Cyclometalated Osmium Compounds and beyond: Synthesis, Properties, Applications
Abstract
:1. Introduction
2. Historical Background
3. Main Precursors
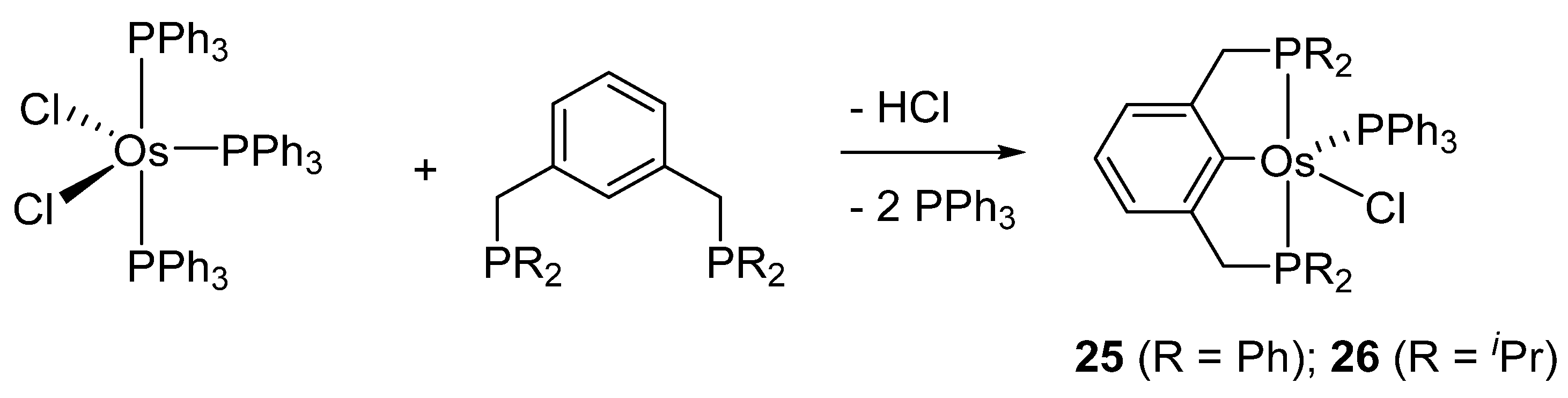
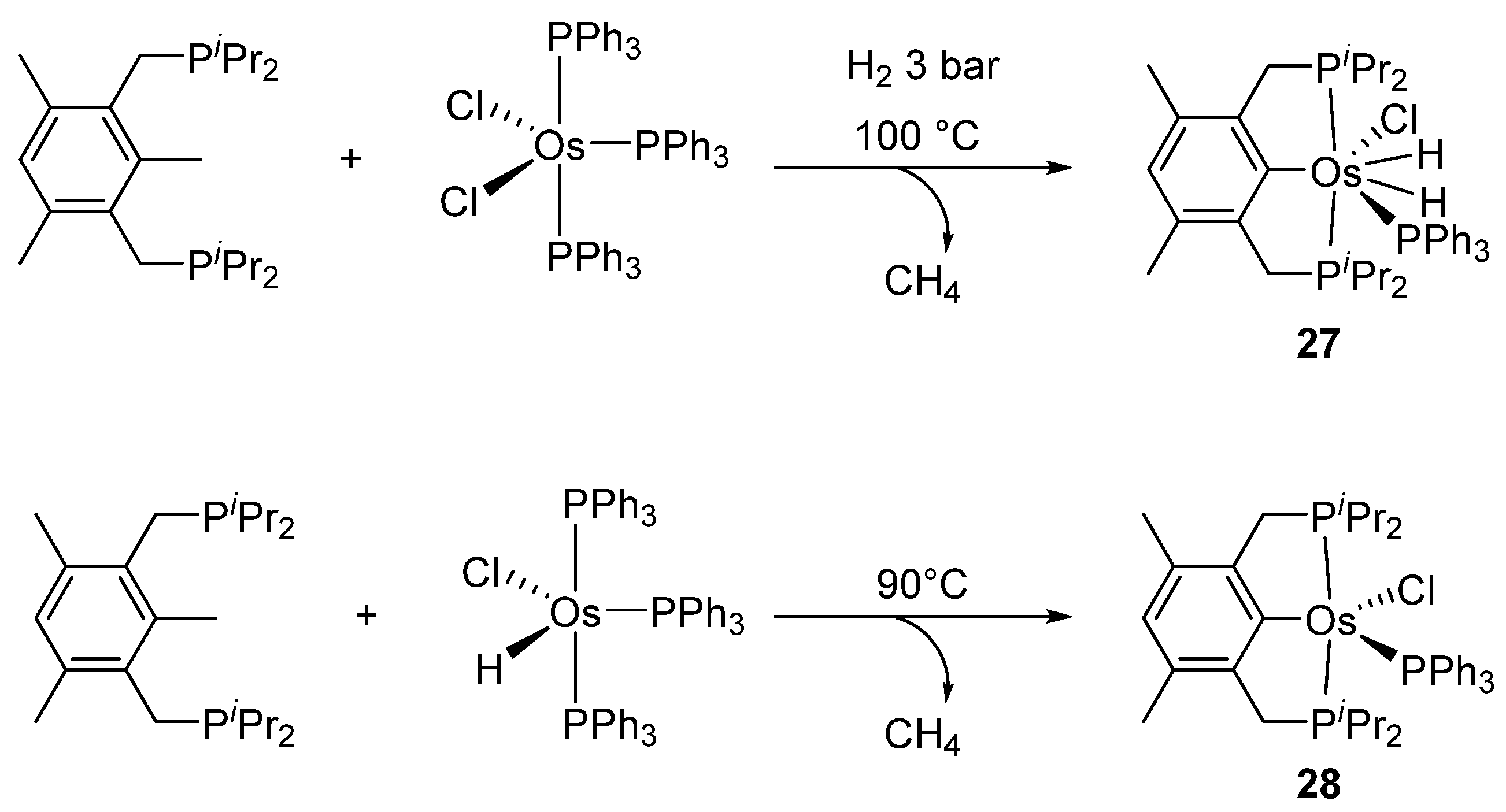
3.1. [OsX2(PR3)3]
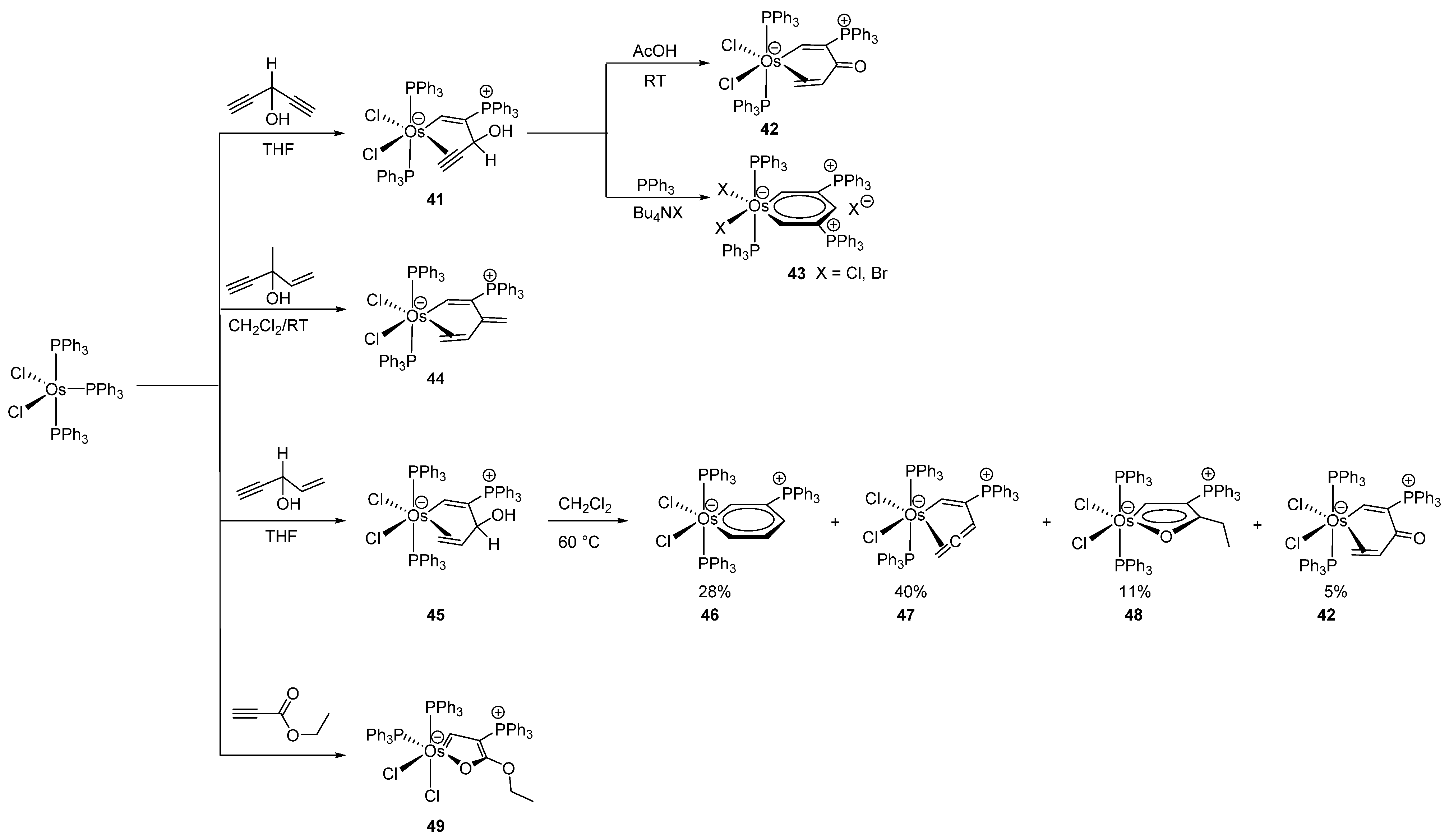
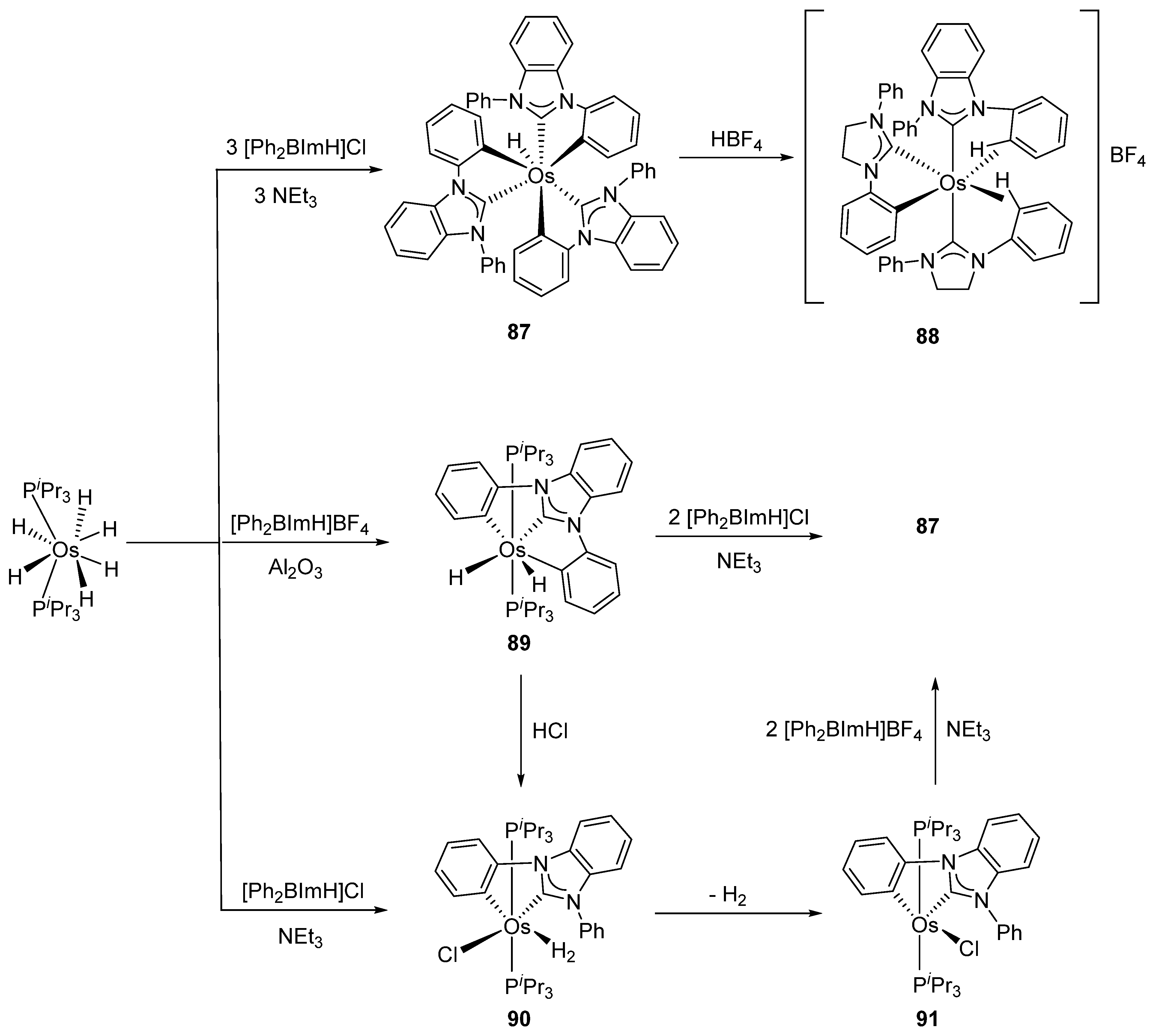
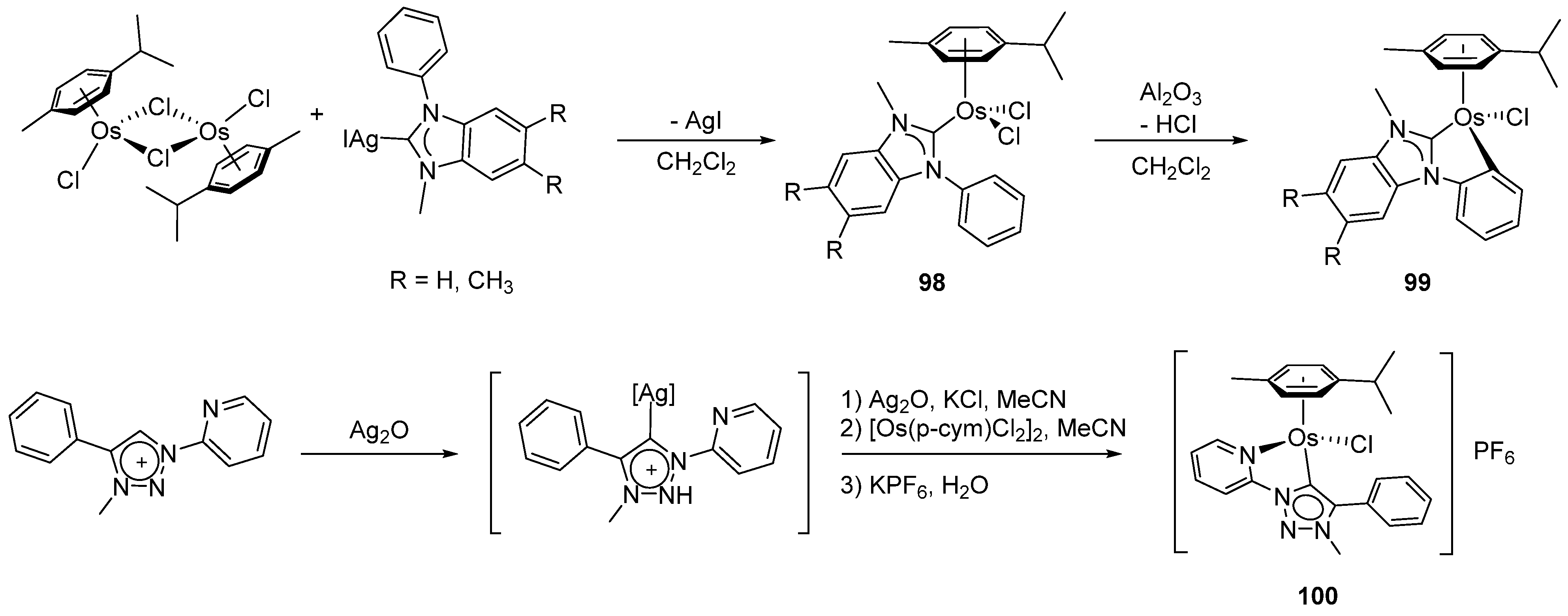
3.1.1. mono-Osmacycles
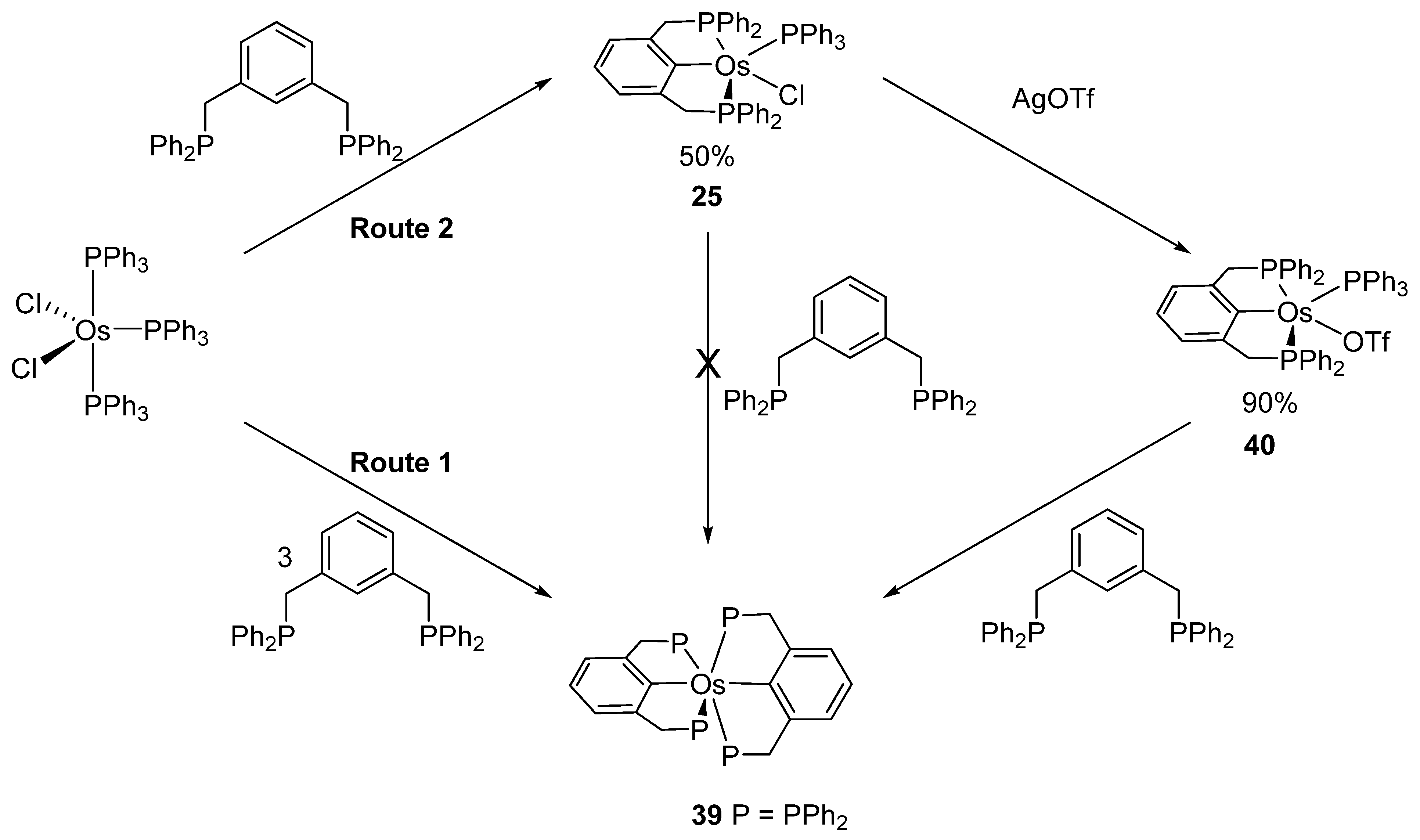
3.1.2. Pincer and bis-Cyclometalated Complexes
3.1.3. Others Osmacycles
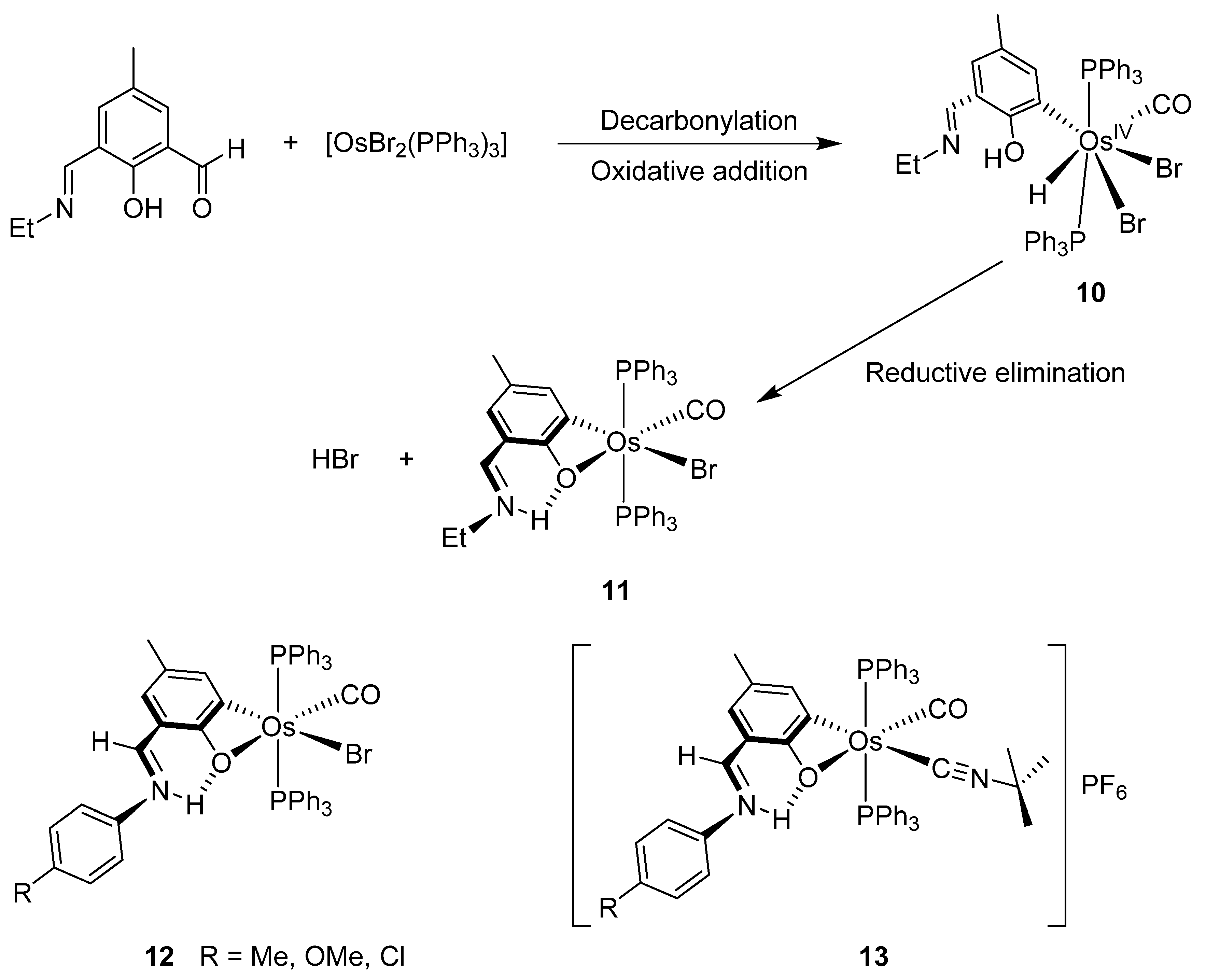
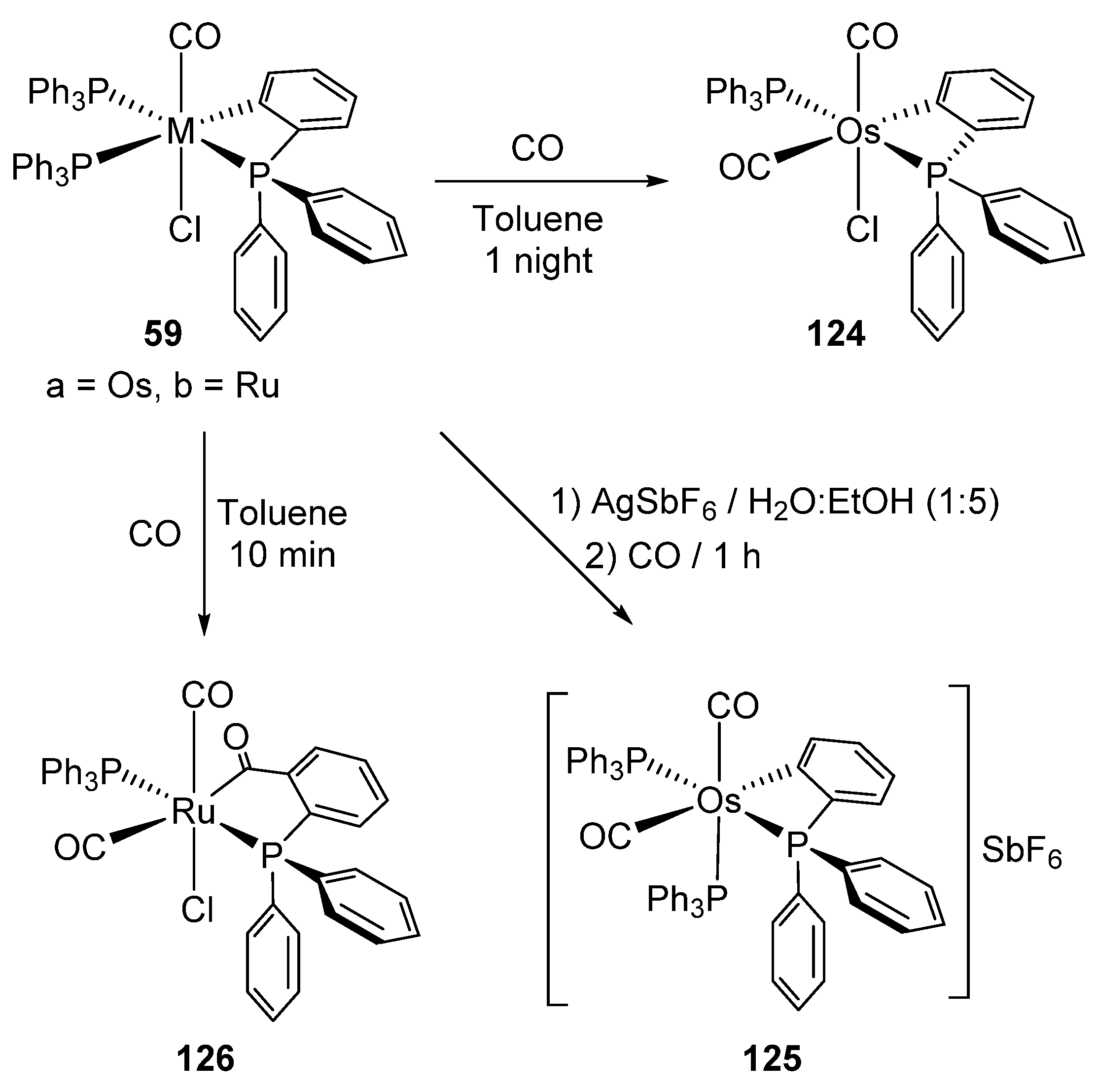
3.2. [OsX2(CO)(PR3)3]
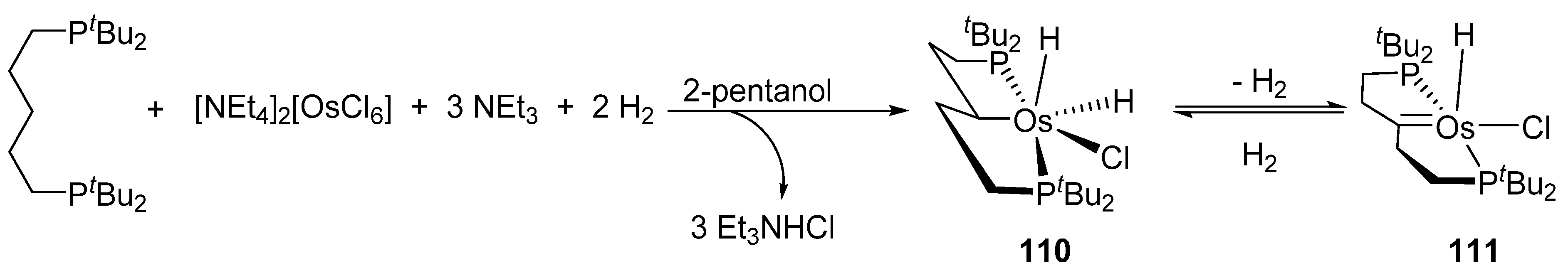
3.3. Osmium Hydride Complexes
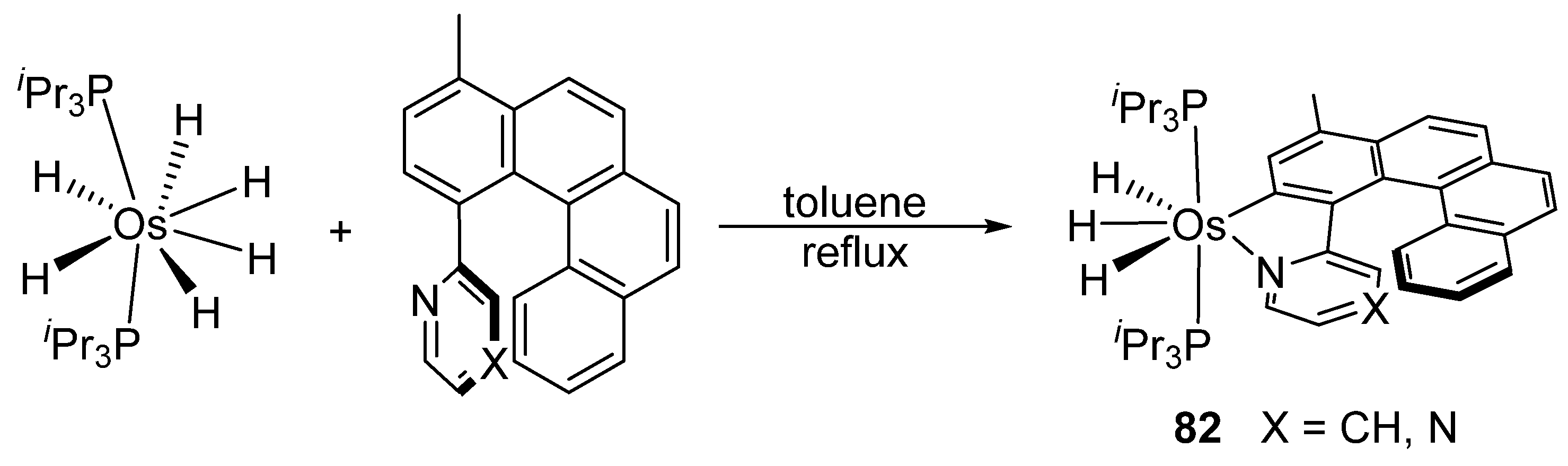
3.3.1. [OsH6(PR3)2]
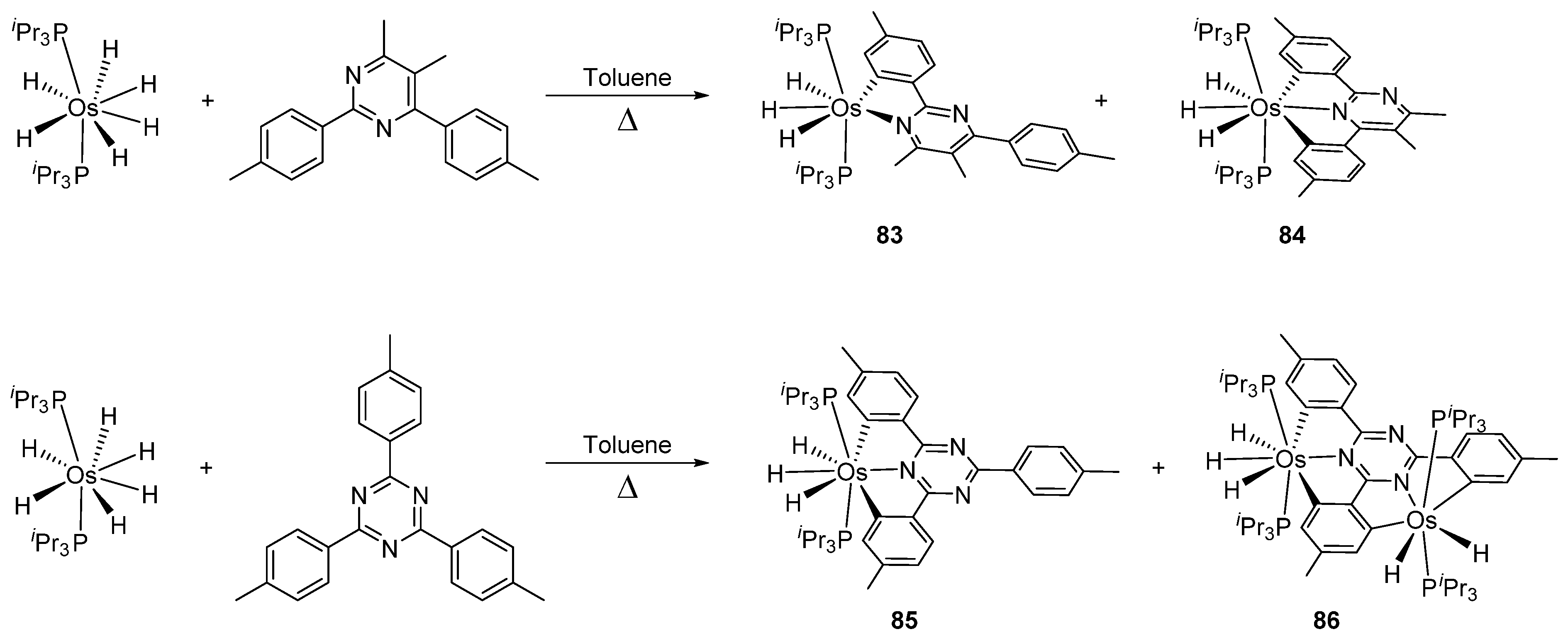
3.3.2. bis- and tris-Osmacycles
3.4. [Os(η6-arene)Cl2]2
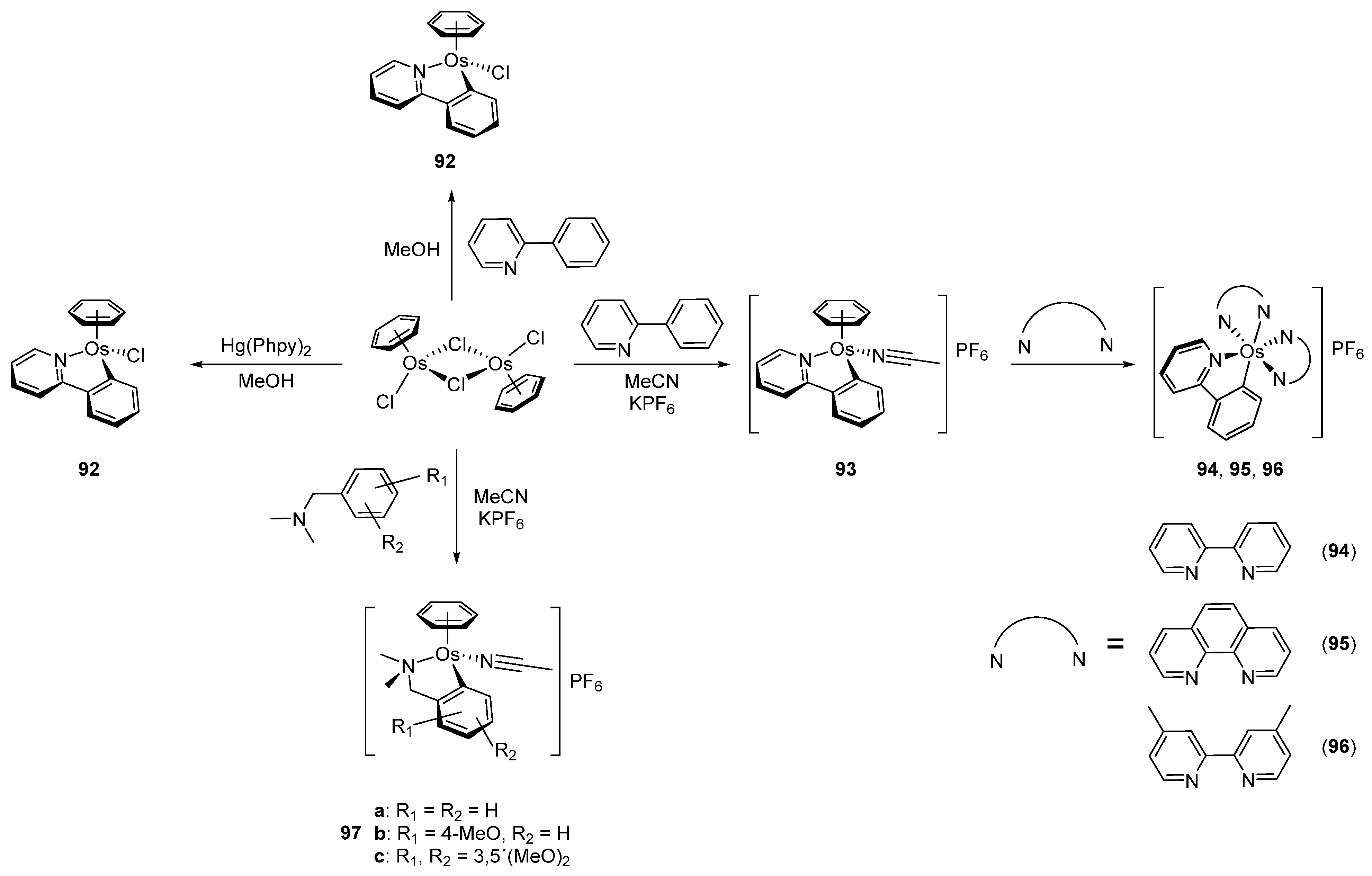
3.4.1. mono-Osmacycles
3.4.2. bis- and tris-Osmacycles
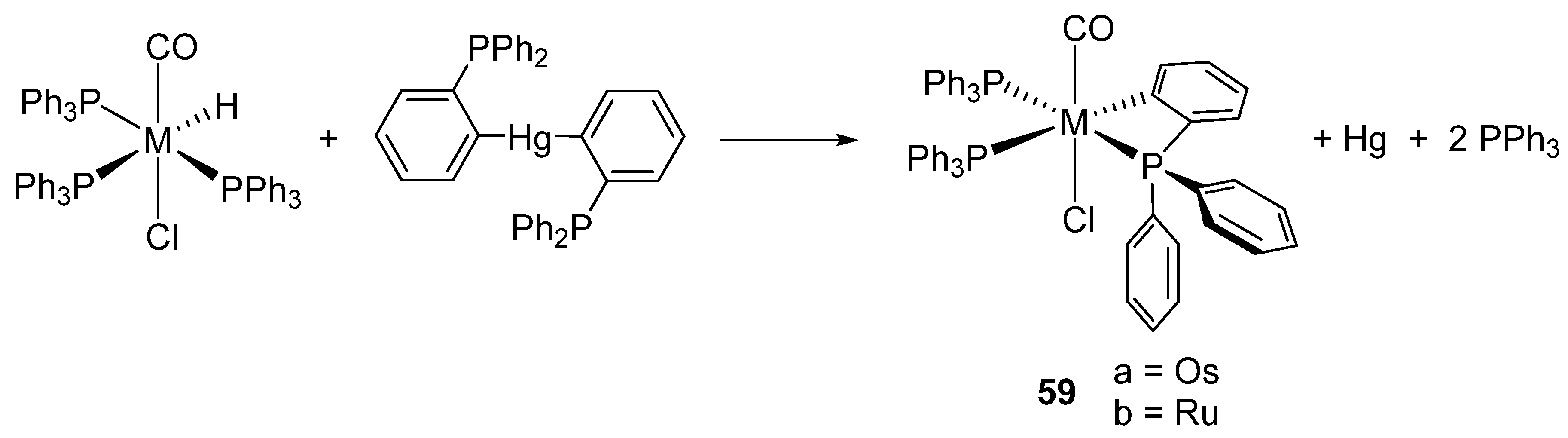
3.5. Other Precursors
4. Representative Reactions of Osmacycles
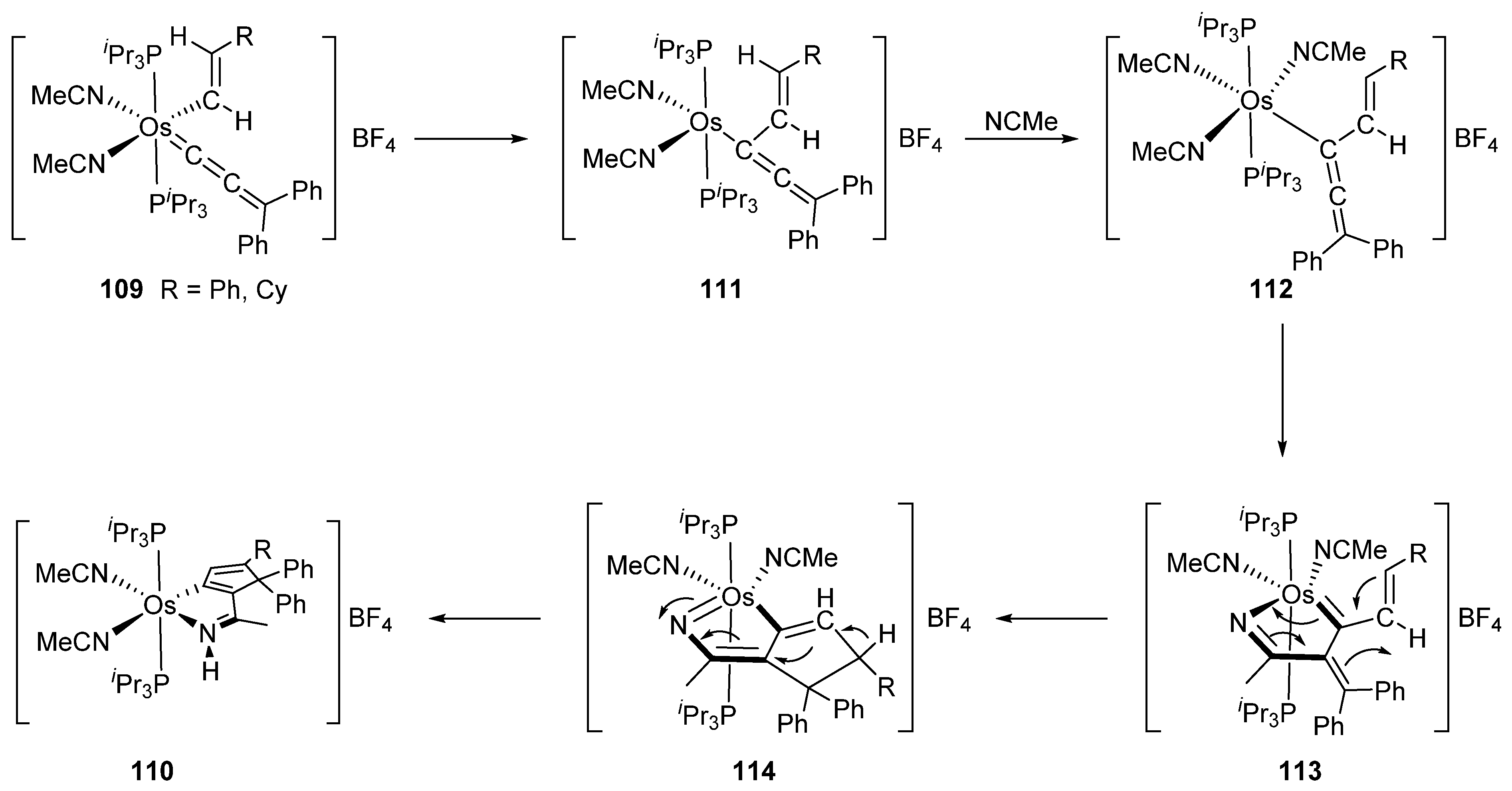
4.1. Reactivity of Chelate and Pincer Complexes
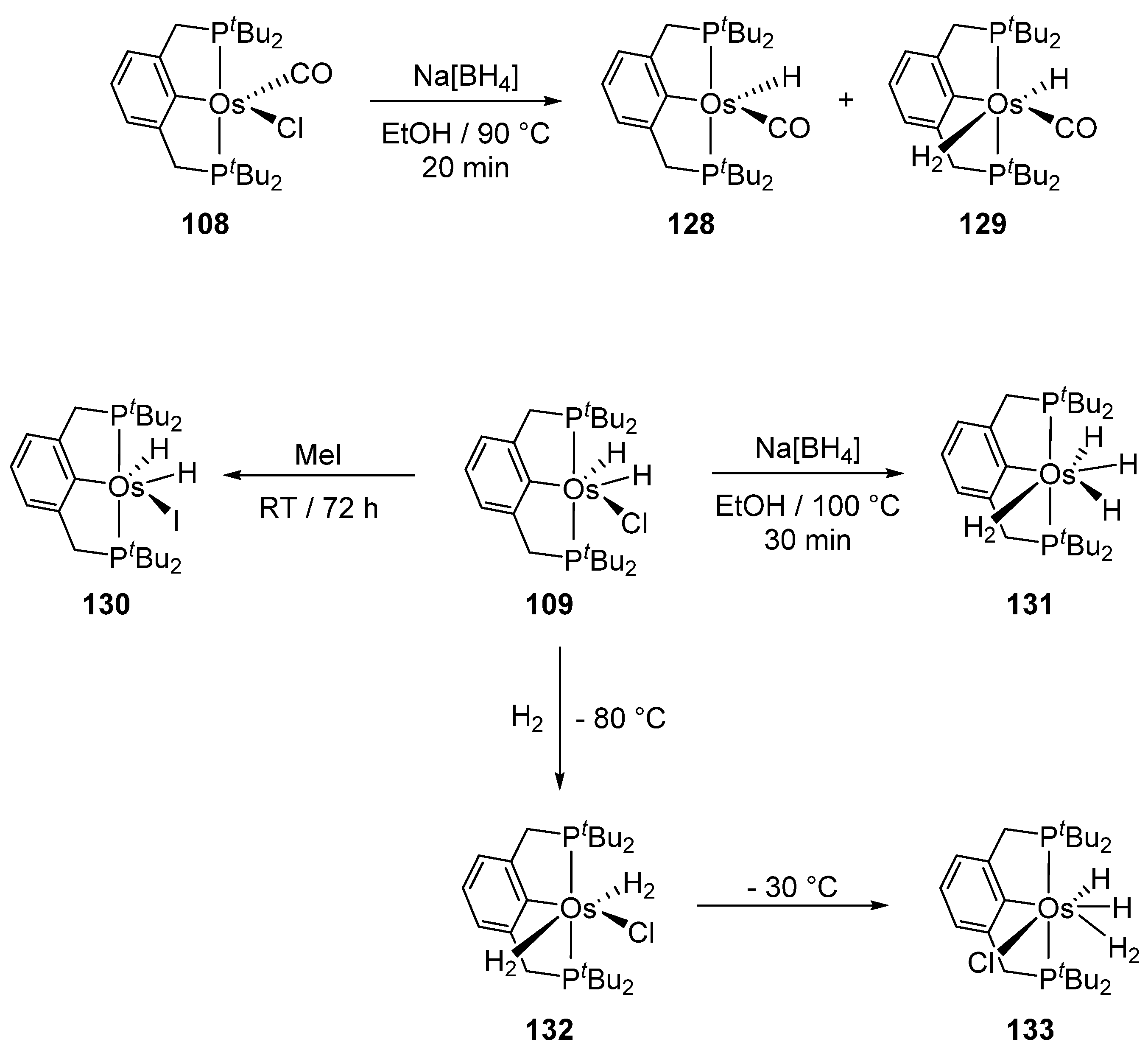
4.2. Reactions of Hydride Complexes
4.3. Ligand Substitution in Osmacycles
5. Applications of Osmacycles
5.1. Catalysis
5.2. Chemical Sensors and Biosensors
5.3. Electronic Properties and Photophysics
5.4. Anticancer and Biological Properties
6. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviation
| 2-MeTHF | 2-methyl-tetrahydrofuran |
| A2780 | Human ovarian cancer cell line |
| A549 | Human lung cancer cell line |
| AND/OR | Boolean operator for browsing in databases |
| ATRP | Atom Transfer Radical Polymerization |
| B3LYP | Becke three-parameter exchange and Lee–Yang–Parr correlation |
| BJAB | Burkitt-like lymphoma |
| Bn | Benzyl substituent |
| bpy | 2,2′-bipyridine |
| C~C’~N | Pincer ligand with Carbon-Carbon-Nitrogen, as donor atoms |
| C~C~C | Pincer ligand with Carbon-Carbon-Carbon, as donor atoms |
| C~C~C~C | Tetradentate Ligand with Carbon-Carbon-Carbon-Carbon as donor atoms |
| C~E | Bidentate ligand with Carbon and E as donor atoms |
| C~N | Bidentate ligand with Carbon and Nitrogen as donor atoms |
| C~N~C | Pincer ligand with Carbon-Nitrogen-Carbon as donor atoms |
| C~N~N | Pincer ligand with Carbon-Nitrogen-Nitrogen as donor atoms |
| C~N~O | Pincer ligand with Carbon-Nitrogen-Oxygen as donor atoms |
| C~O | Bidentate ligand with Carbon and Oxygen as donor atoms |
| CC50 | 50% Cytotoxic concentration |
| CHI/PA-1 | Human ovarian carcinoma cells |
| cod | 1,4-cyclo-octadiene |
| DFT | Density Funtional Theory |
| DMSO | Dimethyl sulphoxide |
| DNA | Deoxyribonucleic acid |
| dpbH | 1,3-di(2-pyridyl)benzene |
| dppb | 1,4-bis(diphenylphosphino)butane |
| E | Donor atom such as: N, P, O, S, As, Se |
| EC50 | 50% Effective concentration |
| GO | Glucose oxidase |
| HBMePI | 1,3-bis(6′-methylpyridyl-2′-imino)isoindoline |
| HEK293 | Kidney adenocarnocinoma cell line |
| HeLa | Cervical cancer cell line |
| HOMO | Highest Occupied Molecular Orbital |
| HRP | Horseradish peroxidase |
| IC50 | 50% Inhibitory concentration |
| ILCT | Intraligand Charge-Transfer |
| log(Po/w) | N-Octanol-water partition coefficient |
| LUMO | Lowest Unoccupied Molecular Orbital |
| Mebib | 2-deprotonated form of 1,3-bis(N-methylbenzimidazolyl)benzene |
| Mebip | bis(N-methylbenzimidazolyl)pyridine) |
| MLCT | Metal-to-Ligand Charge Transfer |
| MT4 | Leukemia cell line |
| N~C~N | Pincer ligand with Nitrogen-Carbon-Nitrogen as donor atoms |
| N~N | Bidentate ligand with Nitrogen-Nitrogen as donor atoms |
| N~N~N | Terdentate ligand where the atom donors are Nitrogen |
| Nalm6 | Leukemia cell line |
| NHC | N-Heterocyclic Carbene |
| NHE | Normal Hydrogen Electrode |
| NMR | Nuclear Magnetic Resonance |
| OLED | Organic Light-Emitting Diode |
| Otf | Triflate |
| P~C(H)~P | Ligand with only phosphorous as donor atoms |
| P~C~P | Pincer ligand with phosphorous–carbon–phosphorous as donor atoms |
| PDT | Photodynamic Therapy |
| PhMeBIm | 1-Phenyl-3-methyl-1H-benzimidazolium |
| phen | 1,10-Phenanthroline |
| phpyH | 2-Phenylpyridine |
| PMMA | Polymethylmethacrylate |
| ROS | Reactive Oxygen Species |
| SCE | Saturated Calomel Electrode |
| SW480 | Colon adenocarcinoma cell line |
| TAML | Tetra-Amido Macrocyclic Ligand |
| TD-DFT | Time-Dependent Density Functional Theory |
| TOF | Turnover Frequency |
| tterpy | 4′-tolyl-2,2′,6′,2”-terpyridine ligand |
References
- Griffith, W.P. Osmium tetroxide and its applications. Platin. Met. Rev. 1974, 18, 94–96. [Google Scholar]
- Smith, M. Organic Synthesis, 4th ed.; Academic Press: Cambridge, MA, USA, 2016; ISBN 9780128007204. [Google Scholar]
- Bozzola, J.J.; Russell, L.D. Electron Microscopy: Principles and Techniques for Biologists, 2nd ed.; Jones and Bartlett: Boston, MA, USA, 1999. [Google Scholar]
- Lay, P.A.; Harman, W.D. Recent advances in Osmium chemistry. Adv. Inorg. Chem. 1991, 37, 219–379. [Google Scholar] [CrossRef]
- Griffith, W.P.; Wilkinson, R.D.G.; McCleverty, J.A. (Eds.) Comprehensive Coordination Chemistry: The Synthesis, Reactions, Properties & Applications of Coordination Compounds; Comprehensive Coordination Chemistry; G. Wilkins; Pergamon: Oxford, UK, 1987; Volume 4. [Google Scholar]
