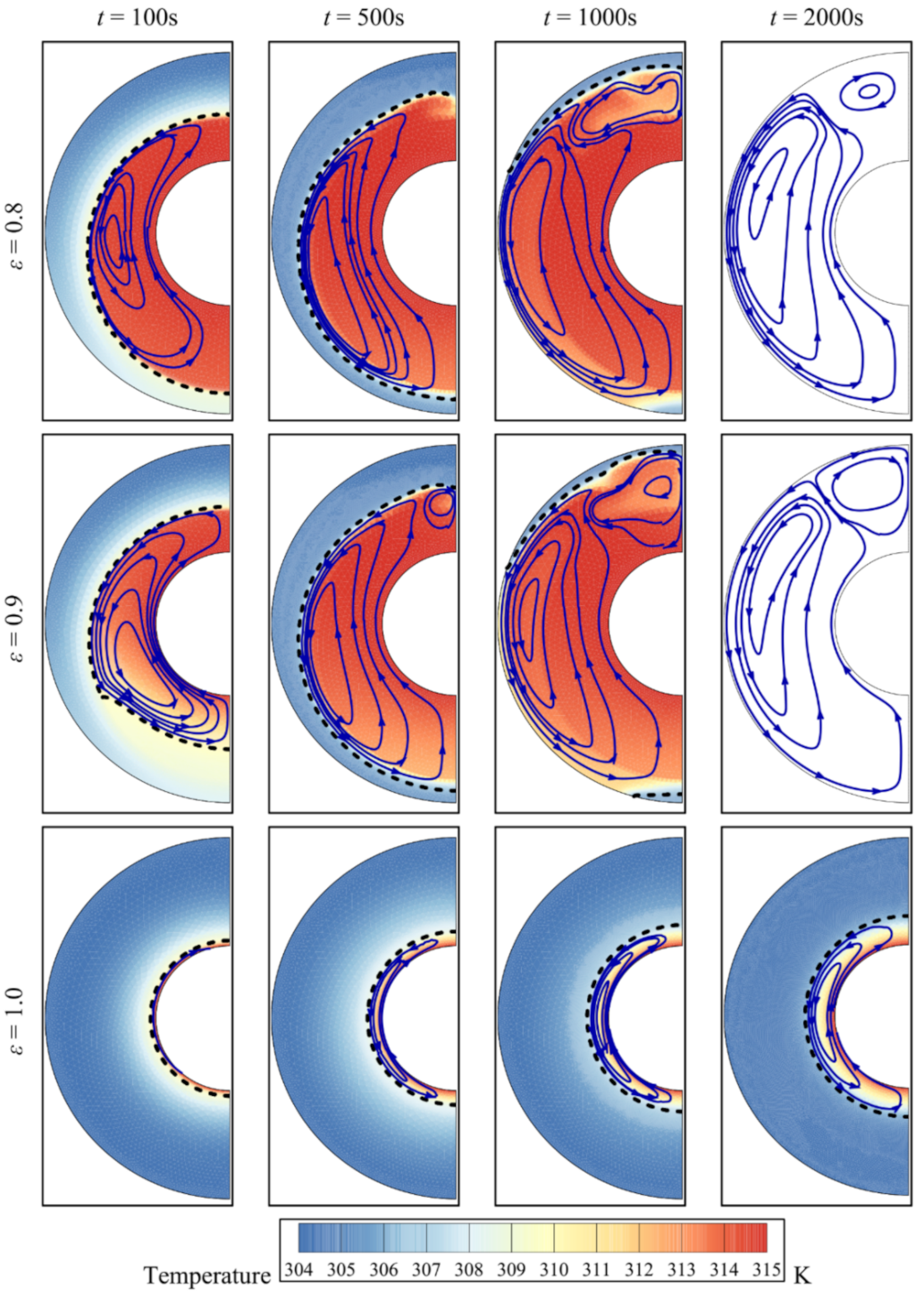
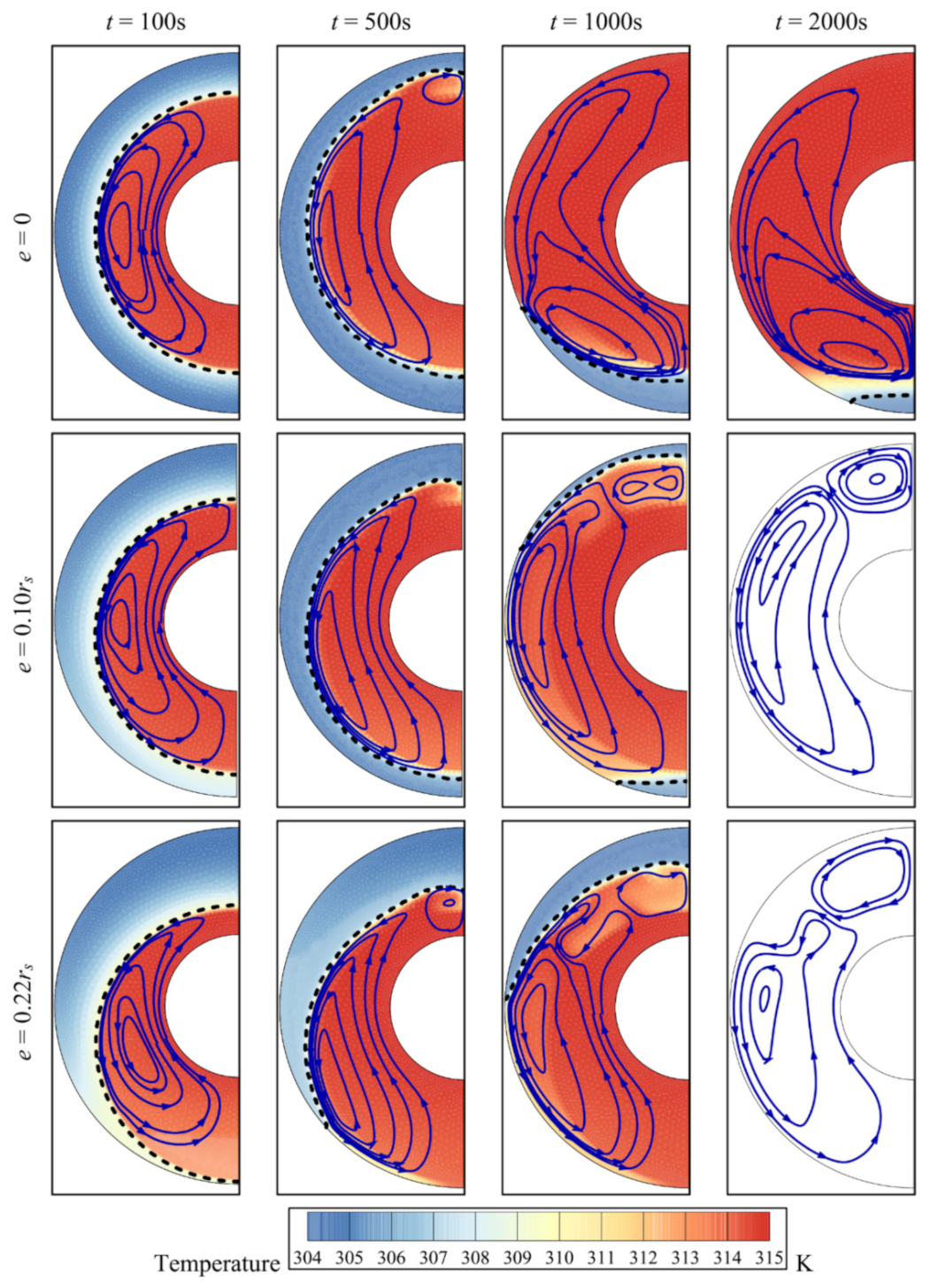
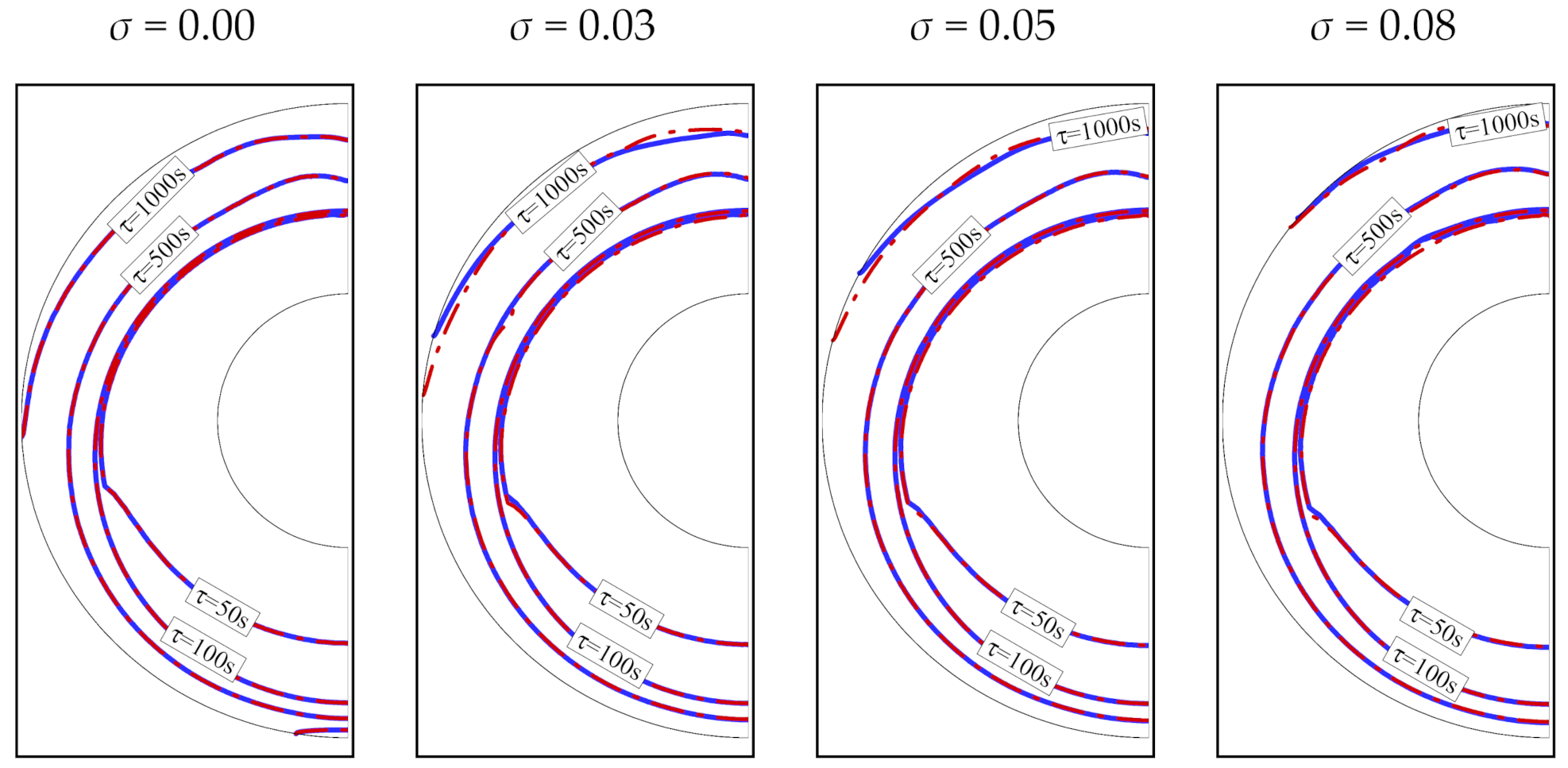
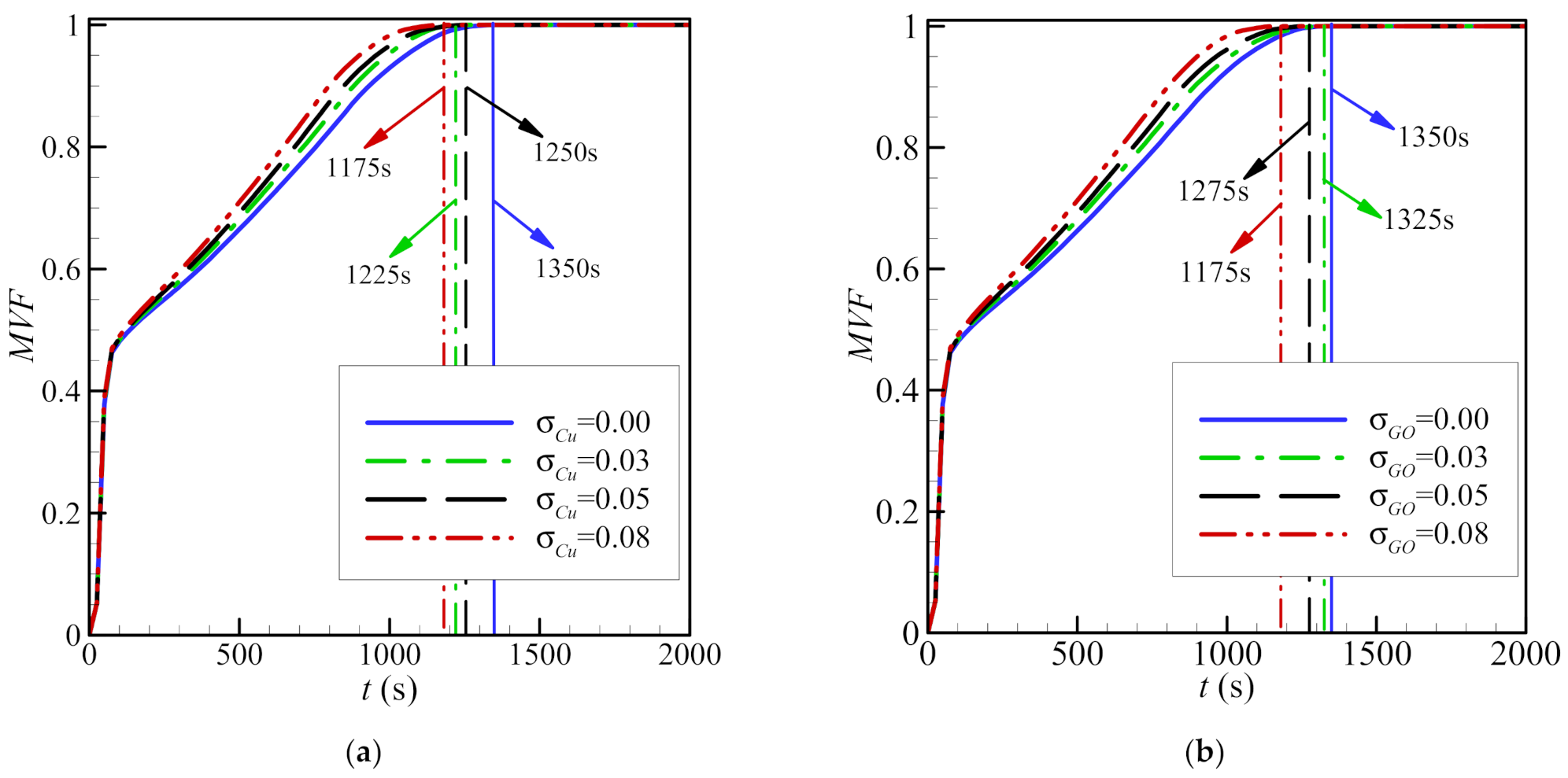
4. Results and Discussion
5. Conclusions
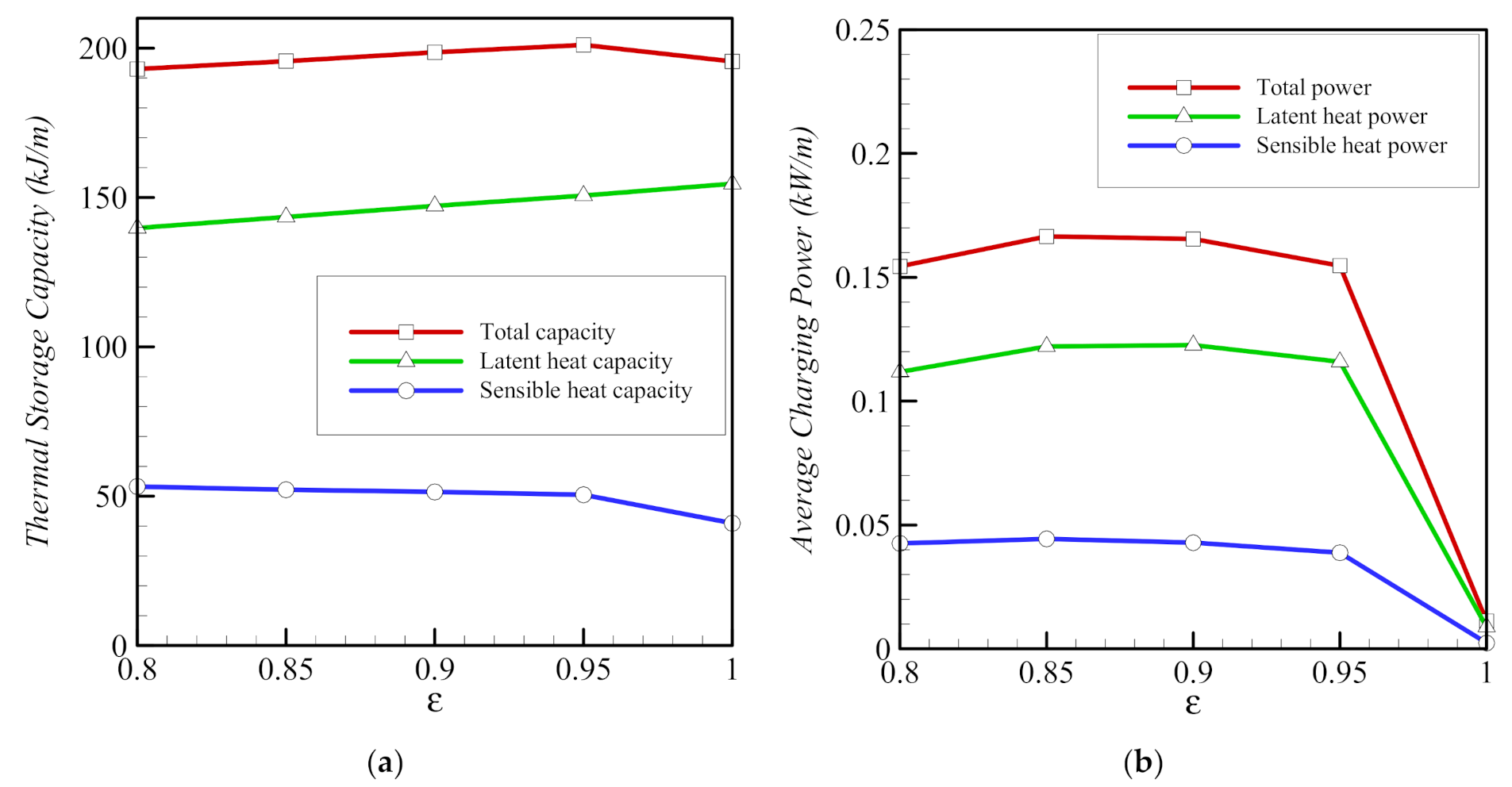