- Warncke, R. Gmelin Handbuch der Anorganischen Chemie; Osmium; Springer: Berlin/Heidelberg, Germany, 1980; Volume 1, No. 66. [Google Scholar]
- Collinson, S.R.; Schröder, M. Osmium: Inorganic & coordination chemistry. Encycl. Inorg. Bioinorg. Chem. 2011, 1–24. [Google Scholar] [CrossRef]
- Shapley, P.A. Osmium: Organometallic chemistry. Encycl. Inorg. Bioinorg. Chem. 2011, 3960–3978. [Google Scholar] [CrossRef]
- Bruce, M.I. Cyclometalation reactions. Angew. Chem. Int. Ed. Engl. 1977, 16, 73–86. [Google Scholar] [CrossRef]
- Omae, I. Organometallic intramolecular-coordination compounds containing a nitrogen donor ligand. Chem. Rev. 1979, 79, 287–321. [Google Scholar] [CrossRef]
- Omae, I. Cyclometalation Reactions: Five-Membered Ring Products as Universal Reagents; Springer: Sayama, Japan, 2014; ISBN 9784431546047. [Google Scholar]
- Michon, C.; Macintyre, K.; Corre, Y.; Agbossou-Niedercorn, F. Pentamethylcyclopentadienyl Iridium(III) metallacycles applied to homogeneous catalysis for fine chemical synthesis. ChemCatChem 2016, 8, 1755–1762. [Google Scholar] [CrossRef]
- Leist, M.; Kerner, C.; Ghoochany, L.T.; Farsadpour, S.; Fizia, A.; Neu, J.P.; Schön, F.; Sun, Y.; Oelkers, B.; Lang, J.; et al. Roll-over cyclometalation: A versatile tool to enhance the catalytic activity of transition metal complexes. J. Organomet. Chem. 2018, 863, 30–43. [Google Scholar] [CrossRef]
- Zi, G. Recent developments in actinide metallacycles. Chem. Commun. 2018, 54, 7412–7430. [Google Scholar] [CrossRef]
- Ryabov, A.D. The exchange of cyclometalated ligands. Molecules 2021, 26, 210. [Google Scholar] [CrossRef]
- Ryabov, A.D. Mechanisms of intramolecular activation of carbon-hydrogen bonds in transition-metal complexes. Chem. Rev. 1990, 90, 403–424. [Google Scholar] [CrossRef]
- Van Der Boom, M.E.; Milstein, D. Cyclometalated phosphine-based pincer complexes: Mechanistic insight in catalysis, coordination, and bond activation. Chem. Rev. 2003, 103, 1759–1792. [Google Scholar] [CrossRef]
- Dupont, J.; Consorti, C.S.; Spencer, J. The potential of palladacycles: More than just precatalysts. Chem. Rev. 2005, 105, 2527–2571. [Google Scholar] [CrossRef]
- Dupont, J.; Pfeffer, M. (Eds.) Palladacycles: Synthesis, Characterization and Applications; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2008; ISBN 9783527623211. [Google Scholar]
- Djukic, J.-P.; Sortais, J.-B.; Barloy, L.; Pfeffer, M. Cycloruthenated compounds—Synthesis and applications. Eur. J. Inorg. Chem. 2009, 2009, 817–853. [Google Scholar] [CrossRef]
- Albrecht, M. Cyclometalation using d-block transition metals: Fundamental Aspects and recent trends. Chem. Rev. 2010, 110, 576–623. [Google Scholar] [CrossRef] [PubMed]
- You, Y.; Nam, W. Photofunctional triplet excited states of cyclometalated Ir(III) complexes: Beyond electroluminescence. Chem. Soc. Rev. 2012, 41, 7061–7084. [Google Scholar] [CrossRef]
- Cutillas, N.; Yellol, G.S.; De Haro, C.; Vicente, C.; Rodríguez, V.; Ruiz, J. Anticancer cyclometalated complexes of platinum group metals and gold. Coord. Chem. Rev. 2013, 257, 2784–2797. [Google Scholar] [CrossRef]
- Dehand, J.; Pfeffer, M. Cyclometallated compounds. Coord. Chem. Rev. 1976, 18, 327–352. [Google Scholar] [CrossRef]
- Dehand, J.; Mutet, C.; Pfeffer, M. Reactivite d’anilines tertiaires vis-a-vis de complexes du palladium: Dealkylation de l’azote et cyclopalladation. J. Organomet. Chem. 1981, 209, 255–270. [Google Scholar] [CrossRef]
- Pfeffer, M. Reactions of cyclopalladated compounds and alkynes: New pathways for organic synthesis? Recl. Des Trav. Chim. Des. Pays-Bas 1990, 109, 567–576. [Google Scholar] [CrossRef]
- Pfeffer, M. Selected applications to organic synthesis of intramolecular C-H activation reactions by transition metals. Pure Appl. Chem. 1992, 64, 335–342. [Google Scholar] [CrossRef]
- Bruce, M.I.; Goodall, B.L.; Stone, F.G. Cyclometallation reactions. J. Organomet. Chem. 1973, 60, 343–349. [Google Scholar] [CrossRef]
- Jameson, G.B.; Muster, A.; Robinson, S.D.; Wingfield, J.N.; Ibers, J.A. Cyclometalated formazan derivatives of ruthenium and Osmium: Structure of Ru((o-C6H4)N=NC(Ph)=NNPh)(CO)(PPH3)2. Inorg. Chem. 1981, 20, 2448–2456. [Google Scholar] [CrossRef]
- Desrosiers, P.J.; Shinomoto, R.S.; Flood, T.C. Intra- and intermolecular activation of carbon-hydrogen bonds in a tetrakis(trimethylphosphine)Osmium(II) system. J. Am. Chem. Soc. 1986, 108, 1346–1347. [Google Scholar] [CrossRef]
- Kisenyi, J.M.; Sunley, G.J.; Cabeza, J.A.; Smith, A.J.; Adams, H.; Salt, N.J.; Maitlis, P.M. The cyclometallation of benzoic acid to give rhodium, iridium, and Osmium C,O-benzoates. X-Ray structure determination of the dibenzoate [(C5Me5)Rh(OOCPh)2(H2O)]. J. Chem. Soc. Dalton Trans. 1987, 2459–2466. [Google Scholar] [CrossRef]
- Beley, M.; Chodorowski, S.; Collin, J.-P.; Sauvage, J.-P.; Flamigni, L.; Barigelletti, F. Luminescent dinuclear complexes containing Ruthenium(II)- and Osmium(II)-terpyridine-type chromophores bridged by a rigid biscyclometalating ligand. Inorg. Chem. 1994, 33, 2543–2547. [Google Scholar] [CrossRef]
- Beley, M.; Collin, J.P.; Sauvage, J.P. Highly coupled mixed-valence dinuclear ruthenium and Osmium complexes with a bis-cyclometalating terpyridine analog as bridging ligand. Inorg. Chem. 1993, 32, 4539–4543. [Google Scholar] [CrossRef]
- Wanandi, P.W.; Tilley, T.D. Osmium alkyl and silyl derivatives with cyclopentadienyl(phosphine) and pentamethylcyclopentadienyl(phosphine) ligand sets. Organometallics 1997, 16, 4299–4313. [Google Scholar] [CrossRef]
- Mamo, A.; Stefio, I.; Poggi, A.; Tringali, C.; DiPietro, C.; Campagna, S. Ruthenium(II) and Osmium(II) complexes with new terdentate polyquinoline and cyclometalating ligands. Synthesis, NMR characterization, luminescence properties, and electrochemical behavior. New J. Chem. 1997, 21, 1173–1185. [Google Scholar]
- Chakravorty, A. New organometallic chemistry of ruthenium and Osmium. Proc. Indian Acad. Sci. Chem. Sci. 1999, 111, 469–477. [Google Scholar] [CrossRef]
- Panda, B.K.; Chakravorty, A. Chemistry of a family of Osmium(II) metallacycles incorporating isonitrile coordination. Indian J. Chem. 2005, 44, 1127–1132. [Google Scholar]
- Zhang, L.; Dang, L.; Wen, T.B.; Sung, H.H.-Y.; Williams, I.D.; Lin, Z.; Jia, G. Cyclometalation of 2-Vinylpyridine with MCl2(PPh3)3 and MHCl(PPh3)3 (M = Ru, Os). Organometallics 2007, 26, 2849–2860. [Google Scholar] [CrossRef]
- Acharyya, R.; Peng, S.-M.; Lee, G.-H.; Bhattacharya, S. An unprecedented oxidative migration of a methyl group from 2-(2′,6′-dimethylphenylazo)-4-methylphenol mediated by ruthenium and Osmium. Inorg. Chem. 2003, 42, 7378–7380. [Google Scholar] [CrossRef] [PubMed]
- Wen, T.B.; Cheung, Y.K.; Yao, J.; Wong, W.-T.; Zhou, Z.Y.; Jia, G. Vinylidene and carbyne complexes derived from the reactions of OsCl(PPh3)(PCP) (PCP = 2,6-(PPh2CH2)2C6H3) with terminal acetylenes. Organometallics 2000, 18, 3803–3809. [Google Scholar] [CrossRef]
- Gauvin, R.M.; Rozenberg, H.; Shimon, L.J.W.; Milstein, D. Synthesis and structure of new Osmium-PCP complexes. Osmium-mediated C-C bond activation. Organometallics 2001, 20, 1719–1724. [Google Scholar] [CrossRef]
- McQueen, C.M.A.; Hill, A.F.; Ma, C.; Ward, J.S. Ruthenium and Osmium complexes of dihydroperimidine-based N-heterocyclic carbene pincer ligands. Dalton Trans. 2015, 44, 20376–20385. [Google Scholar] [CrossRef]
- Majumder, K.; Peng, S.-M.; Bhattacharya, S. Cyclometallation and N=N bond cleavage of 2-(arylazo)phenols by Osmium. Synthesis, structure and redox properties. J. Chem. Soc. Dalton Trans. 2001, 33, 284–288. [Google Scholar] [CrossRef]
- Das, A.; Basuli, F.; Falvello, L.R.; Bhattacharya, S. Unusual transformation of N-arylbenzohydroxamic acids mediated by Osmium. Formation of organometallic complexes of Osmium(III). Inorg. Chem. 2001, 40, 4085–4088. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Djurovich, P.I.; Haiges, R.; Thompson, M.E. Synthesis and photophysical characterization of a bis-pincer Osmium complex. Polyhedron 2014, 84, 136–143. [Google Scholar] [CrossRef]
- Gong, L.; Lin, Y.; Wen, T.B.; Zhang, H.; Zeng, B.; Xia, H. Formation of four conjugated osmacyclic species in a one-pot reaction. Organometallics 2008, 27, 2584–2589. [Google Scholar] [CrossRef]
- Xia, H.; He, G.; Zhang, H.; Wen, T.B.; Herman, H.; Sung, Y.; Williams, I.D.; Jia, G. Osmabenzenes from the reactions of HC≡CCH(OH)C≡CH with OsX2(PPh3)3 (X = Cl, Br). J. Am. Chem. Soc. 2004, 126, 6862–6863. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Gong, L.; Xu, H.; He, X.; Wen, T.B.; Xia, H. Nine-membered osmacycles derived from metathesis reactions between Alkynes and an osmafuran. Organometallics 2009, 28, 1524–1533. [Google Scholar] [CrossRef]
- Buil, M.L.; Esteruelas, M.A.; Goni, E.; Oliván, M.; Oñate, E. Displacement of phenyl and styryl ligands by benzophenone imine and 2-vinylpyridine on Ruthenium and Osmium. Organometallics 2006, 25, 3076–3083. [Google Scholar] [CrossRef]
- Clark, A.M.; Rickard, C.E.F.; Roper, W.R.; Wright, L.J. Electrophilic substitution reactions at the phenyl ring of the chelated 2-(2‘-Pyridyl)phenyl ligand bound to Ruthenium(II) or Osmium(II). Organometallics 1999, 18, 2813–2820. [Google Scholar] [CrossRef]
- Bennett, M.A.; Clark, A.M.; Contel, M.; Rickard, C.E.F.; Roper, W.R.; Wright, L.J. Cyclometallated complexes of ruthenium and Osmium containing the o-C6H4PPh2 ligand. J. Organomet. Chem. 2000, 601, 299–304. [Google Scholar] [CrossRef]
- Rickard, C.E.F.; Roper, W.R.; Taylor, G.E.; Waters, J.M.; Wright, L.J. Coordinatively unsaturated σ-aryl complexes of Ruthenium(II) and Osmium(II). J. Organomet. Chem. 1990, 389, 375–388. [Google Scholar] [CrossRef]
- Esteruelas, M.A.; López, A.M.; Oliván, M. Polyhydrides of platinum group metals: Nonclassical interactions and σ-bond activation reactions. Chem. Rev. 2016, 116, 8770–8847. [Google Scholar] [CrossRef] [Green Version]
- Barrio, P.; Castarlenas, R.; Esteruelas, M.A.; Lledós, A.; Maseras, F.; Oñate, E.; Tomàs, J. Reactions of a hexahydride-Osmium Complex with aromatic ketones: C−H activation versus C−F activation. Organometallics 2001, 20, 442–452. [Google Scholar] [CrossRef]
- Barrio, P.; Castarlenas, R.; Esteruelas, M.A.; Oñate, E. Triple C-H activation of a cycloalkyl ketone using an Osmium-hexahydride complex. Organometallics 2001, 20, 2635–2638. [Google Scholar] [CrossRef]
- Buil, M.L.; Esteruelas, M.A.; Garcés, K.; Oliván, M.; Oñate, E. Understanding the formation of N-H tautomers from α-substituted pyridines: Tautomerization of 2-ethylpyridine promoted by Osmium. J. Am. Chem. Soc. 2007, 129, 10998–10999. [Google Scholar] [CrossRef] [PubMed]