- The presence of a metal foam around the inner pipe contributes significantly to heat transfer and to the melting of the PCM. Reducing the porosity of the foam increases the amount of heat transmitted through the foam and enhances the melting of the PCM.
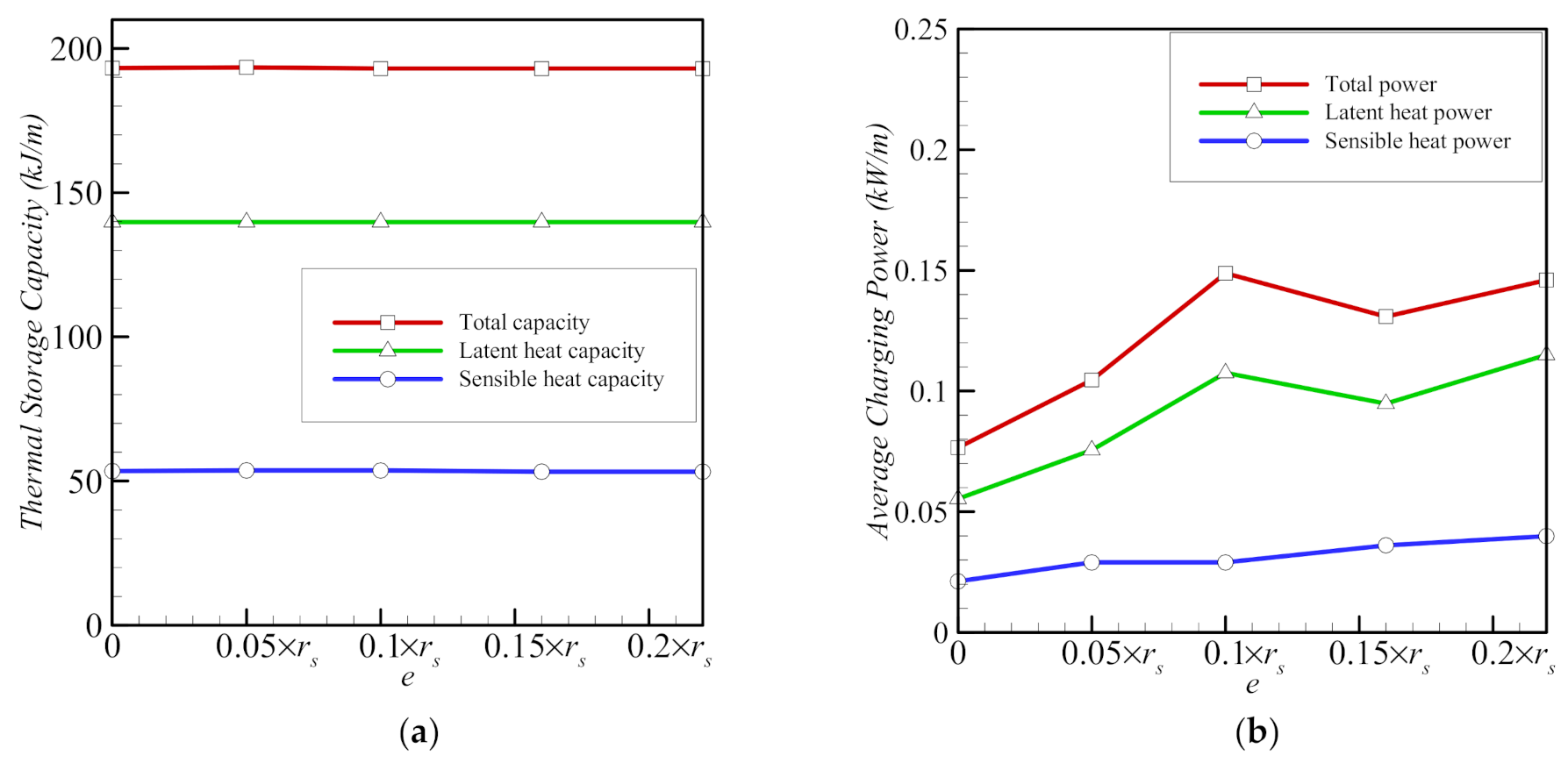
- The eccentricity of the system affects heat transfer by changing the location of the foam around the inner pipe. The melting of the PCM is limited by convection in the upper part of the cavity and by conduction through the metal foam in the lower region of the cavity. The melting of the PCM is slowest, when the inner pipe and the porous zone are concentric; the total melting time is halved for the eccentricity e = 0.1rs.