- Baya, M.; Eguillor, B.; Esteruelas, M.A.; Lledós, A.; Olivan, M.; Oñate, E. Coordination and rupture of methyl C(sp3)-H bonds in Osmium-polyhydride complexes with δ agostic interaction. Organometallics 2007, 26, 5140–5152. [Google Scholar] [CrossRef]
- Esteruelas, M.A.; Masamunt, A.B.; Oliván, M.; Oñate, E.; Valencia, M. Aromatic diosmatricyclic nitrogen-containing compounds. J. Am. Chem. Soc. 2008, 130, 11612–11613. [Google Scholar] [CrossRef]
- Esteruelas, M.A.; Fernández, I.; Gómez-Gallego, M.; Martín-Ortíz, M.; Molina, P.; Oliván, M.; Otón, F.; Sierra, M.A.; Valencia, M. Mono- and dinuclear Osmium N,N′-di- and tetraphenylbipyridyls and extended bipyridyls. Synthesis, structure and electrochemistry. Dalton Trans. 2013, 42, 3597. [Google Scholar] [CrossRef]
- Casarrubios, L.; Esteruelas, M.A.; Larramona, C.; Muntaner, J.G.; Oñate, E.; Sierra, M.A. 2-Azetidinones as precursors of pincer ligands: Preparation, structure, and spectroscopic properties of CC′N-Osmium complexes. Inorg. Chem. 2015, 54, 10998–11006. [Google Scholar] [CrossRef] [Green Version]
- Casarrubios, L.; Esteruelas, M.A.; Larramona, C.; Lledós, A.; Muntaner, J.G.; Oñate, E.; Ortuño, M.A.; Sierra, M.A. Mechanistic insight into the facilitation of β-lactam fragmentation through metal assistance. Chem. A Eur. J. 2015, 21, 16781–16785. [Google Scholar] [CrossRef] [PubMed]
- Eguillor, B.; Esteruelas, M.A.; Lezáun, V.; Oliván, M.; Oñate, E. Elongated dihydrogen versus compressed dihydride in Osmium complexes. Chem. A Eur. J. 2017, 23, 1526–1530. [Google Scholar] [CrossRef] [Green Version]
- Eguillor, B.; Esteruelas, M.A.; Fernández, I.; Gómez-Gallego, M.; Lledós, A.; Martín-Ortiz, M.; Oliván, M.; Oñate, E.; Sierra, M.A. Azole assisted C-H bond activation promoted by an Osmium-polyhydride: Discerning between N and NH. Organometallics 2015, 34, 1898–1910. [Google Scholar] [CrossRef] [Green Version]
- Buil, M.L.; Cardo, J.J.F.; Esteruelas, M.A.; Fernández, I.; Oñate, E. An entry to stable mixed phosphine-Osmium-NHC polyhydrides. Inorg. Chem. 2016, 55, 5062–5070. [Google Scholar] [CrossRef]
- Esteruelas, M.A.; Larramona, C.; Oñate, E. Osmium-mediated direct C-H bond activation at the 8-position of quinolines. Organometallics 2016, 35, 1597–1600. [Google Scholar] [CrossRef] [Green Version]
- Baya, M.; Esteruelas, M.A.; Oñate, E. Efficient concatenation of C═C reduction, C-H bond activation, and C-C and C-N coupling reactions on Osmium: Assembly of two allylamines and an allene. Organometallics 2010, 29, 6298–6307. [Google Scholar] [CrossRef]
- Crespo, O.; Eguillor, B.; Esteruelas, M.A.; Fernández, I.; García-Raboso, J.; Gómez-Gallego, M.; Martín-Ortiz, M.; Oliva, M.; Sierra, M.A. Synthesis and characterisation of [6]-azaosmahelicenes: The first d4-heterometallahelicenes. Chem. Commun. 2012, 48, 5328–5330. [Google Scholar] [CrossRef]
- Esteruelas, M.A.; Fernández, I.; Herrera, A.; Martín-Ortiz, M.; Martínez-Álvarez, R.; Oliván, M.; Oñate, E.; Sierra, M.A.; Valencia, M. Multiple C-H bond activation of phenyl-substituted pyrimidines and triazines promoted by an Osmium polyhydride: Formation of osmapolycycles with three, five, and eight fused rings. Organometallics 2010, 29, 976–986. [Google Scholar] [CrossRef]
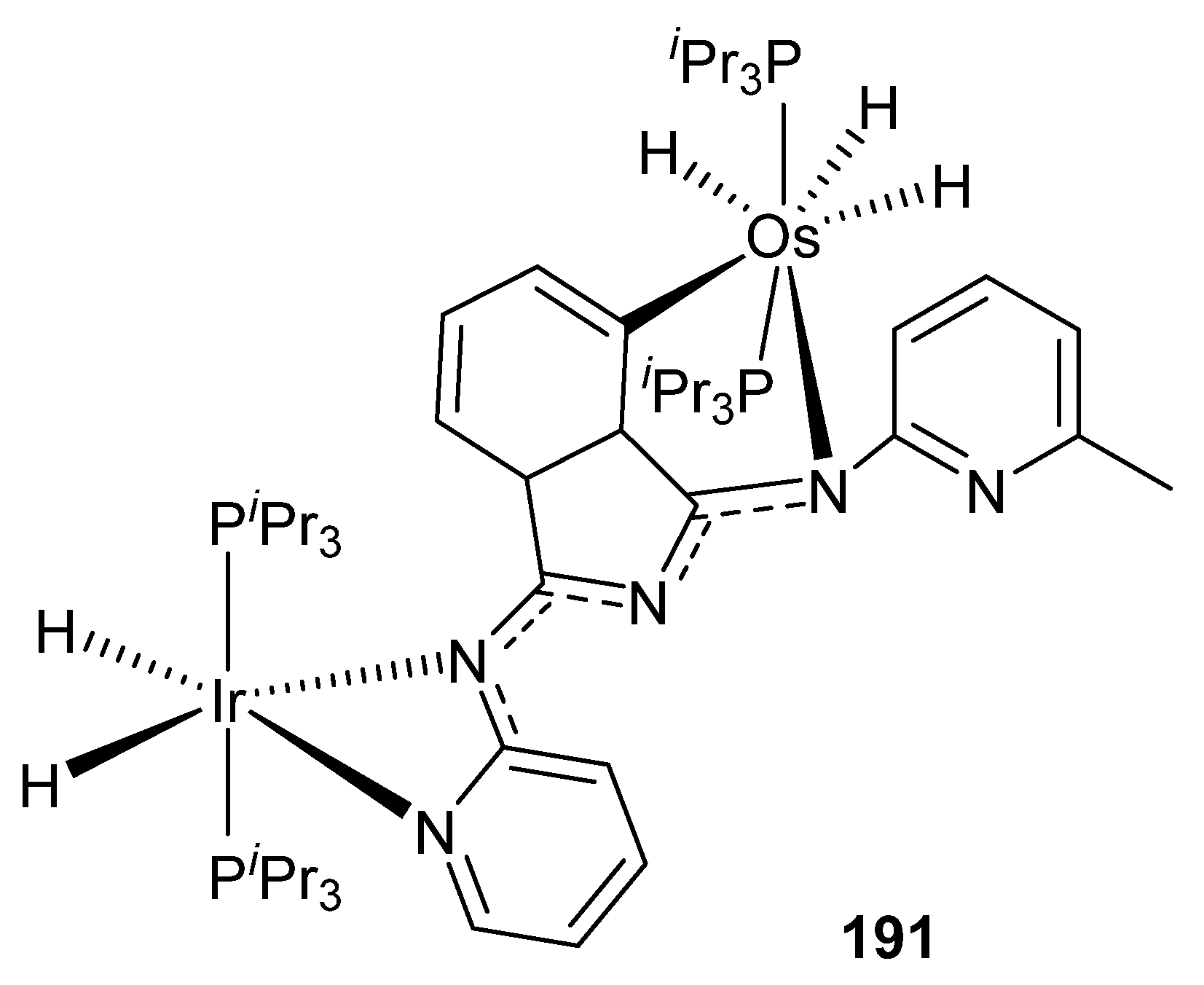
- Eguillor, B.; Esteruelas, M.A.; Lezáun, V.; Oliván, M.; Oñate, E.; Tsai, J.-Y.; Xia, C. A capped octahedral MHC6 compound of a Platinum group metal. Chem. A Eur. J. 2016, 22, 9106–9110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arthur, T.; Stephenson, T.A. Synthesis of triple halide-bridged arene complexes of Ruthenium(II) and Osmium(II). J. Organomet. Chem. 1981, 208, 369–387. [Google Scholar] [CrossRef]
- Ryabov, A.D.; Soukharev, V.S.; Alexandrova, L.; Le Lagadec, R.; Pfeffer, M. Low-potential cyclometalated Osmium(II) mediators of glucose oxidase. Inorg. Chem. 2003, 42, 6598–6600. [Google Scholar] [CrossRef]
- Abbenhuis, H.C.L.; Pfeffer, M.; Sutter, J.P.; de Cian, A.; Fischer, J.; Ji, H.L.; Nelson, J.H. Carbon-carbon and carbon-nitrogen bond formation mediated by Ruthenium(II) complexes: Synthesis of (1H)-isoquinolinium derivatives. Organometallics 1993, 12, 4464–4472. [Google Scholar] [CrossRef]
- Ferstl, W.; Sakodinskaya, I.K.; Beydoun-Sutter, N.; Le Borgne, G.; Pfeffer, M.; Ryabov, A.D. Mechanism of alkyne insertion into the Ru-C bonds of orthoruthenated compounds featuring similarity of the Ru(II) and Pd(II) reactions. Organometallics 1997, 16, 411–418. [Google Scholar] [CrossRef]
- Pfeffer, M.; Sutter, J.-P.; Urriolabeitia, E.P. Ruthenium mediated carbon-carbon and carbon-nitrogen bond formation: Parameters governing the reactivity of the metal centre. Bull. Soc. Chim. Fr. 1997, 134, 947–954. [Google Scholar]
- Fernandez, S.; Pfeffer, M.; Ritleng, V.; Sirlin, C. An effective route to cycloruthenated N-ligands under mild conditions. Organometallics 1999, 18, 2390–2394. [Google Scholar] [CrossRef]
- Cerón-Camacho, R.; Morales-Morales, D.; Hernandez, S.; Le Lagadec, R.; Ryabov, A.D. Easy access to bio-inspired Osmium(II) complexes through electrophilic intramolecular C(sp2)-H bond cyclometalation. Inorg. Chem. 2008, 47, 4988–4995. [Google Scholar] [CrossRef]
- Esteruelas, M.A.; Oñate, E.; Palacios, A.; Tsai, J.Y.; Xia, C. Preparation of capped octahedral OsHC6 complexes by sequential carbon-directed C-H bond activation reactions. Organometallics 2016, 35, 2532–2542. [Google Scholar] [CrossRef] [Green Version]
- Bolje, A.; Hohloch, S.; van der Meer, M.; Košmrlj, J.; Sarkar, B. RuII, OsII, and IrII complexes with chelating pyridyl-mesoionic carbene ligands: Structural characterization and applications in transfer hydrogenation catalysis. Chem. A Eur. J. 2015, 21, 6756–6764. [Google Scholar] [CrossRef]
- Cerón-Camacho, R.; Hernández, S.; Le Lagadec, R.; Ryabov, A.D. Cyclometalated [Os(C-N)x(N-N)3-x]m+ mimetics of tris(2,2′-bipyridine)Osmium(II): Covering a 2 V potential range by known (x = 0, 1) and new (x = 2, 3) species (C-N = o-2-phenylpyridinato). Chem. Commun. 2011, 47, 2823–2825. [Google Scholar] [CrossRef] [PubMed]
- Cerón-Camacho, R.; Le Lagadec, R.; Kurnikov, I.V.; Ryabov, A.D. A glance at the reactivity of osma(II)cycles [Os(C-N)x(bpy)3-x]m+ (x=0-3) covering a 1.8 V potential range toward peroxidase through monte carlo simulations (-C-N = o-2-phenylpyridinato, bpy=2,2′-bipyridine). J. Inorg. Biochem. 2014, 134, 20–24. [Google Scholar] [CrossRef] [PubMed]
- Gusev, D.G.; Dolgushin, F.M.; Antipin, M.Y. Cyclometalated Osmium complexes containing a tridentate PCP ligand. Organometallics 2001, 20, 1001–1007. [Google Scholar] [CrossRef]
- Gusev, D.G.; Lough, A.J. Double C-H activation on Osmium and Ruthenium centers: Carbene vs. olefin products. Organometallics 2002, 21, 2601–2603. [Google Scholar] [CrossRef]
- Bolaño, T.; Castarlenas, R.; Esteruelas, M.A.; Oñate, E. Assembly of an allenylidene ligand, a terminal alkyne, and an acetonitrile molecule: Formation of osmacyclopentapyrrole derivatives. J. Am. Chem. Soc. 2006, 128, 3965–3973. [Google Scholar] [CrossRef] [PubMed]
- Shao, J.-Y.; Zhong, Y.-W. Monometallic Osmium(II) complexes with bis(N-methylbenzimidazolyl)benzene or -pyridine: A comparison study with Ruthenium(II) analogues. Inorg. Chem. 2013, 52, 6464–6472. [Google Scholar] [CrossRef]
- Wen, T.B.; Zhou, Z.Y.; Jia, G. Coupling reaction of phenylacetylene with OsHn(PPh3)(2,6-(PPh2CH2)2C6H3) (n = 1, 3). Organometallics 2003, 22, 4947–4951. [Google Scholar] [CrossRef]
- Barrio, P.; Esteruelas, M.A.; Lledós, A.; Oñate, E.; Tomàs, J. Influence of the cis ligand on the H-H separation and the rotation barrier of the dihydrogen in Osmium-elongated dihydrogen complexes containing an ortho-metalated ketone. Organometallics 2004, 23, 3008–3015. [Google Scholar] [CrossRef]
- Gong, L.; Chen, Z.; Lin, Y.; He, X.; Wen, T.B.; Xu, X.; Xia, H. Osmabenzenes from Osmacycles containing an η2-coordinated olefin. Chem. A Eur. J. 2009, 15, 6258–6266. [Google Scholar] [CrossRef]
- Arockiam, P.B.; Bruneau, C.; Dixneuf, P.H. Ruthenium(II)-catalyzed C-H bond activation and functionalization. Chem. Rev. 2012, 112, 5879–5918. [Google Scholar] [CrossRef] [PubMed]
- Simonetti, M.; Cannas, D.M.; Just-Baringo, X.; Vitorica-Yrezabal, I.J.; Larrosa, I. Cyclometallated Ruthenium catalyst enables late-stage directed arylation of pharmaceuticals. Nat. Chem. 2018, 10, 724–731. [Google Scholar] [CrossRef]
- Kapdi, A.; Maiti, D. (Eds.) Palladacycles: Catalysis and Beyond; Elsevier: Amsterdam, The Netherlands, 2019; ISBN 9780128155059. [Google Scholar]
- Liu, S.H.; Lo, S.T.; Wen, T.B.; Williams, I.D.; Zhou, Z.Y.; Lau, C.P.; Jia, G. Reactions of hydrogen with Ruthenium and Osmium complexes containing tridentate ligands Cy2PCH2CH(CH2)2PCy2 and 2,6-(Ph2PCH2)2C6H3. Inorg. Chim. Acta 2002, 334, 122–130. [Google Scholar] [CrossRef]
- Wen, T.B.; Zhou, Z.Y.; Jia, G. Osmium-mediated hexamerization of phenylacetylene. Angew. Chem. Int. Ed. 2006, 45, 5842–5846. [Google Scholar] [CrossRef] [PubMed]
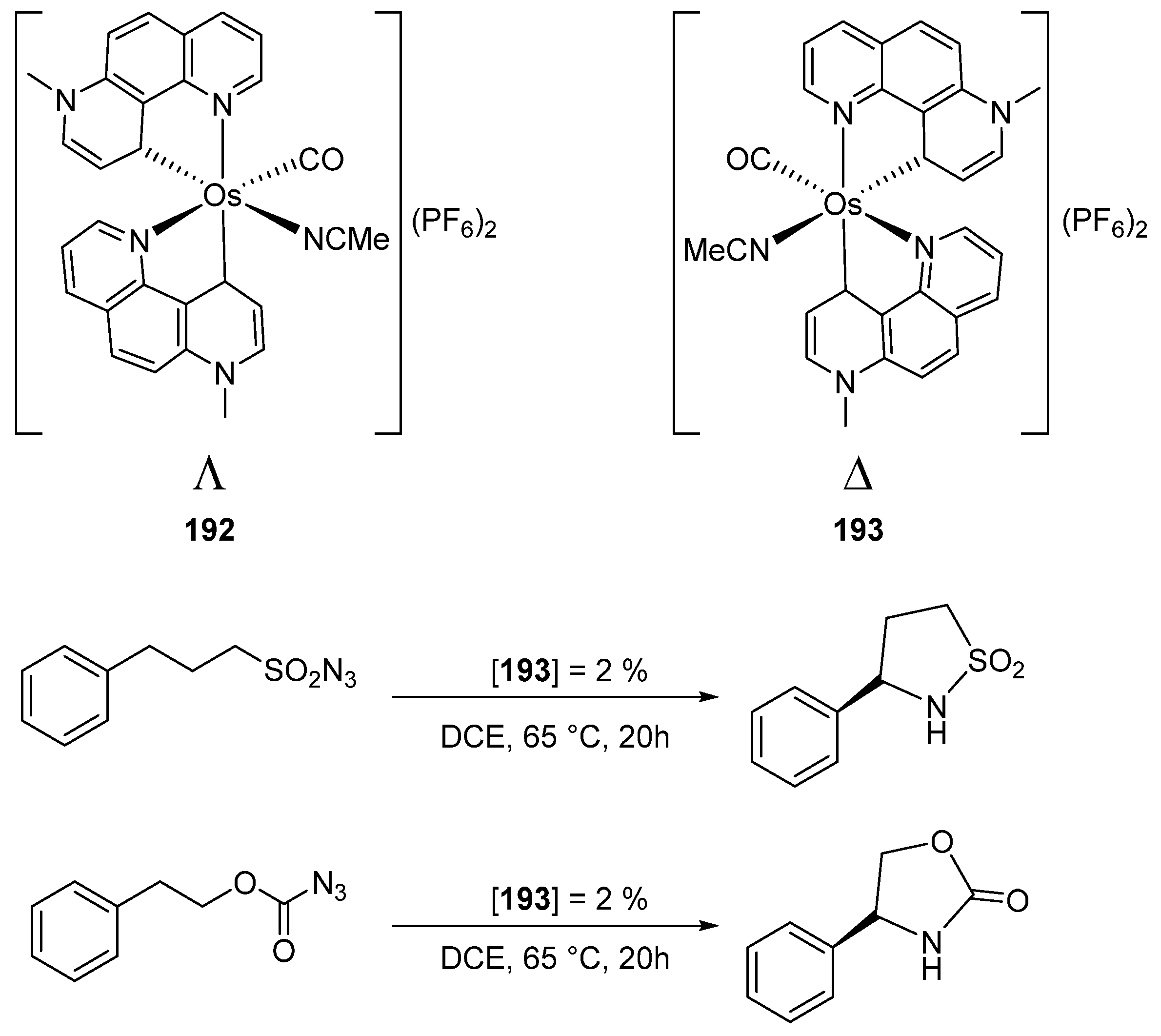
- Baratta, W.; Ballico, M.; Baldino, S.; Chelucci, G.; Herdtweck, E.; Siega, K.; Magnolia, S.; Rigo, P. New benzo[h]quinoline-based ligands and their Pincer Ru and Os complexes for efficient catalytic transfer hydrogenation of carbonyl compounds. Chem. A Eur. J. 2008, 14, 9148–9160. [Google Scholar] [CrossRef]
- Baratta, W.; Ballico, M.; Chelucci, G.; Siega, K.; Rigo, P. Osmium(II) CNN pincer complexes as efficient catalysts for both asymmetric transfer and H2 hydrogenation of ketones. Angew. Chem. Int. Ed. 2008, 47, 4362–4365. [Google Scholar] [CrossRef] [PubMed]
- Baratta, W.; Fanfoni, L.; Magnolia, S.; Siega, K.; Rigo, P. Benzo[h]quinoline pincer Ruthenium and Osmium catalysts for hydrogenation of ketones. Eur. J. Inorg. Chem. 2010, 1419–1423. [Google Scholar] [CrossRef]
- Baratta, W.; Benedetti, F.; Del Zotto, A.; Fanfoni, L.; Felluga, F.; Magnolia, S.; Putignano, E.; Rigo, P. Chiral pincer Ruthenium and Osmium complexes for the fast and efficient hydrogen transfer reduction of ketones. Organometallics 2010, 29, 3563–3570. [Google Scholar] [CrossRef]
- Baratta, W.; Bossi, G.; Putignano, E.; Rigo, P. Pincer and diamine Ru and Os diphosphane complexes as efficient catalysts for the dehydrogenation of alcohols to ketones. Chem. A Eur. J. 2011, 17, 3474–3481. [Google Scholar] [CrossRef]
- Bossi, G.; Putignano, E.; Rigo, P.; Baratta, W. Pincer Ru and Os complexes as efficient catalysts for racemization and deuteration of alcohols. Dalton Trans. 2011, 40, 8986–8995. [Google Scholar] [CrossRef] [PubMed] [Green Version]
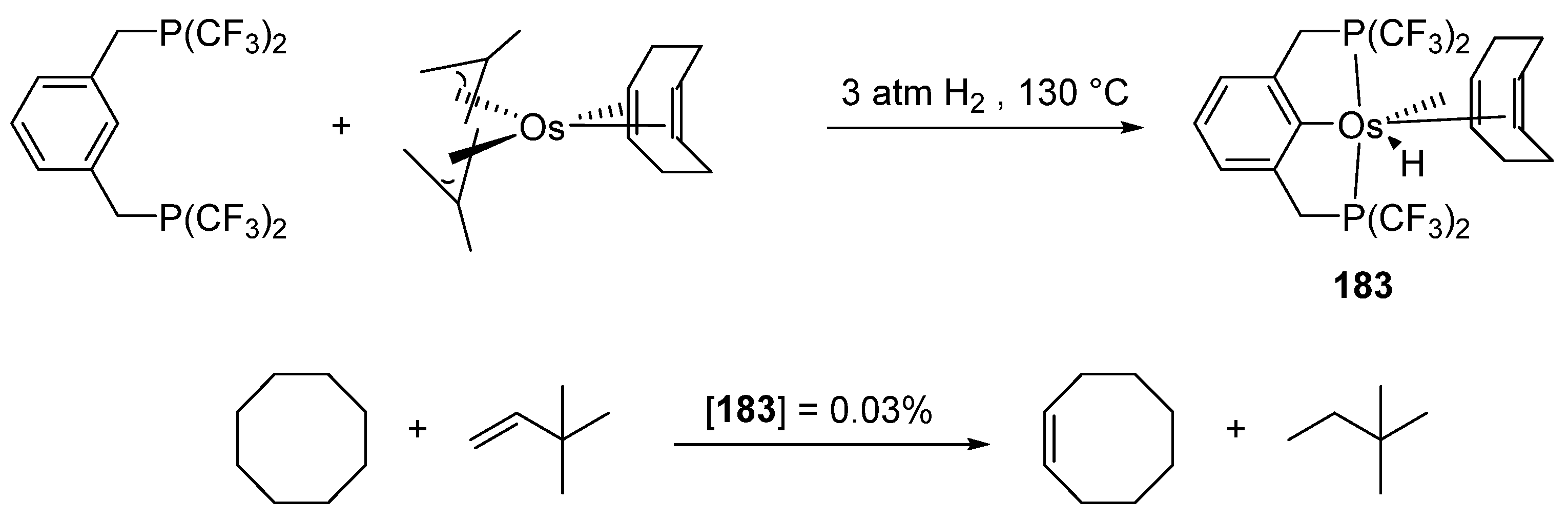