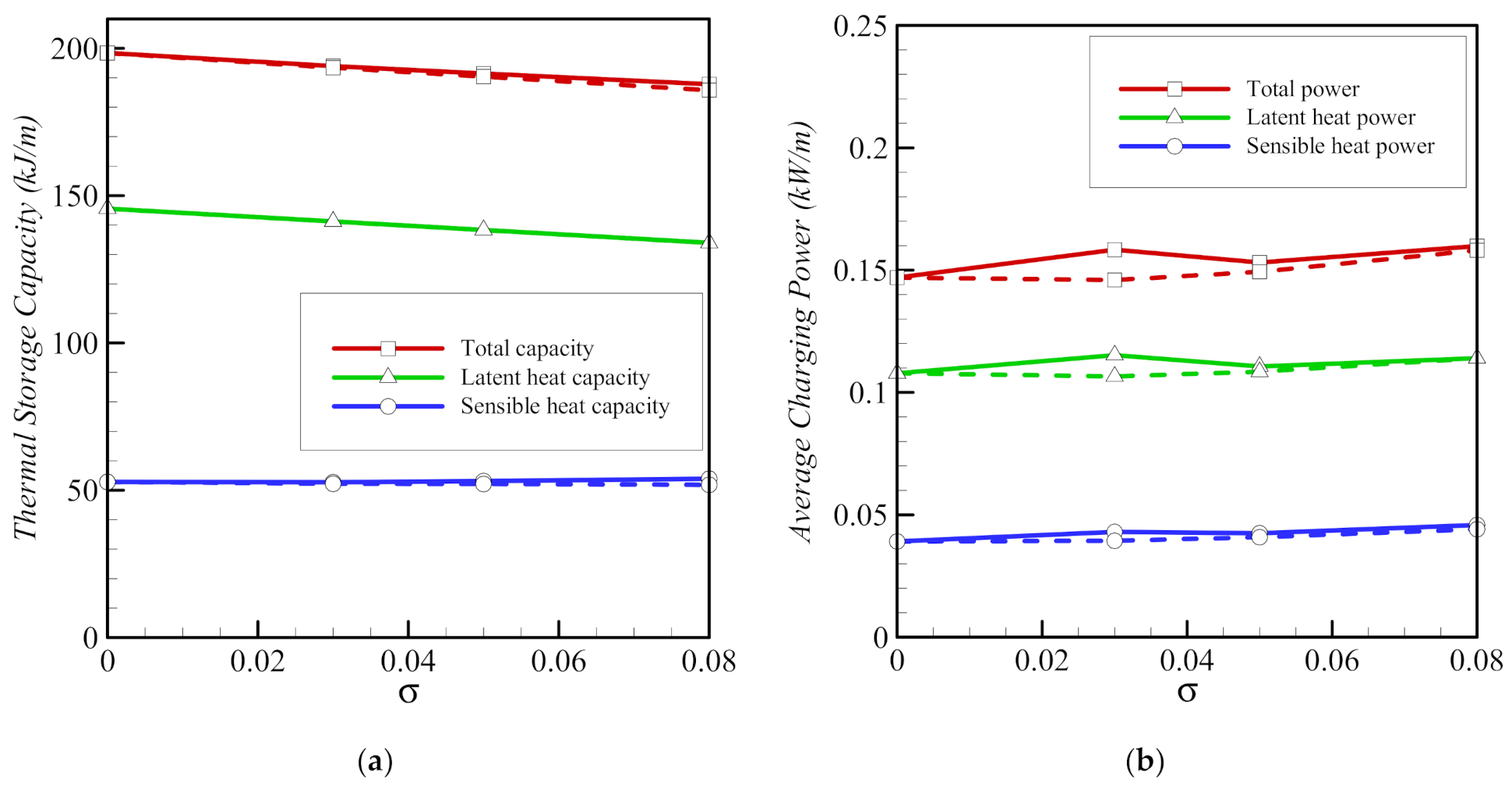
- Using a higher volume fraction, σ, of nanoparticles enhances melting. Copper nanoparticles are slightly more effective in transferring heat than graphite oxide nanoparticles. For both types of nanoparticles, the time required to melt the PCM completely is 13% shorter for an 8% volume fraction of nanoparticles (σ = 0.08).