- Gruver, B.C.; Adams, J.J.; Arulsamy, N.; Roddick, D.M. Acceptor pincer chemistry of Osmium: Catalytic alkane dehydrogenation by (CF3 PCP)Os(cod)(H). Organometallics 2013, 32, 6468–6475. [Google Scholar] [CrossRef]
- Bolaño, T.; Esteruelas, M.A.; Fernández, I.; Oñate, E.; Palacios, A.; Tsai, J.Y.; Xia, C. Osmium(II)-bis(dihydrogen) complexes containing Caryl, CNHC-chelate ligands: Preparation, bonding situation, and acidity. Organometallics 2015, 34, 778–789. [Google Scholar] [CrossRef] [Green Version]
- Bolaño, T.; Esteruelas, M.A.; Gay, M.P.; Oñate, E.; Pastor, I.M.; Yus, M. An acyl-NHC Osmium cooperative system: Coordination of small molecules and heterolytic B-H and O-H bond activation. Organometallics 2015, 34, 3902–3908. [Google Scholar] [CrossRef] [Green Version]
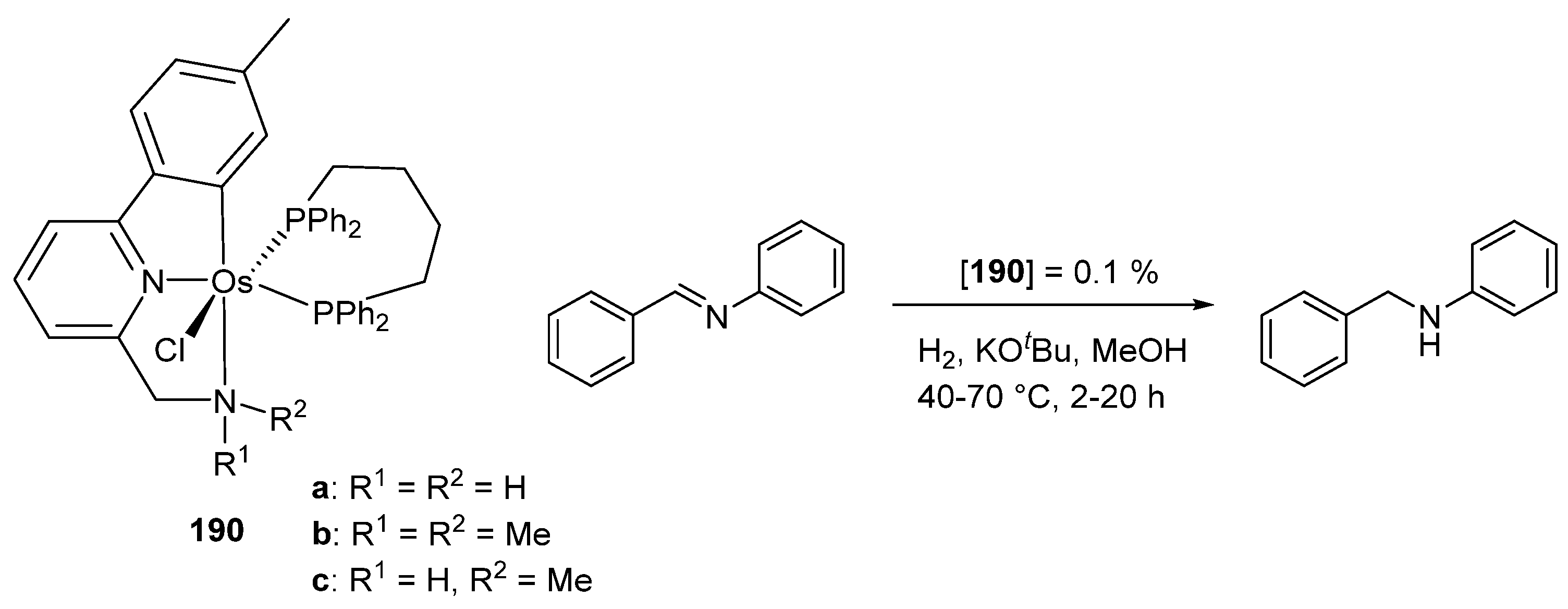
- Solinas, M.; Sechi, B.; Baldino, S.; Baratta, W.; Chelucci, G. Hydrogenation of imines catalyzed by 2-(Aminomethyl)pyridine-based ruthenium and Osmium complexes. ChemistrySelect 2016, 1, 2492–2497. [Google Scholar] [CrossRef]
- Buil, M.L.; Esteruelas, M.A.; Izquierdo, S.; Nicasio, A.I.; Oñate, E. N-H and C-H bond activations of an isoindoline promoted by Iridium- and Osmium-polyhydride complexes: A noninnocent bridge ligand for acceptorless and Base-Free Dehydrogenation of secondary alcohols. Organometallics 2020, 39, 2719–2731. [Google Scholar] [CrossRef]
- Wang, G.; Zhou, Z.; Shen, X.; Ivlev, S.; Meggers, E. Asymmetric catalysis with a chiral-at-Osmium complex. Chem. Commun. 2020, 56, 7714–7717. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Chen, L.A.; Huo, H.; Shen, X.; Harms, K.; Gong, L.; Meggers, E. Asymmetric lewis acid catalysis directed by octahedral rhodium centrochirality. Chem. Sci. 2015, 6, 1094–1100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, Y.; Tan, Y.; Harms, K.; Marsch, M.; Riedel, R.; Zhang, L.; Meggers, E. Octahedral Ruthenium complex with exclusive metal-centered chirality for highly effective asymmetric catalysis. J. Am. Chem. Soc. 2017, 139, 4322–4325. [Google Scholar] [CrossRef]
- Zhou, Z.; Chen, S.; Hong, Y.; Winterling, E.; Tan, Y.; Hemming, M.; Harms, K.; Houk, K.N.; Meggers, E. Non-C2-symmetric chiral-at-Ruthenium catalyst for highly efficient enantioselective intramolecular C(sp3)-H amidation. J. Am. Chem. Soc. 2019, 141, 19048–19057. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Lugo, C.; Le Lagadec, R.; Ryabov, A.D.; Valverde, G.C.; Morales, S.L.; Alexandrova, L. “Living” radical polymerization of styrene catalyzed by cyclometalated Ruthenium(II) complexes bearing nonlabile ligands. J. Polym. Sci. Part A Polym. Chem. 2009, 47, 3814–3828. [Google Scholar] [CrossRef]
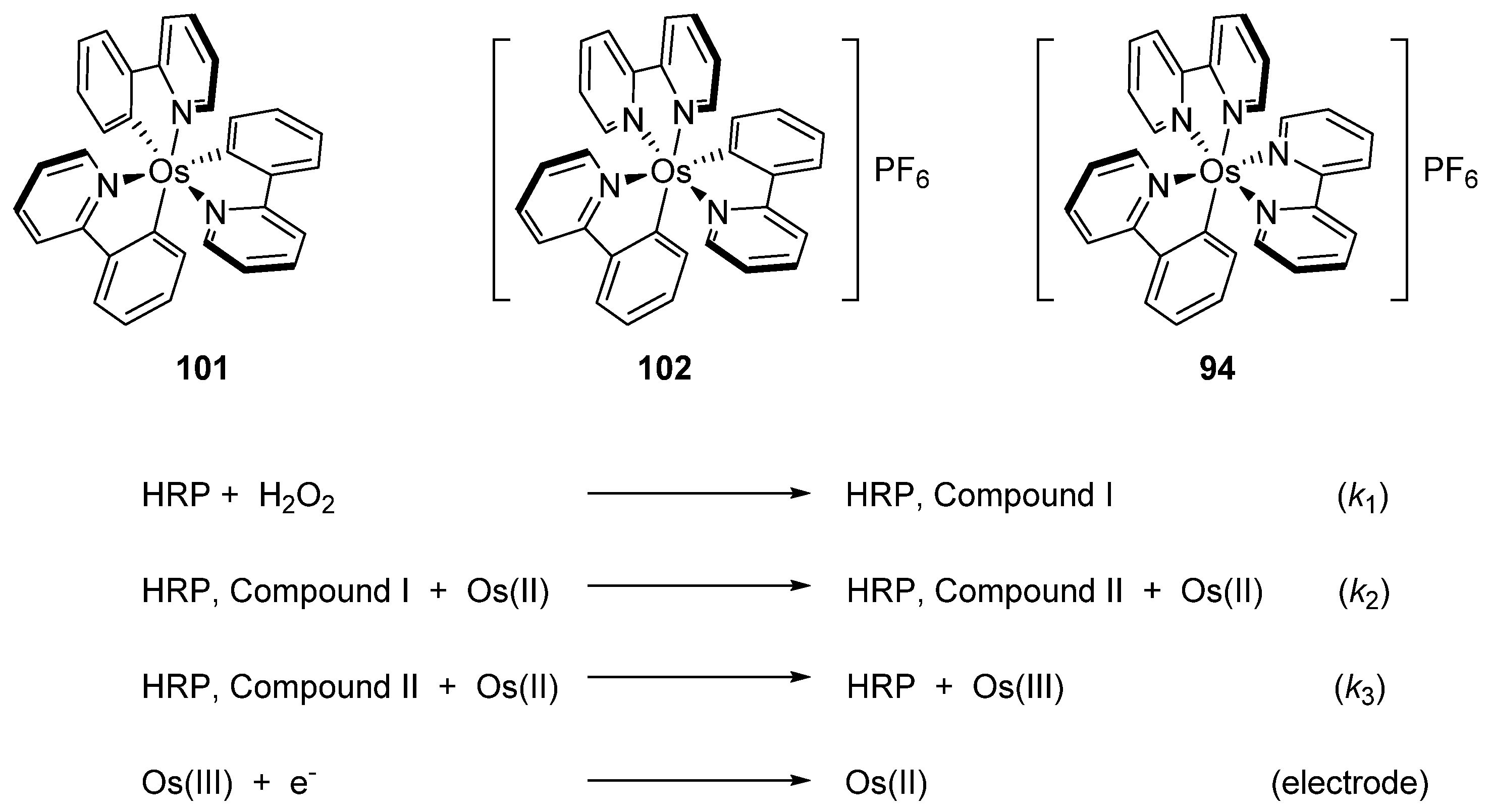
- Ryabov, A.D. Transition metal chemistry of glucose oxidase, horseradish peroxidase, and related enzymes. Adv. Inorg. Chem. 2004, 55, 201–269. [Google Scholar] [CrossRef]
- Ryabov, A.D.; Sukbarev, V.S.; Alexandrova, L.; Le Lagadec, R.; Pfeffer, M. New synthesis and new bio-application of cyclometalated Ruthenium(II) complexes for fast mediated electron transfer with peroxidase and glucose oxidase. Inorg. Chem. 2001, 40, 6529–6532. [Google Scholar] [CrossRef]
- Le Lagadec, R.; Rubio, L.; Alexandrova, L.; Toscano, R.A.; Ivanova, E.V.; Meškys, R.; Laurinavičius, V.; Pfeffer, M.; Ryabov, A.D. Cyclometalated N,N-dimethylbenzylamine Ruthenium(II) complexes [Ru(C6HR1R2R3-o-CH2NMe2)(bpy)(RCN)2]PF6 for bioapplications: Synthesis, characterization, crystal structures, redox properties, and reactivity toward PQQ-dependent glucose dehydrogenase. J. Organomet. Chem. 2004, 689, 4820–4832. [Google Scholar] [CrossRef]
- Ryabov, A.D.; Kurova, V.S.; Ivanova, E.V.; Le Lagadec, R.; Alexandrova, L. Redox mediation and photomechanical oscillations involving photosensitive cyclometalated Ru(II) complexes, glucose oxidase, and peroxidase. Anal. Chem. 2005, 77, 1132–1139. [Google Scholar] [CrossRef] [PubMed]
- Saji, T.; Aoyagui, S. Polarographic studies on bipyridines complexes. II. Correlation between charge-transfer frequencies and oxidation potentials of tris (2,2′-bipyridine) complexes of iron, Ruthenium, Osmium, Cobalt and Chromium. J. Electroanal. Chem. 1975, 60, 1–10. [Google Scholar] [CrossRef]
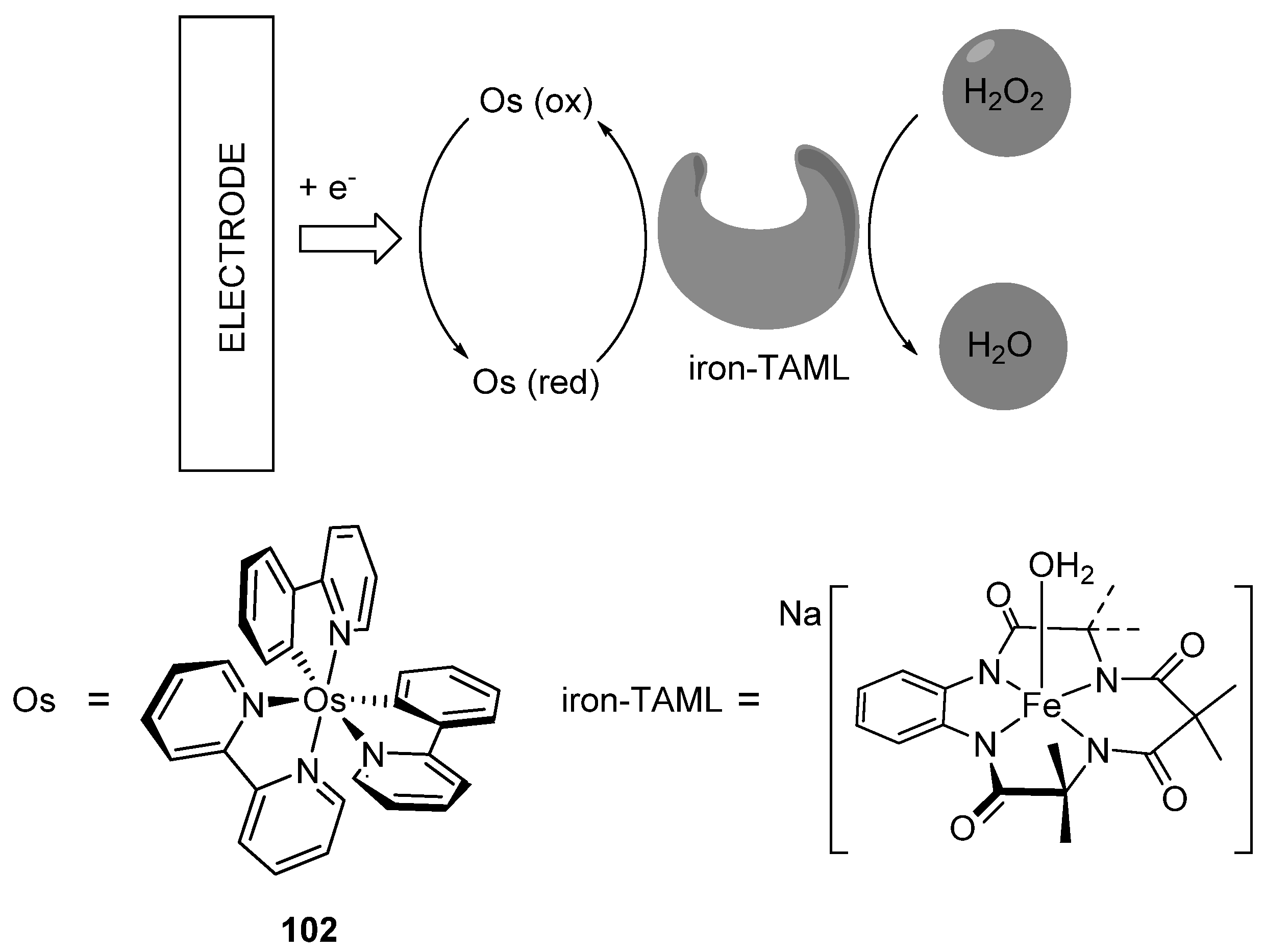
- Ryabov, A.D.; Cerón-Camacho, R.; Saavedra-Díaz, O.; Denardo, M.A.; Ghosh, A.; Le Lagadec, R.; Collins, T.J. TAML activator-based amperometric analytical devices as alternatives to peroxidase biosensors. Anal. Chem. 2012, 84, 9096–9100. [Google Scholar] [CrossRef] [PubMed]
- Demas, J.N.; DeGraff, Β.A. Design and applications of highly luminescent transition metal complexes. Anal. Chem. 1991, 63, 829A–837A. [Google Scholar] [CrossRef]
- Bhaumik, C.; Das, S.; Maity, D.; Baitalik, S. Luminescent bis-tridentate Ruthenium(II) and Osmium(II) complexes based on terpyridyl-imidazole ligand: Synthesis, structural characterization, photophysical, electrochemical, and solvent dependence studies. Dalton Trans. 2012, 41, 2427–2438. [Google Scholar] [CrossRef]
- Wilkinson, A.J.; Puschmann, H.; Howard, J.A.K.; Foster, C.E.; Williams, J.A.G. Luminescent complexes of Iridium(III) containing N∧C∧N-coordinating terdentate ligands. Inorg. Chem. 2006, 45, 8685–8699. [Google Scholar] [CrossRef] [PubMed]
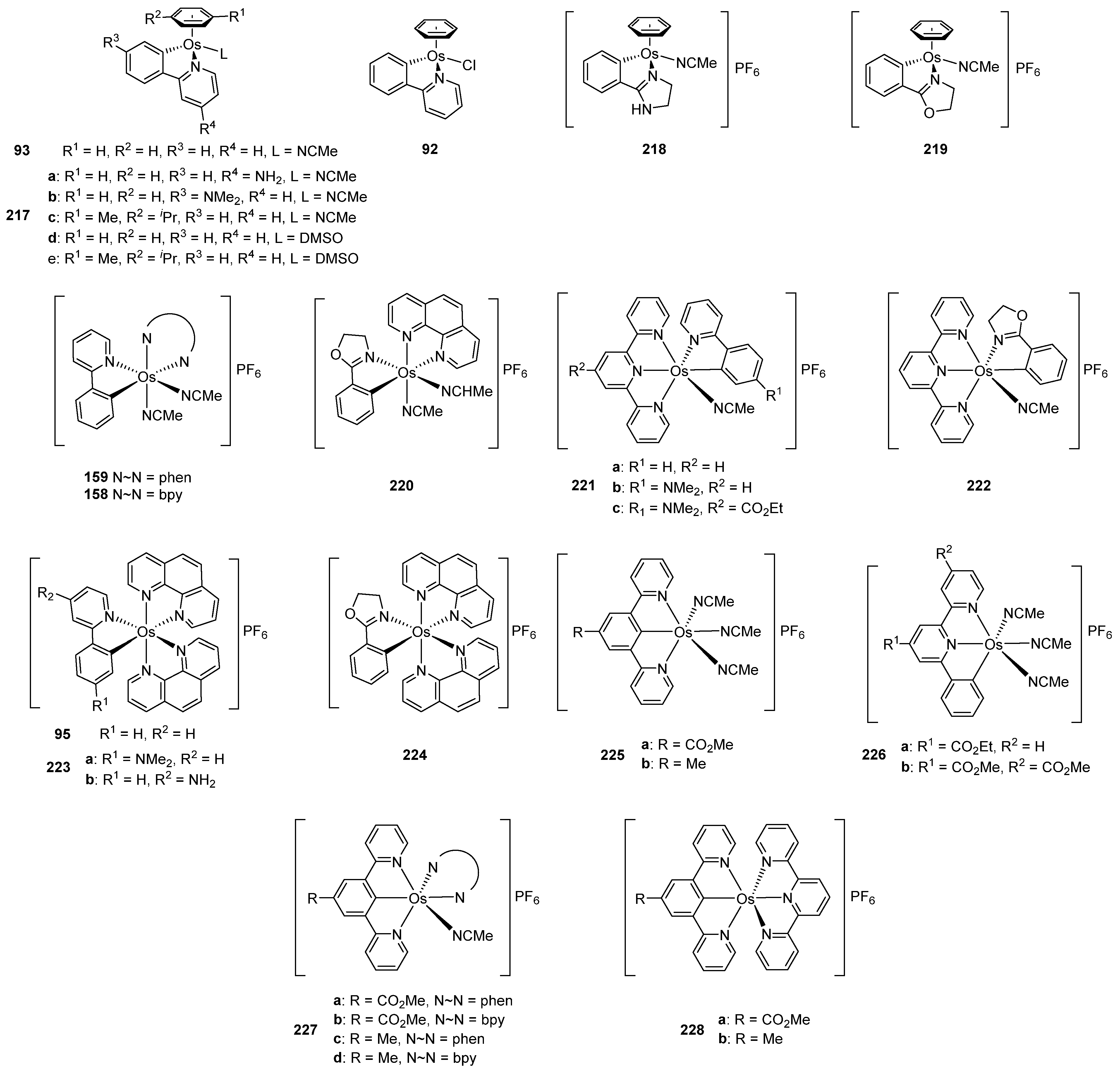
- Wadman, S.H.; Lutz, M.; Tooke, D.M.; Spek, A.L.; František, H.; Havenith, R.W.A.; Van Klink, G.P.M.; Van Koten, G. Consequences of N, C, N′- and C, N, N′-coordination modes on electronic and photophysical properties of cyclometalated aryl ruthenium(II) complexes. Inorg. Chem. 2009, 48, 1887–1900. [Google Scholar] [CrossRef] [PubMed]
- Demovič, L.; Kellö, V.; Urban, M. Role of relativity in energy pattern of low-lying terms of Fe, Ru and Os. Comput. Chem. 2016, 1084, 157–161. [Google Scholar] [CrossRef]
- Woźna, A.; Kapturkiewicz, A.; Angulo, G. Synthesis and characterization of heteroleptic cyclometalated divalent Osmium Os[P(C6H5)3]2(CO)(N∩C−)Cl complexes. Inorg. Chem. Commun. 2013, 37, 26–29. [Google Scholar] [CrossRef]
- Chung, L.H.; Lo, H.S.; Ng, S.W.; Ma, D.L.; Leung, C.H.; Wong, C.Y. Luminescent Iridium(III) complexes supported by N-heterocyclic carbene-based C^C^C-pincer ligands and aromatic diimines. Sci. Rep. 2015, 5, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chung, L.H.; Chan, S.C.; Lee, W.C.; Wong, C.Y. Emissive Osmium(II) complexes supported by N-heterocyclic carbene-based C^C^C-pincer ligands and aromatic diimines. Inorg. Chem. 2012, 51, 8693–8703. [Google Scholar] [CrossRef]
- Alabau, R.G.; Eguillor, B.; Esler, J.; Esteruelas, M.A.; Oliván, M.; Oñate, E.; Tsai, J.-Y.; Xia, C. CCC-pincer-NHC Osmium complexes: New types of blue-green emissive neutral compounds for organic light-emitting devices (OLEDs). Organometallics 2014, 33, 5582–5596. [Google Scholar] [CrossRef]
- Alabau, R.G.; Esteruelas, M.A.; Oliván, M.; Oñate, E.; Palacios, A.U.; Tsai, J.Y.; Xia, C. Osmium(II) complexes containing a dianionic CCCC-donor tetradentate ligand. Organometallics 2016, 35, 3981–3995. [Google Scholar] [CrossRef] [Green Version]
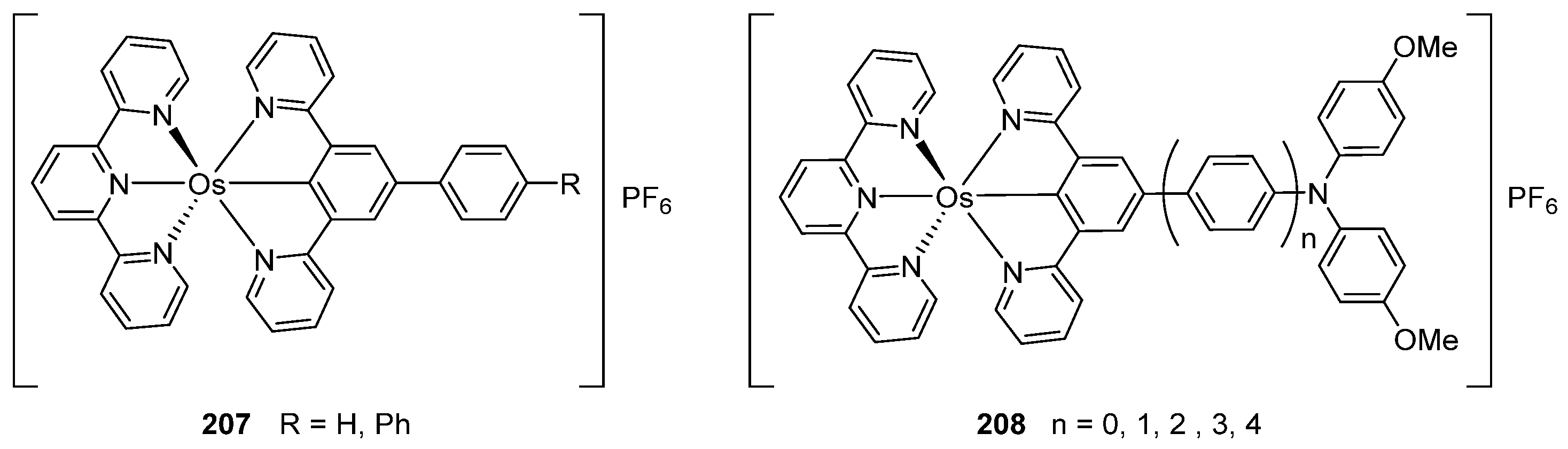
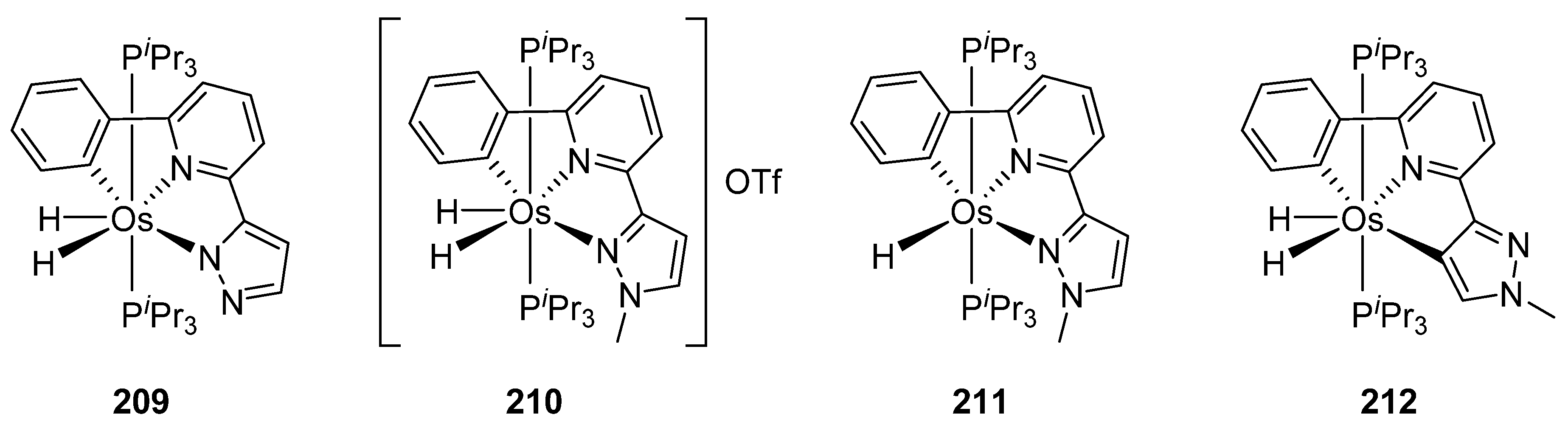
- Shen, J.-J.; Shao, J.-Y.; Gong, Z.-L.; Zhong, Y.-W. Cyclometalated Osmium-amine electronic communication through the p-oligophenylene Wire. Inorg. Chem. 2015, 54, 10776–10784. [Google Scholar] [CrossRef]
- Castro-Rodrigo, R.; Esteruelas, M.A.; Gómez-Bautista, D.; Lezáun, V.; López, A.M.; Oliván, M.; Oñate, E. Influence of the bite angle of dianionic C,N,C-pincer ligands on the chemical and photophysical properties of Iridium(III) and Osmium(IV) hydride complexes. Organometallics 2019, 38, 3707–3718. [Google Scholar] [CrossRef]
- Alabau, R.G.; Esteruelas, M.A.; Oliván, M.; Oñate, E. Preparation of phosphorescent Osmium(IV) complexes with N,N′,C- and C,N,C′-pincer ligands. Organometallics 2017, 36, 1848–1859. [Google Scholar] [CrossRef] [Green Version]
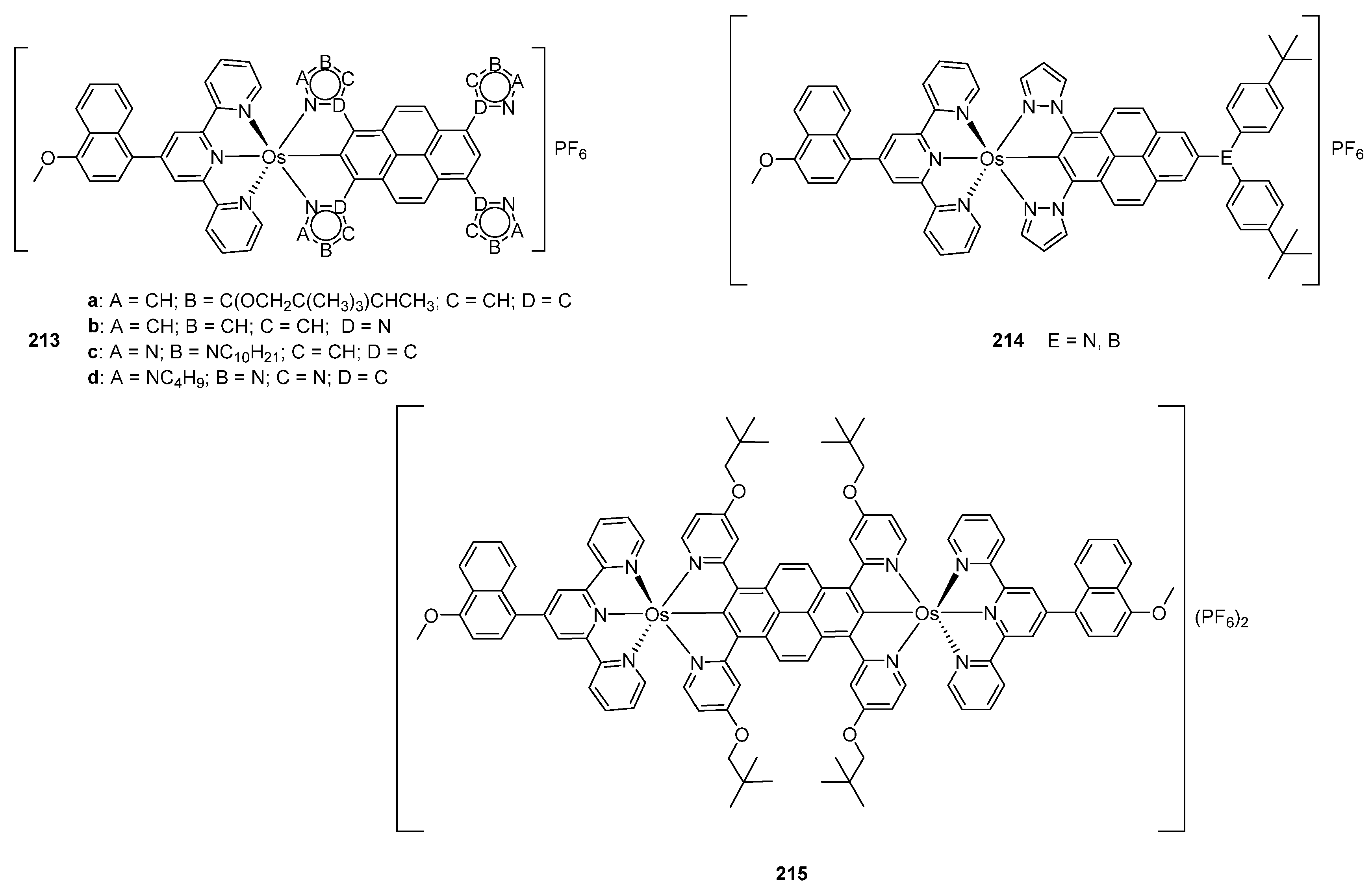
- Zych, D.; Slodek, A.; Matuszczyk, D.; Golba, S. Comprehensive study of mononuclear Osmium complexes with various pyrene ligands. Eur. J. Inorg. Chem. 2018, 5117–5128. [Google Scholar] [CrossRef]
- Zych, D. 1,3-Di(hetero)aryl-7-substituted pyrenes—An undiscovered area of important pyrene derivatives. Proceedings 2019, 41, 28. [Google Scholar] [CrossRef] [Green Version]
- Zych, D.; Slodek, A.; Golba, S.; Krompiec, S. Cyclometalated Ruthenium, Osmium, and Iridium complexes bridged by an NCN-pyrene-NCN derivative-synthesis and comparison of optical, thermal, and electrochemical properties. Eur. J. Inorg. Chem. 2018, 1581–1588. [Google Scholar] [CrossRef]
- Zhang, P.; Huang, H. Future potential of Osmium complexes as anticancer drug candidates, photosensitizers and organelle-targeted probes. Dalton Trans. 2018, 47, 14841–14854. [Google Scholar] [CrossRef] [PubMed]
- Omae, I. Applications of five-membered ring products of cyclometalation reactions as anticancer agents. Coord. Chem. Rev. 2014, 280, 84–95. [Google Scholar] [CrossRef]
- Zhang, P.; Sadler, P.J. Advances in the design of organometallic anticancer complexes. J. Organomet. Chem. 2017, 839, 5–14. [Google Scholar] [CrossRef]
- Jia, P.; Ouyang, R.; Cao, P.; Tong, X.; Zhou, X.; Lei, T.; Zhao, Y.; Guo, N.; Chang, H.; Miao, Y.; et al. Review: Recent advances and future development of metal complexes as anticancer agents. J. Coord. Chem. 2017, 70, 2175–2201. [Google Scholar] [CrossRef]
- Alessio, E. (Ed.) Bioinorganic Medicinal Chemistry; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2011; ISBN 9783527633104. [Google Scholar]
- Jaouen, G.; Salmain, M. (Eds.) Bioorganometallic Chemistry: Applications in Drug Discovery, Biocatalysis, and Imaging; John Wiley & Sons: Weinheim, Germany, 2015; ISBN1 3527335277. ISBN2 9783527335275. [Google Scholar]
- Casini, A.; Vessières, A.; Meier-Menches, S.M. (Eds.) Metal-Based Anticancer Agents; Royal Society of Chemistry: Cambridge, UK, 2019; ISBN 978-1-78801-406-9. [Google Scholar]
- Zeng, L.; Gupta, P.; Chen, Y.; Wang, E.; Ji, L.; Chao, H.; Chen, Z.-S. The development of anticancer ruthenium(II) complexes: From single molecule compounds to nanomaterials. Chem. Soc. Rev. 2017, 46, 5771–5804. [Google Scholar] [CrossRef]
- Alessio, E.; Messori, L. Anticancer drug candidates face-to-face: A case story in medicinal inorganic chemistry. Molecules 2019, 24, 1995. [Google Scholar] [CrossRef] [Green Version]
- Browne, W.R.; Holder, A.A.; Lawrence, M.A.; Bullock, J.L., Jr.; Lilge, L. (Eds.) Ruthenium Complexes: Photochemical and Biomedical Applications; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2018; ISBN1 9783527339570. ISBN2 9783527695225. [Google Scholar]