- The three parameters, the porosity ε, the eccentricity e, and the volume fraction of nanoparticles, σ, have limited effects on the total storage capacity. However, the average charging power and the total melting time are affected more strongly by these parameters. Overall, the charging power decreases for longer total melting time, mainly for smaller values of eccentricity e and for lower volume fractions of foam (higher values of ε). The charging power is highest, when eccentricity takes the value e of 0.1rs.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
Nomenclature
| A | surface area of domain, m2 |
| Amush | mushy zone parameter, Pa·s·m−2 |
| Cp | specific heat capacity, J·kg−1·K−1 |
| dl | ligament diameter of foam, m |
| dp | pore diameter of foam, m |
| e | eccentricity, m |
| ES | total energy stored, k·m−1 |
| g | gravitational acceleration, m·s−2 |
| hsf | interfacial heat transfer coefficient, W·m−2·K−1 |
| MVF | melting volume fraction |
| n | normal direction of the surfaces |
| p | pressure, Pa |
| Pr | Prandtl number |
| R | radial coordinates, m |
| Ra | Rayleigh number |
| rh | radius of the heated tube, mm |
| rp | the radius of the average porous layer, mm |
| rs | radius of the shell of LHTES unit, mm |
| s | source term |
| T | temperature field, K |
| t | time, s |
| Tc | initial temperature of LHTES unit, K |
| Th | temperature of hot wall, K |
| u, v | x- and y -direction velocities, respectively, m·s−1 |
| Fo | nondimensional time (tρCp)/(4λrs2) |
| Greek | |
| µ | dynamic viscosity, kg·s−1·m−1 |
| β | coefficient of thermal expansion, K−1 |
| δT | temperature increment window for fusion, K |
| Δλ | thermal conductivity difference, W·m−1·K−1 |
| ε | porosity |
| η | liquid volume fraction |
| η0 | dummy liquid volume fraction for an adaptive mesh |
| ϑ | computational constant |
| κ | permeability, m2 |
| λ | thermal conductivity, W·m−1·K−1 |
| ρ | density, kg·m−3 |
| σ | volume fraction of nanoadditives |
| τ | nondimensional time |
| χ | a variable defined as (1 − ε)/(3π) |
| ω | pore density (PPI) |
| Subscripts | |
| cz | clear zone (no metal foam) |
| eff | effective |
| fu | fusion |
| i | index variable |
| j | index variable |
| l | liquid |
| m | metal foam |
| mush | mushy zone |
| NePCM | nano-enhanced phase change material |
| PCM | phase change material with no nanoadditive |
| pz | porous zone (metal foam) |
| s | solid |
References
- Cabeza, L.F.; Palacios, A.; Serrano, S.; Ürge-Vorsatz, D.; Barreneche, C. Comparison of past projections of global and regional primary and final energy consumption with historical data. Renew. Sustain. Energy Rev. 2018, 82, 681–688. [Google Scholar] [CrossRef] [Green Version]
- Sharma, S.S. Determinants of carbon dioxide emissions: Empirical evidence from 69 countries. Appl. Energy 2011, 88, 376–382. [Google Scholar] [CrossRef]
- Chua, K.; Chou, S.; Yang, W.; Yan, J. Achieving better energy-efficient air conditioning-A review of technologies and strategies. Appl. Energy 2013, 104, 87–104. [Google Scholar] [CrossRef]
- Shahsavar, A.; Goodarzi, A.; Mohammed, H.I.; Shirneshan, A.; Talebizadehsardari, P. Thermal performance evaluation of non-uniform fin array in a finned double-pipe latent heat storage system. Energy 2020, 193, 116800. [Google Scholar] [CrossRef]
- Ma, Z.; Yang, W.-W.; Yuan, F.; Jin, B.; He, Y.-L. Investigation on the thermal performance of a high-temperature latent heat storage system. Appl. Therm. Eng. 2017, 122, 579–592. [Google Scholar] [CrossRef]
- Wang, W.; Li, H.; Guo, S.; He, S.; Ding, J.; Yan, J.; Yang, J. Numerical simulation study on discharging process of the direct-contact phase change energy storage system. Appl. Energy 2015, 150, 61–68. [Google Scholar] [CrossRef]
- Atal, A.; Wang, Y.; Harsha, M.; Sengupta, S. Effect of porosity of conducting matrix on a phase change energy storage device. Int. J. Heat Mass Transf. 2016, 93, 9–16. [Google Scholar] [CrossRef]
- Liu, Q.; He, Y.-L.; Li, Q. Enthalpy-based multiple-relaxation-time lattice Boltzmann method for solid-liquid phase-change heat transfer in metal foams. Phys. Rev. E 2017, 96, 023303. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Yang, L.; Xu, C.; Du, X. Experimental study on enhancement of thermal energy storage with phase-change material. Appl. Energy 2016, 169, 164–176. [Google Scholar] [CrossRef]
- Xiao, X.; Zhang, P.; Li, M. Preparation and thermal characterization of paraffin/metal foam composite phase change material. Appl. Energy 2013, 112, 1357–1366. [Google Scholar]
- Abujas, C.R.; Jové, A.; Prieto, C.; Gallas, M.; Cabeza, L.F. Performance comparison of a group of thermal conductivity enhancement methodology in phase change material for thermal storage application. Renew. Energy 2016, 97, 434–443. [Google Scholar] [CrossRef] [Green Version]
- Tao, Y.; Liu, Y.; He, Y.-L. Effects of PCM arrangement and natural convection on charging and discharging performance of shell-and-tube LHS unit. Int. J. Heat Mass Transf. 2017, 115, 99–107. [Google Scholar] [CrossRef]
- Kousha, N.; Hosseini, M.; Aligoodarz, M.; Pakrouh, R.; Bahrampoury, R. Effect of inclination angle on the performance of a shell and tube heat storage unit-An experimental study. Appl. Therm. Eng. 2017, 112, 1497–1509. [Google Scholar] [CrossRef]
- Liu, C.; Groulx, D. Experimental study of the phase change heat transfer inside a horizontal cylindrical latent heat energy storage system. Int. J. Therm. Sci. 2014, 82, 100–110. [Google Scholar] [CrossRef]
- Mehryan, S.; Ghalambaz, M.; Gargari, L.S.; Hajjar, A.; Sheremet, M. Natural convection flow of a suspension containing nano-encapsulated phase change particles in an eccentric annulus. J. Energy Storage 2020, 28, 101236. [Google Scholar] [CrossRef]
- Cao, X.; Yuan, Y.; Xiang, B.; Haghighat, F. Effect of natural convection on melting performance of eccentric horizontal shell and tube latent heat storage unit. Sustain. Cities Soc. 2018, 38, 571–581. [Google Scholar] [CrossRef]
- Senthil, R. Effect of position of heat transfer fluid tube on the melting of phase change material in cylindrical thermal energy storage. Energy Sources Part A Recover. Util. Environ. Eff. 2019, 10, 1–11. [Google Scholar] [CrossRef]
- Sciacovelli, A.; Gagliardi, F.; Verda, V. Maximization of performance of a PCM latent heat storage system with innovative fins. Appl. Energy 2015, 137, 707–715. [Google Scholar] [CrossRef]
- Rajabifar, B.; Seyf, H.R.; Zhang, Y.; Khanna, S.K. Flow and Heat Transfer in Micro Pin Fin Heat Sinks with Nano-Encapsulated Phase Change Materials. J. Heat Transf. 2016, 138, 062401. [Google Scholar] [CrossRef] [Green Version]
- Srikanth, R.; Balaji, C. Experimental investigation on the heat transfer performance of a PCM based pin fin heat sink with discrete heating. Int. J. Therm. Sci. 2017, 111, 188–203. [Google Scholar] [CrossRef]
- Wang, Z.; Li, Y. Layer pattern thermal design and optimization for multistream plate-fin heat exchangers—A review. Renew. Sustain. Energy Rev. 2016, 53, 500–514. [Google Scholar] [CrossRef]
- Ye, W.-B.; Zhu, D.-S.; Wang, N. Numerical simulation on phase-change thermal storage/release in a plate-fin unit. Appl. Therm. Eng. 2011, 31, 3871–3884. [Google Scholar] [CrossRef]
- Rahimi, M.; Ranjbar, A.; Ganji, D.; Sedighi, K.; Hosseini, M.; Bahrampoury, R. Analysis of geometrical and operational parameters of PCM in a fin and tube heat exchanger. Int. Commun. Heat Mass Transf. 2014, 53, 109–115. [Google Scholar] [CrossRef]
- Saha, S.K.; Dutta, P. Heat transfer correlations for PCM-based heat sinks with plate fins. Appl. Therm. Eng. 2010, 30, 2485–2491. [Google Scholar] [CrossRef]
- Pakrouh, R.; Hosseini, M.J.; Ranjbar, A.A. A parametric investigation of a PCM-based pin fin heat sink. Mech. Sci. 2015, 6, 65–73. [Google Scholar] [CrossRef] [Green Version]
- Baby, R.; Balaji, C. Thermal optimization of PCM based pin fin heat sinks: An experimental study. Appl. Therm. Eng. 2013, 54, 65–77. [Google Scholar] [CrossRef]
- Zhao, C.Y.; Zhou, D.; Wu, Z.G. Heat Transfer Enhancement of Phase Change Materials (PCMs) in Low and High Temperature Thermal Storage by Using Porous Materials. In Proceedings of the 2010 14th International Heat Transfer Conference, Washington, DC, USA, 8–13August 2010; ASME International: New York, NY, USA, 2010; Volume 8, pp. 435–441. [Google Scholar]
- Mesalhy, O.; Lafdi, K.; Elgafy, A.; Bowman, K. Numerical study for enhancing the thermal conductivity of phase change material (PCM) storage using high thermal conductivity porous matrix. Energy Convers. Manag. 2005, 46, 847–867. [Google Scholar] [CrossRef]
- Kumar, A.; Saha, S.K. Energy and exergy analyses of medium temperature latent heat thermal storage with high porosity metal matrix. Appl. Therm. Eng. 2016, 109, 911–923. [Google Scholar] [CrossRef] [Green Version]
- Buonomo, B.; Celik, H.; Ercole, D.; Manca, O.; Mobedi, M. Numerical study on latent thermal energy storage systems with aluminum foam in local thermal equilibrium. Appl. Therm. Eng. 2019, 159, 113980. [Google Scholar] [CrossRef]
- Sheikholeslami, M.; Mahian, O. Enhancement of PCM solidification using inorganic nanoparticles and an external magnetic field with application in energy storage systems. J. Clean. Prod. 2019, 215, 963–977. [Google Scholar] [CrossRef]
- Mahdi, J.M.; Nsofor, E.C. Solidification of a PCM with nanoparticles in triplex-tube thermal energy storage system. Appl. Therm. Eng. 2016, 108, 596–604. [Google Scholar] [CrossRef]
- Heyhat, M.M.; Mousavi, S.; Siavashi, M. Battery thermal management with thermal energy storage composites of PCM, metal foam, fin and nanoparticle. J. Energy Storage 2020, 28, 101235. [Google Scholar] [CrossRef]
- Mahdi, J.M.; Mohammed, H.I.; Hashim, E.T.; Talebizadehsardari, P.; Nsofor, E.C. Solidification enhancement with multiple PCMs, cascaded metal foam and nanoparticles in the shell-and-tube energy storage system. Appl. Energy 2020, 257, 113993. [Google Scholar] [CrossRef]
- Sheikholeslami, M.; Jafaryar, M.; Shafee, A.; Babazadeh, H. Acceleration of discharge process of clean energy storage unit with insertion of porous foam considering nanoparticle enhanced paraffin. J. Clean. Prod. 2020, 261, 121206. [Google Scholar] [CrossRef]
- Gupta, M.; Singh, V.; Kumar, R.; Said, Z. A review on thermophysical properties of nanofluids and heat transfer applications. Renew. Sustain. Energy Rev. 2017, 74, 638–670. [Google Scholar]
- Hussanan, A.; Salleh, M.Z.; Khan, I.; Shafie, S. Convection heat transfer in micropolar nanofluids with oxide nanoparticles in water, kerosene and engine oil. J. Mol. Liq. 2017, 229, 482–488. [Google Scholar] [CrossRef] [Green Version]
- Kant, K.; Shukla, A.; Sharma, A.; Biwole, P.H. Heat transfer study of phase change materials with graphene nano particle for thermal energy storage. Sol. Energy 2017, 146, 453–463. [Google Scholar] [CrossRef]
- Nield, D.A.; Bejan, A. Convection in Porous Media, 4th ed.; Springer Science & Business Media: Berlin, Germany, 2013. [Google Scholar]
- Buongiorno, J. Convective Transport in Nanofluids. J. Heat Transf. 2005, 128, 240–250. [Google Scholar] [CrossRef]
- Sheikholeslami, M.; Shamlooei, M.; Moradi, R. Fe3O4 -Ethylene glycol nanofluid forced convection inside a porous enclosure in existence of Coulomb force. J. Mol. Liq. 2018, 249, 429–437. [Google Scholar] [CrossRef]
- Mahdi, J.M.; Nsofor, E.C. Melting enhancement in triplex-tube latent heat energy storage system using nanoparticles-metal foam combination. Appl. Energy 2017, 191, 22–34. [Google Scholar] [CrossRef]
- Sardari, P.T.; Mohammed, H.I.; Giddings, D.; Walker, G.S.; Gillott, M.; Grant, D. Numerical study of a multiple-segment metal foam-PCM latent heat storage unit: Effect of porosity, pore density and location of heat source. Energy 2019, 189, 116108. [Google Scholar] [CrossRef]
- Ranut, P. On the effective thermal conductivity of aluminum metal foams: Review and improvement of the available empirical and analytical models. Appl. Therm. Eng. 2016, 101, 496–524. [Google Scholar] [CrossRef]
- Zheng, H.; Wang, C.; Liu, Q.; Tian, Z.; Fan, X. Thermal performance of copper foam/paraffin composite phase change material. Energy Convers. Manag. 2018, 157, 372–381. [Google Scholar] [CrossRef]
- Reddy, J.N.; Gartling, D. The Finite Element Method in Heat Transfer and Fluid Dynamics; Informa UK Limited: London, UK, 2010. [Google Scholar]
- Zienkiewicz, O.; Taylor, R.; Nithiarasu, P. The Finite Element Method for Fluid Dynamics; Elsevier: Oxford, UK, 2014; Volume 6. [Google Scholar] [CrossRef]
- Reyes, J.C.D.L.; Andrade, S.G. A combined BDF-semismooth Newton approach for time-dependent Bingham flow. Numer. Methods Partial. Differ. Equ. 2011, 28, 834–860. [Google Scholar] [CrossRef]
- Schenk, O.; Gärtner, K. Solving unsymmetric sparse systems of linear equations with PARDISO. Future Gener. Comput. Syst. 2004, 20, 475–487. [Google Scholar]
- Wriggers, P. Nonlinear Finite Element Methods; Springer International Publishing: Berlin, Germany, 2008. [Google Scholar]
- Verbosio, F.; De Coninck, A.; Kourounis, D.; Schenk, O. Enhancing the scalability of selected inversion factorization algorithms in genomic prediction. J. Comput. Sci. 2017, 22, 99–108. [Google Scholar]
- Kumar, L.; Manjunath, B.; Patel, R.; Markandeya, S.; Agrawal, R.; Agrawal, A.; Kashyap, Y.; Sarkar, P.; Sinha, A.; Iyer, K.; et al. Experimental investigations on melting of lead in a cuboid with constant heat flux boundary condition using thermal neutron radiography. Int. J. Therm. Sci. 2012, 61, 15–27. [Google Scholar] [CrossRef]
- Bertrand, O.; Binet, B.; Combeau, H.; Couturier, S.; Delannoy, Y.; Gobin, D.; Lacroix, M.; Le Quéré, P.; Médale, M.; Mencinger, J.; et al. Melting driven by natural convection A comparison exercise: First results. Int. J. Therm. Sci. 1999, 38, 5–26. [Google Scholar] [CrossRef]
- Kuehn, T.H.; Goldstein, R.J. An experimental and theoretical study of natural convection in the annulus between horizontal concentric cylinders. J. Fluid Mech. 1976, 74, 695–719. [Google Scholar] [CrossRef]
- Kashani, S.; Ranjbar, A.A.; Abdollahzadeh, M.; Sebti, S.; Abdollahzadehsangroudi, M. Solidification of nano-enhanced phase change material (NEPCM) in a wavy cavity. Heat Mass Transf. 2012, 48, 1155–1166. [Google Scholar] [CrossRef]






| Material | P (kg·m−3) | Cp (J·kg−1·K−1) | β (K−1) | Tfu (°C) | λ (W·m−1·K−1) | hsf (kJ·kg−1) | μ (m2·s−1) |
|---|---|---|---|---|---|---|---|
| PCM | Solid: 1018 Liquid: 888 | Solid: 1900 Liquid: 2400 | 1.51 × 10−3 | 32 | Solid: 0.372 Liquid: 0.153 | 152.7 | 3 × 10−6 |
| Metal foam | 8900 | 386 | / | / | 380 | / | / |
| Graphite oxide | 1800 | 0.717 | 2.84 × 10−4 | / | 5000 | / | / |
| Copper | 8933 | 0.385 | 1.67 × 10−5 | / | 401 | / | / |
| Cases | Elements in the Domain (Boundary) | Time to Run |
|---|---|---|
| Case I | 2246 (276) | 20 min 30 s |
| Case II | 3703 (312) | 32 min 4 s |
| Case III | 5104 (335) | 43 min 52 s |
| Case IV | 20,153 (801) | 1 h 48 min 47 s |
| Case V | 50,092 (1895) | 3 h 34 min 9 s |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghalambaz, M.; Mehryan, S.A.M.; Ayoubloo, K.A.; Hajjar, A.; El Kadri, M.; Younis, O.; Pour, M.S.; Hulme-Smith, C. Thermal Energy Storage and Heat Transfer of Nano-Enhanced Phase Change Material (NePCM) in a Shell and Tube Thermal Energy Storage (TES) Unit with a Partial Layer of Eccentric Copper Foam. Molecules 2021, 26, 1491. https://doi.org/10.3390/molecules26051491
Ghalambaz M, Mehryan SAM, Ayoubloo KA, Hajjar A, El Kadri M, Younis O, Pour MS, Hulme-Smith C. Thermal Energy Storage and Heat Transfer of Nano-Enhanced Phase Change Material (NePCM) in a Shell and Tube Thermal Energy Storage (TES) Unit with a Partial Layer of Eccentric Copper Foam. Molecules. 2021; 26(5):1491. https://doi.org/10.3390/molecules26051491
Chicago/Turabian StyleGhalambaz, Mohammad, Seyed Abdollah Mansouri Mehryan, Kasra Ayoubi Ayoubloo, Ahmad Hajjar, Mohamad El Kadri, Obai Younis, Mohsen Saffari Pour, and Christopher Hulme-Smith. 2021. "Thermal Energy Storage and Heat Transfer of Nano-Enhanced Phase Change Material (NePCM) in a Shell and Tube Thermal Energy Storage (TES) Unit with a Partial Layer of Eccentric Copper Foam" Molecules 26, no. 5: 1491. https://doi.org/10.3390/molecules26051491