- Thota, S.; Rodrigues, D.A.; Crans, D.C.; Barreiro, E.J. Ru(II) compounds: Next-generation anticancer metallotherapeutics? J. Med. Chem. 2018, 61, 5805–5821. [Google Scholar] [CrossRef]
- Gaiddon, C.; Pfeffer, M. The Fate of Cycloruthenated compounds: From C-H activation to innovative anticancer therapy. Eur. J. Inorg. Chem. 2017, 2017, 1639–1654. [Google Scholar] [CrossRef] [Green Version]
- He, X.; Gong, L.; Kräling, K.; Gründler, K.; Frias, C.; Webster, R.D.; Meggers, E.; Prokop, A.; Xia, H. Unusual η2-allene osmacycle with apoptotic properties. ChemBioChem 2010, 11, 1607–1613. [Google Scholar] [CrossRef] [PubMed]
- Boff, B.; Gaiddon, C.; Pfeffer, M. Cancer cell cytotoxicity of cyclometalated compounds obtained with Osmium(II) complexes. Inorg. Chem. 2013, 52, 2705–2715. [Google Scholar] [CrossRef]
- Licona, C.; Delhorme, J.-B.; Riegel, G.; Vidimar, V.; Cerón-Camacho, R.; Boff, B.; Venkatasamy, A.; Tomasetto, C.; Da Silva Figueiredo Celestino Gomes, P.; Rognan, D.; et al. Anticancer activity of Ruthenium and Osmium cyclometalated compounds: Identification of ABCB1 and EGFR as resistance mechanisms. Inorg. Chem. Front. 2020, 7, 678–688. [Google Scholar] [CrossRef]
- Riedl, C.A.; Flocke, L.S.; Hejl, M.; Roller, A.; Klose, M.H.M.M.; Jakupec, M.A.; Kandioller, W.; Keppler, B.K. Introducing the 4-phenyl-1,2,3-triazole moiety as a versatile scaffold for the development of cytotoxic Ruthenium(II) and Osmium(II) arene cyclometalates. Inorg. Chem. 2017, 56, 528–541. [Google Scholar] [CrossRef] [PubMed]
- Rono, C.K.; Chu, W.K.; Darkwa, J.; Meyer, D.; Makhubela, B.C.E. Triazolyl RuII, RhIII OsII, and IrIII complexes as potential anticancer agents: Synthesis, structure elucidation, cytotoxicity, and DNA model interaction studies. Organometallics 2019, 38, 3197–3211. [Google Scholar] [CrossRef]
- Ortega, E.; Yellol, J.G.; Rothemund, M.; Ballester, F.J.; Rodríguez, V.; Yellol, G.; Janiak, C.; Schobert, R.; Ruiz, J. A new C,N-cyclometalated Osmium(II) arene anticancer scaffold with a handle for functionalization and antioxidative properties. Chem. Commun. 2018, 54, 11120–11123. [Google Scholar] [CrossRef] [Green Version]
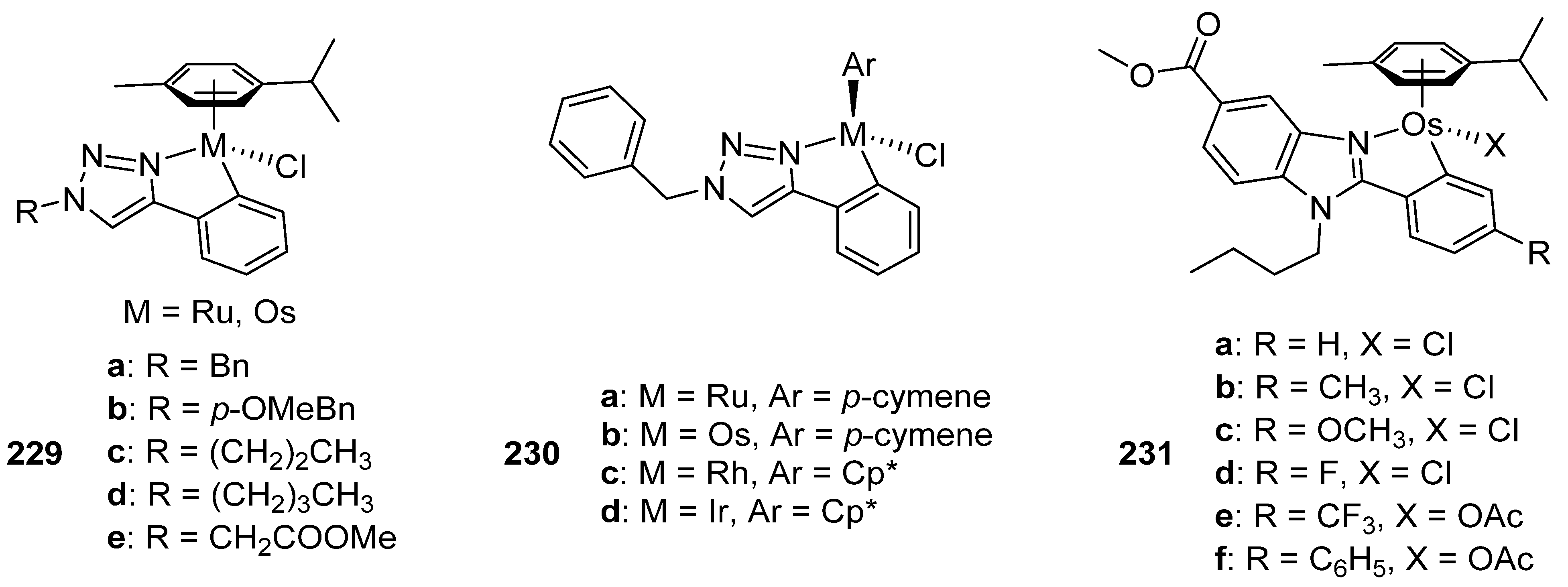
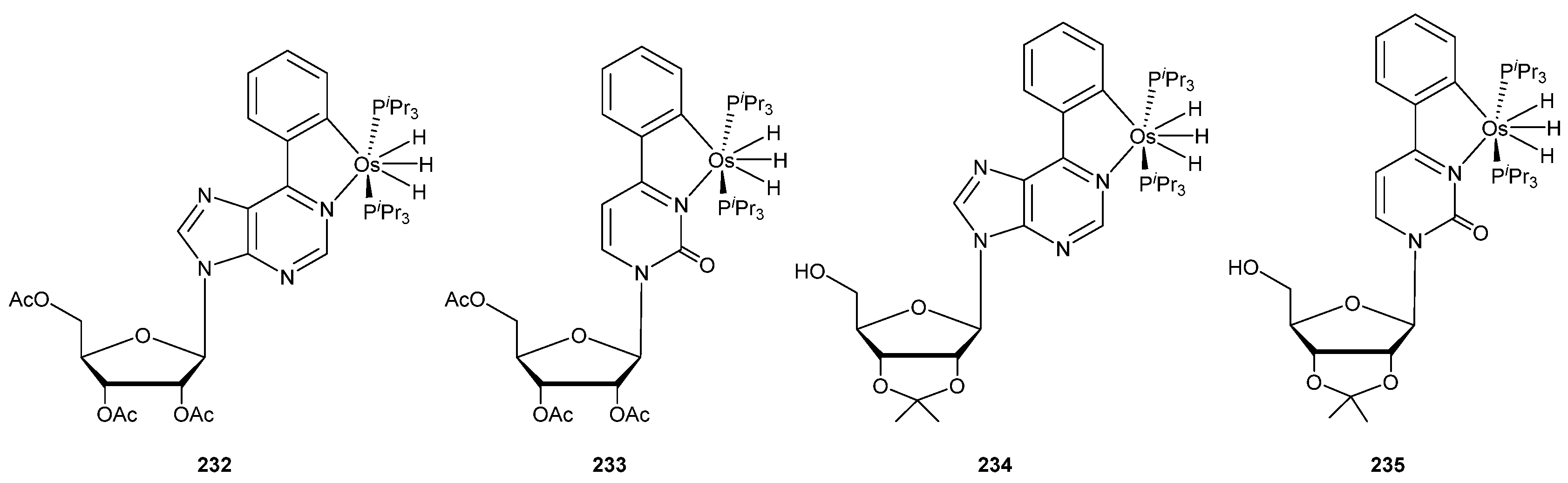
- Valencia, M.; Merinero, A.D.; Lorenzo-Aparicio, C.; Gómez-Gallego, M.; Sierra, M.A.; Eguillor, B.; Esteruelas, M.A.; Oliván, M.; Oñate, E. Osmium-promoted σ-bond activation reactions on nucleosides. Organometallics 2020, 39, 312–323. [Google Scholar] [CrossRef]














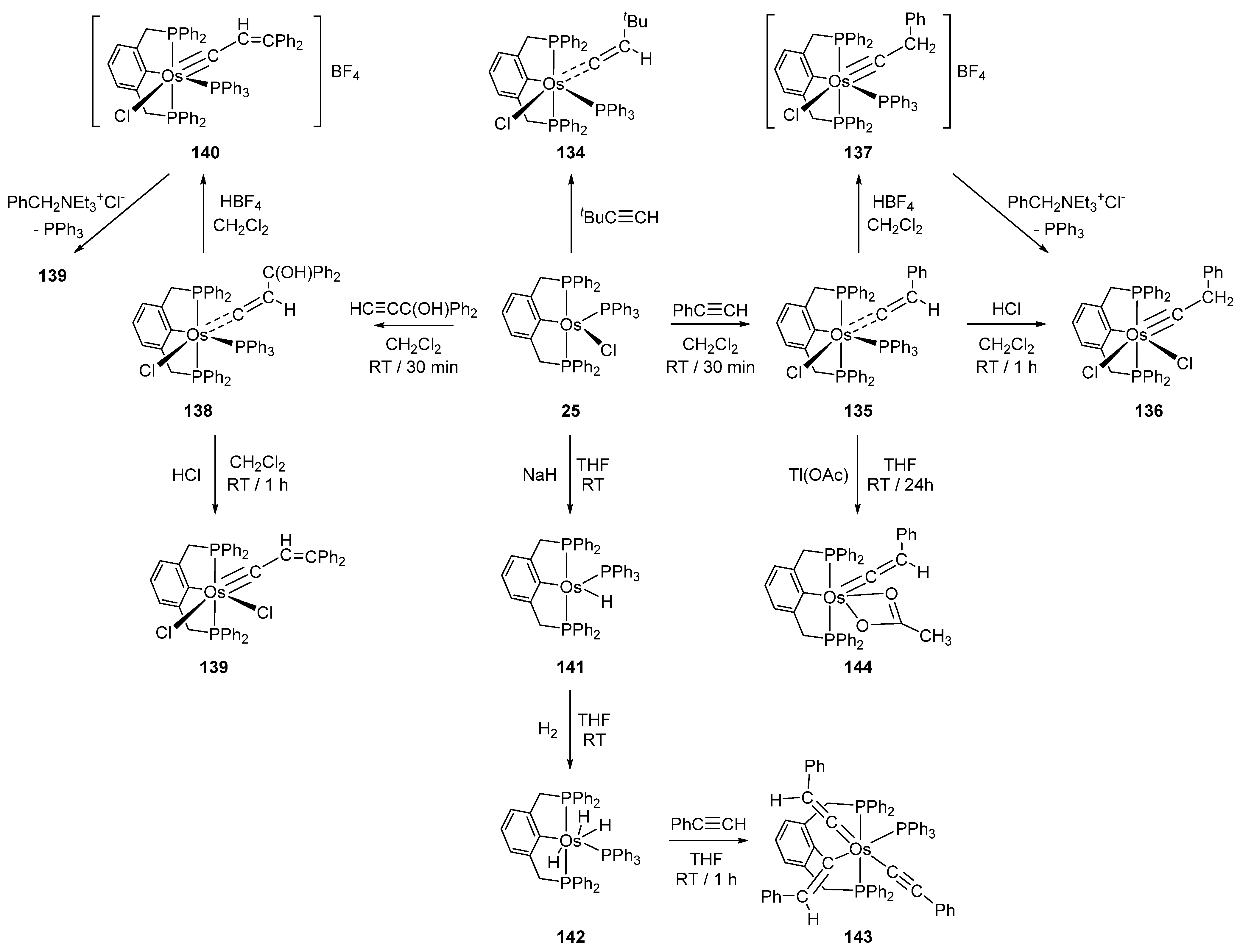
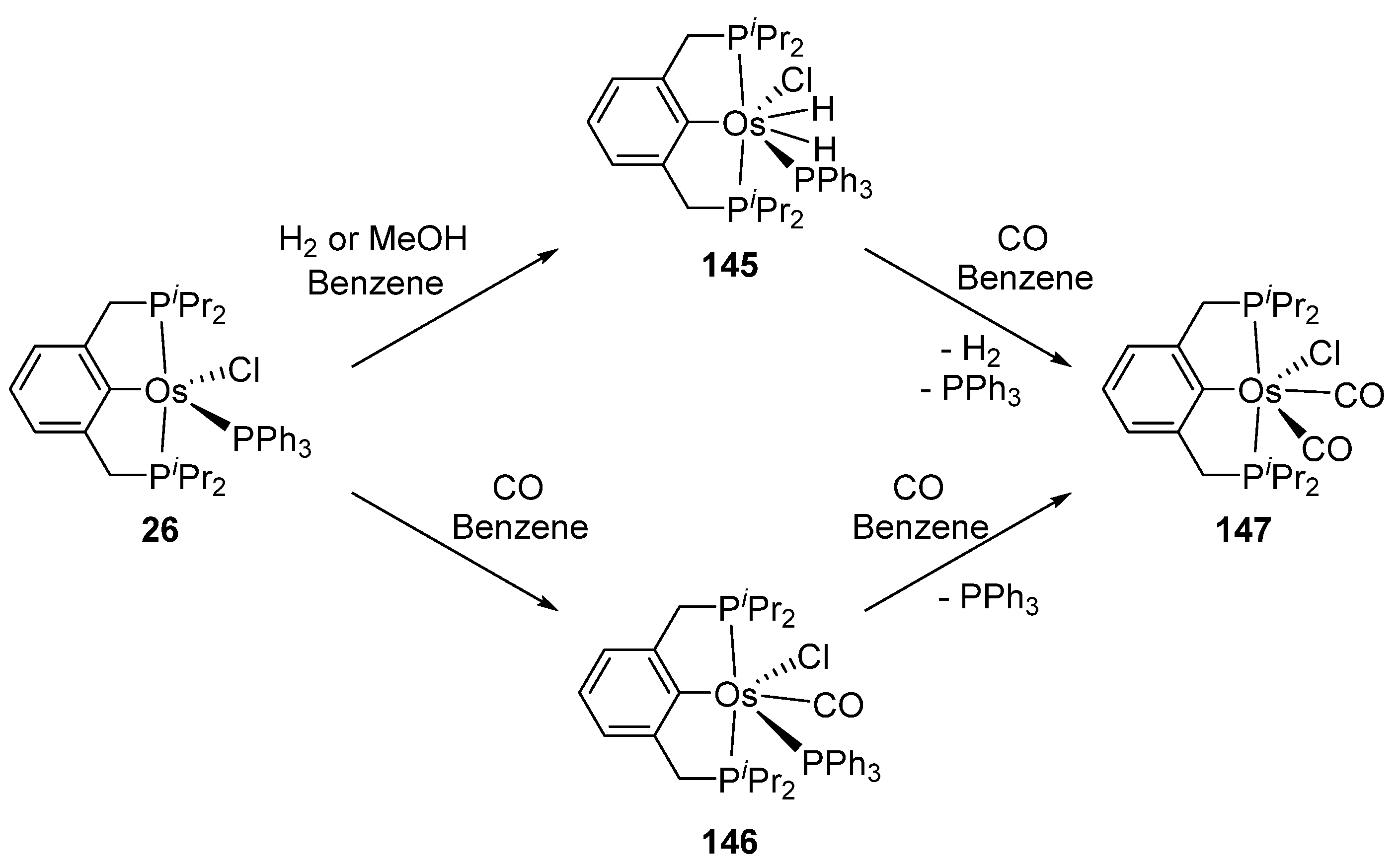

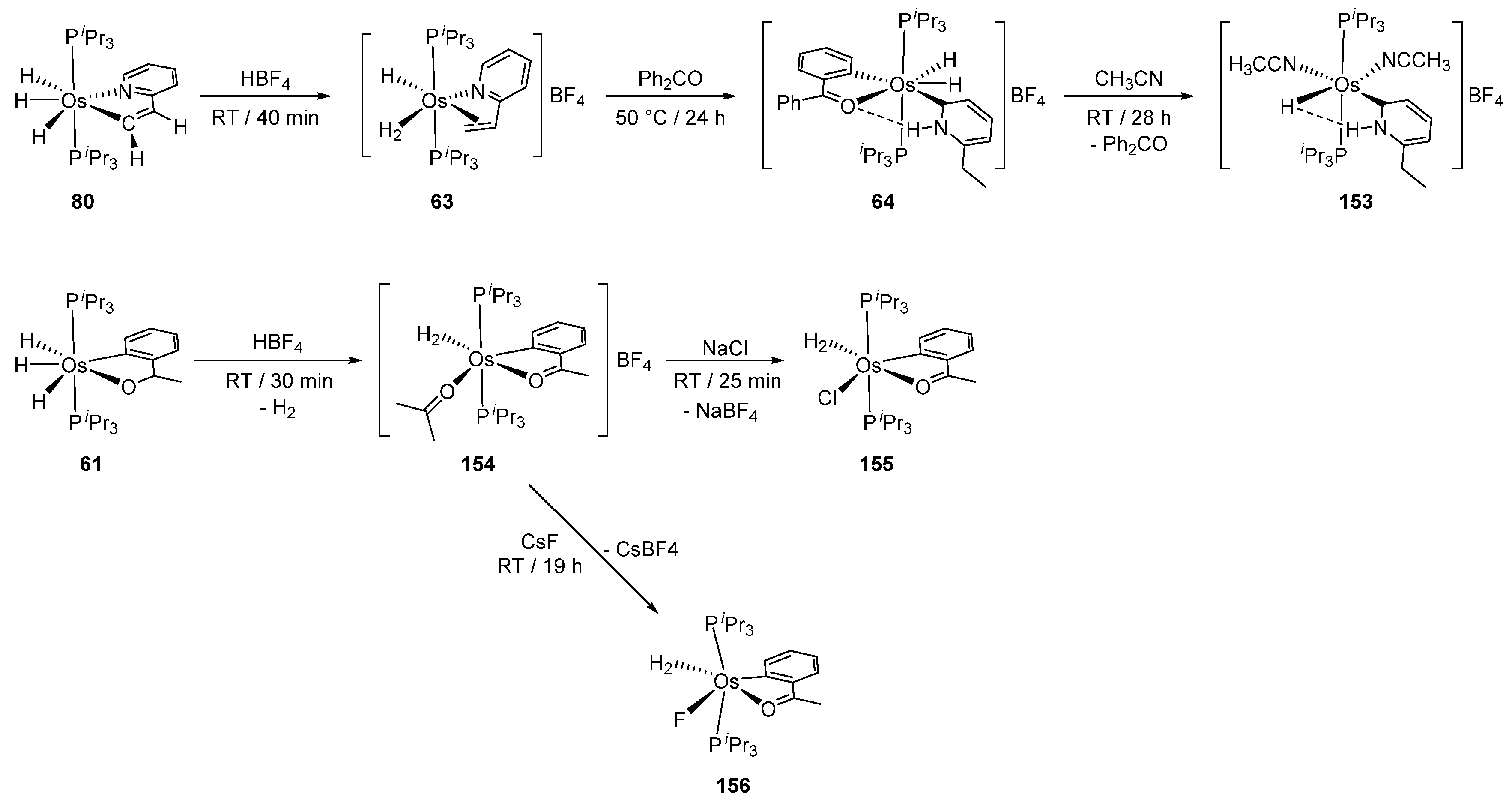
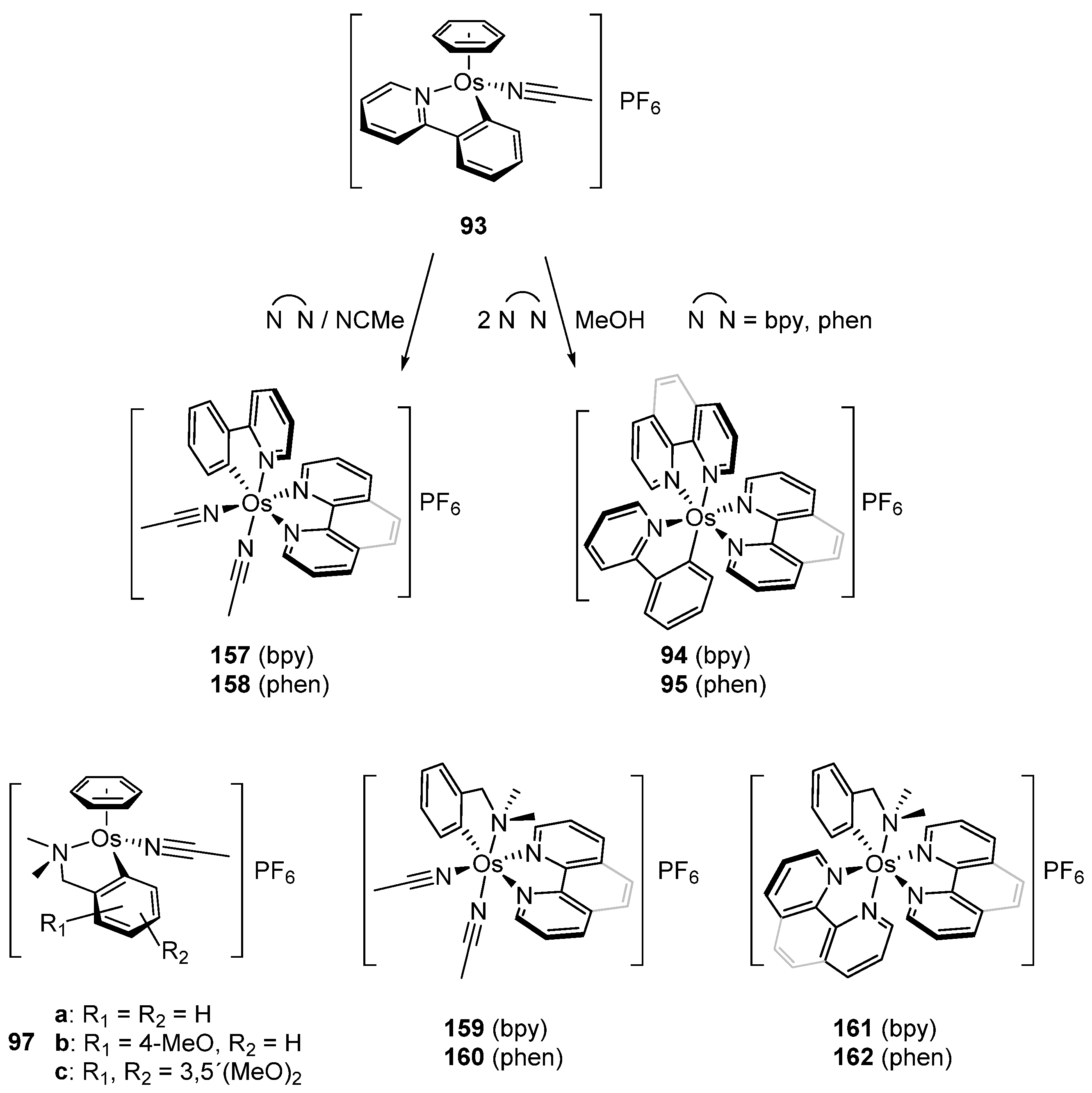


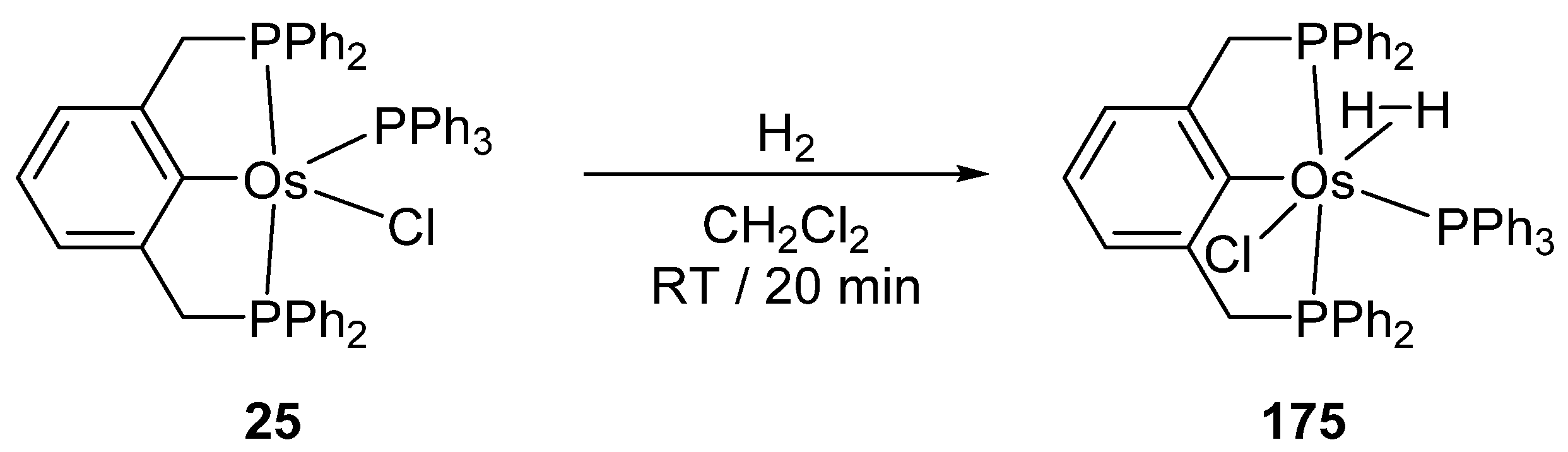
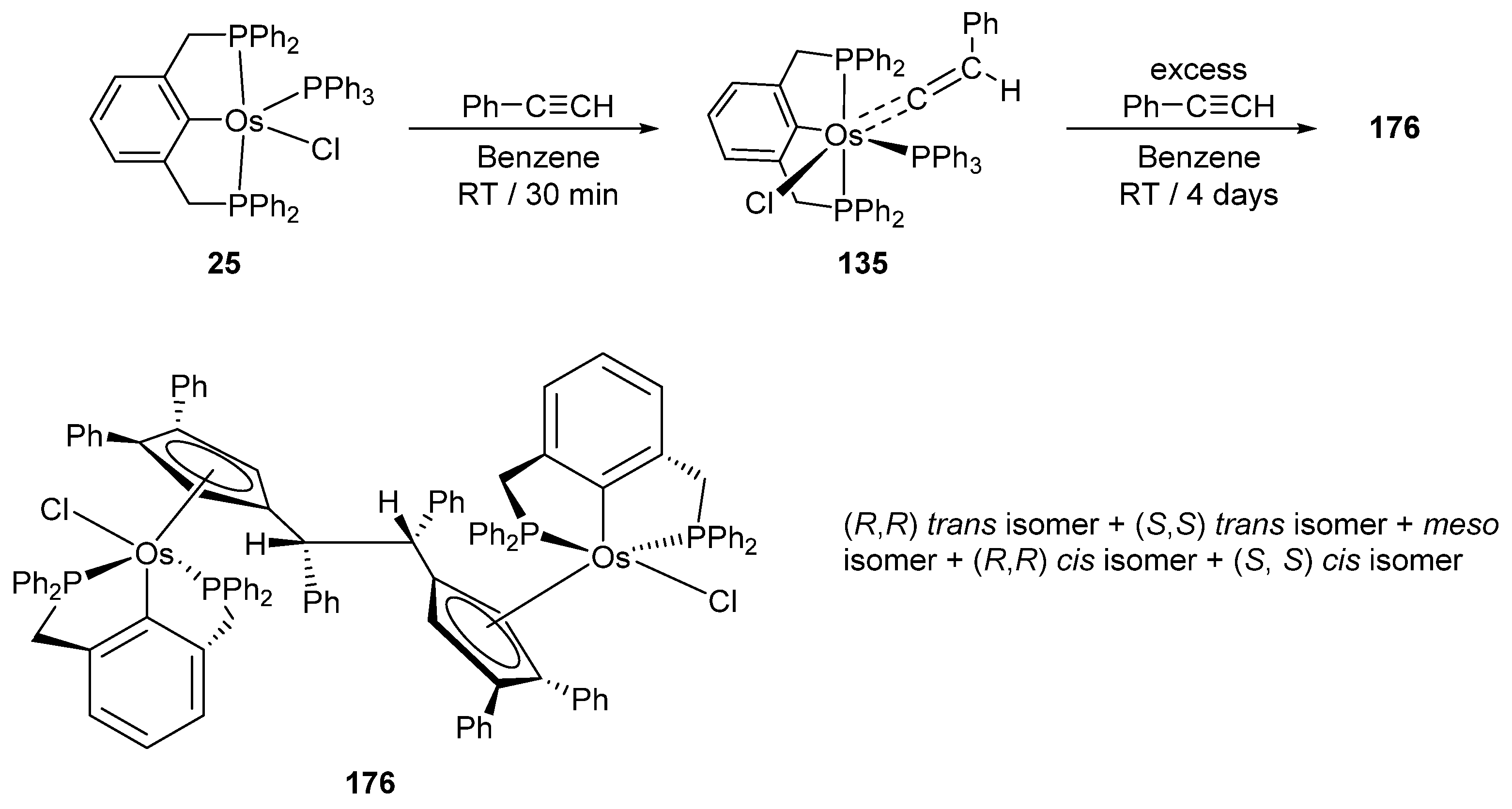
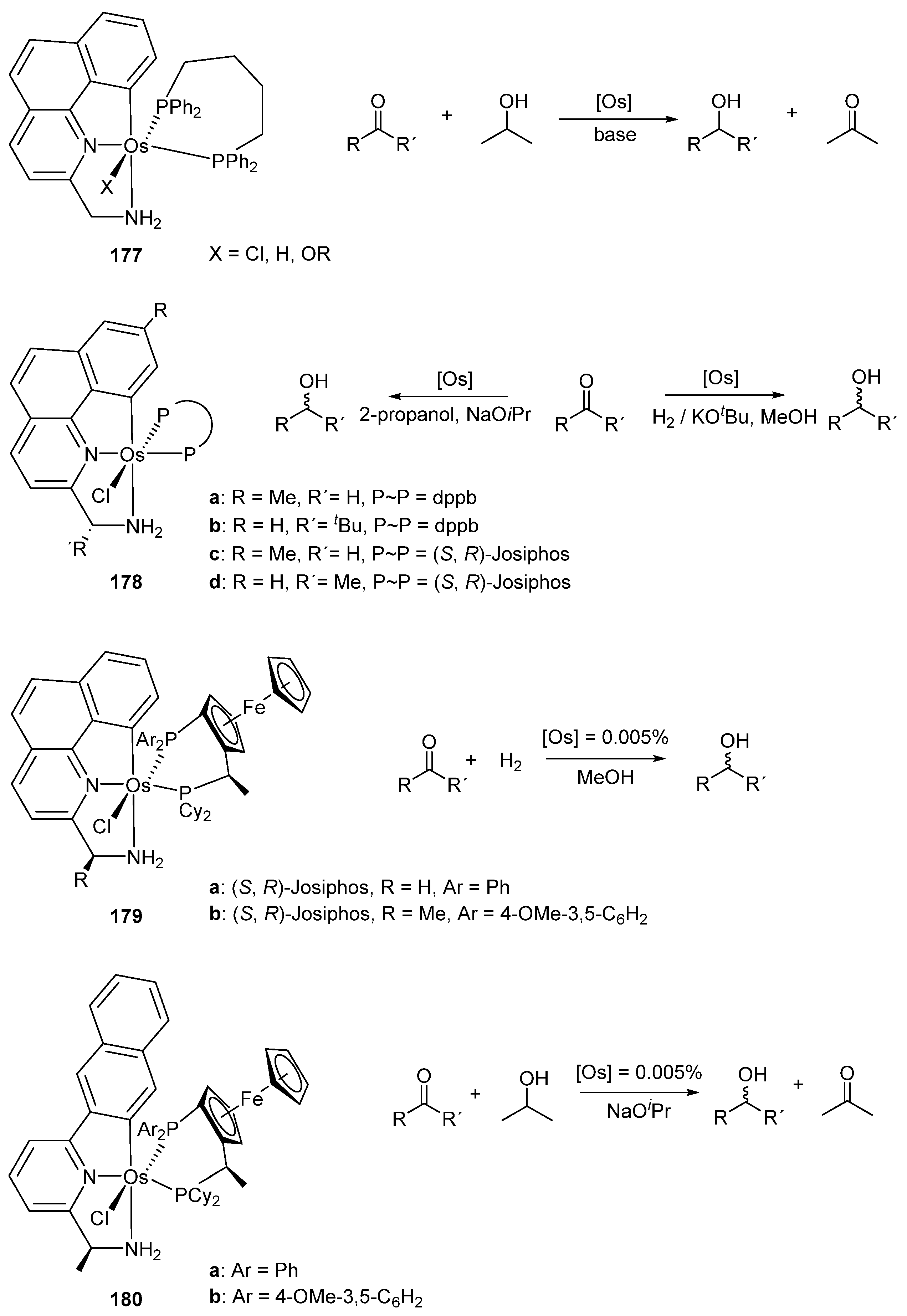
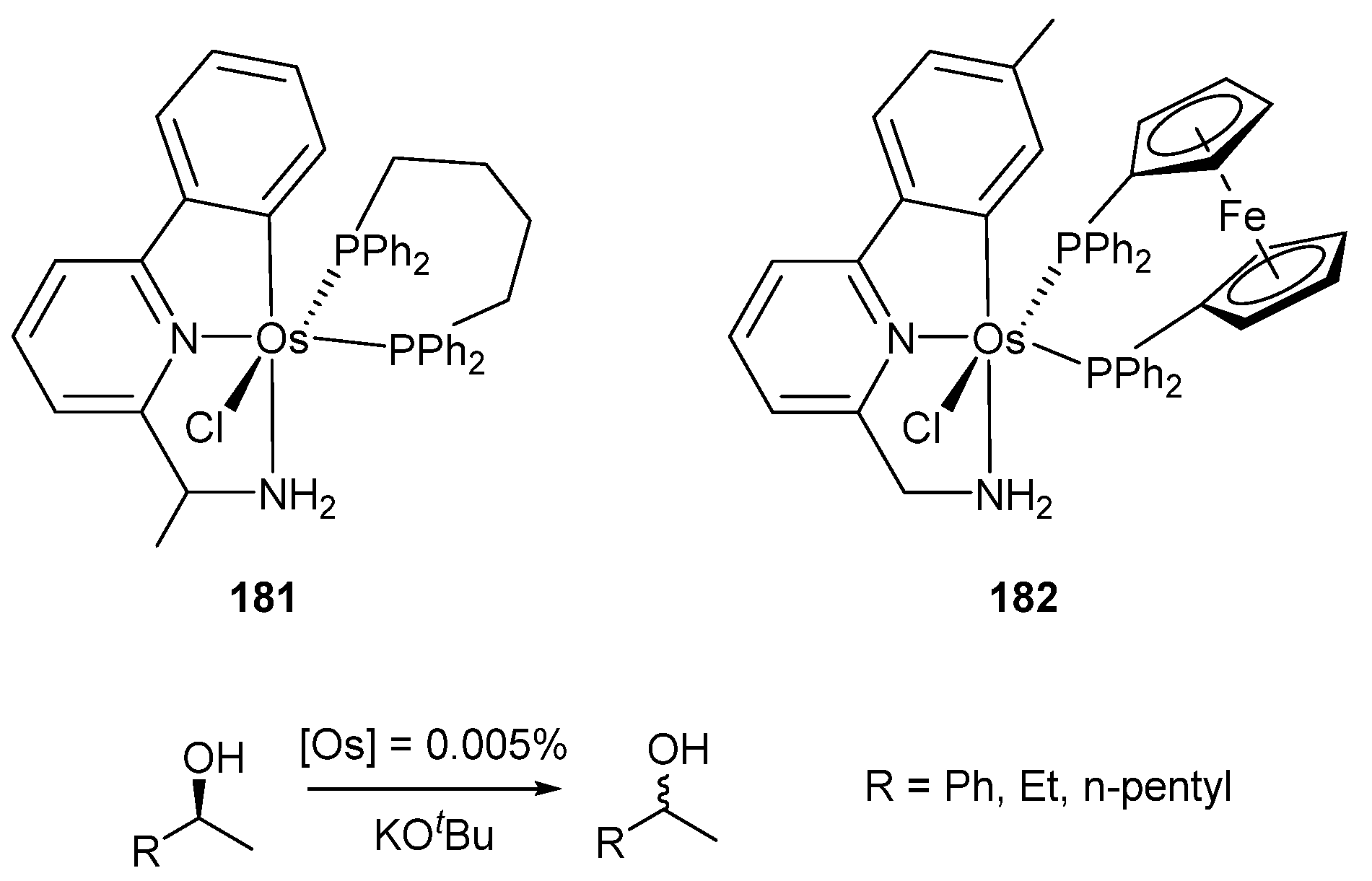

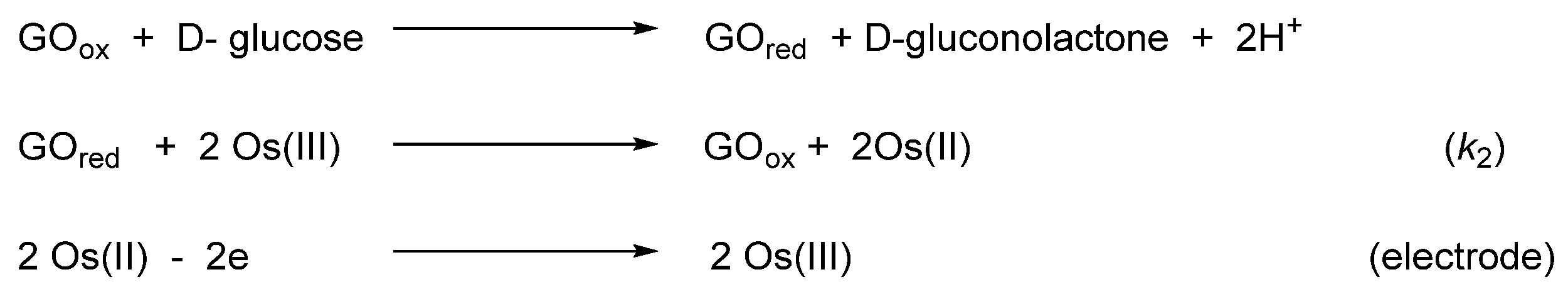



| Precursor | Cyclometalated Ligand | Osmacycle | Ref. |
|---|---|---|---|
| [Os3(CO)12] |  L1 |  1 speculative mixture of cis or trans | [28] |
| [OsH(O2CCF3)(CO)(PPh3)2] |  L2 |  2 | [29] |
| cis-[Os(PMe3)4H(CH2C(Me)3)] | PMe3 L3 |  3 | [30] |
| [Os(p-cymene)Me(Cl)(Me2SO)] prepared from [Os(p-cymene)Cl2]2 |  L4 | 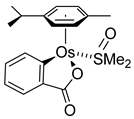 4 | [31] |
| [Os(tterpy)(O)2(OH)(NO3)] prepared from K2[Os(O)2(OH)4] | 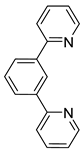 L5 | 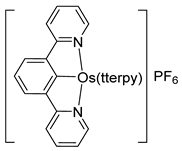 5 | [32] |
| [Os(tterpy)(O)2(OH)(NO3)] prepared from K2[Os(O)2(OH)4] |  L6 |  6 | [32] |
| [Os(tterpy)C13] prepared from [OsCl3] |  L6 | 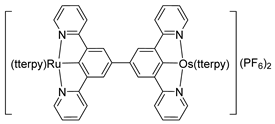 7 * | [33] |
| [Os(Cp)(PPh3)2]OTf |  L7 | 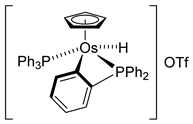 8 | [34] |
[Os(N~N~N)Cl3] prepared from [OsCl3]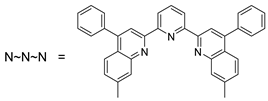 |  L8 | 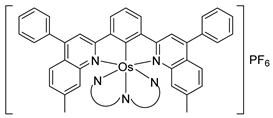 9 | [35] |
| Cyclometalated Ligand | Osmacycle | Yield | Ref. | ||
|---|---|---|---|---|---|
 L9 | 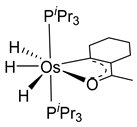 60 | 62% | [55] | ||
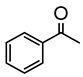 L10 | 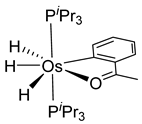 61 | 61% | [54] | ||
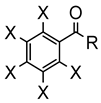 X = H, F; R = Ph, CH3 L11 | 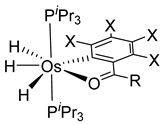 62 | 62% (X = H, R = Ph) 74% (X = F, R = CH3) 60% (X = F, R = Ph) | [54] | ||
 L12 |  63 | 92% | [56] | ||
 L13 | 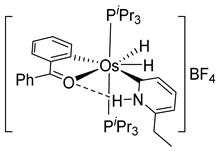 64 | 55% | [56] | ||
 L14 | 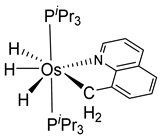 65 | 97% | [57] | ||
  L15, L16 | 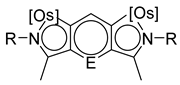 66 | 23% (E = N) 45% (E = CH) | [58] | ||
 L17 |  67 | 77% | [59] | ||
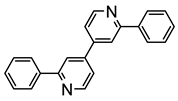 L18 | 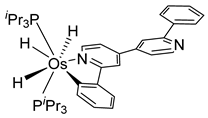 68 | 50% | [59] | ||
 L18 |  69 | 91% | [59] | ||
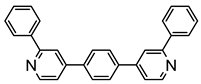 L19 | 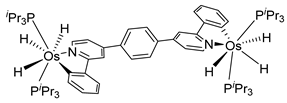 70 | 99% | [59] | ||
 L20 | 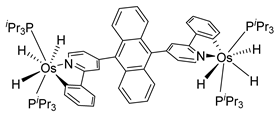 71 | 90% | [59] | ||
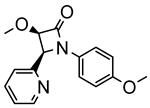 L21 | 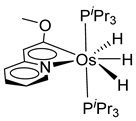 72 | 60% | [60] | ||
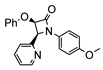 L22 | 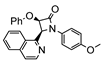 L23 |  L24 | 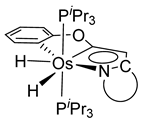 73 | 71% (L22) 60% (L23) 63% (L24) | [61] |
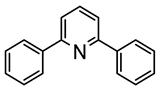 L25 | 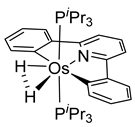 74 | 64% | [62] | ||
 L26 | 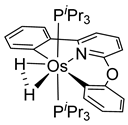 75 | 80% | [62] | ||
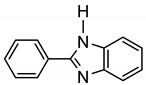 L27 |  76 | 53% | [63] | ||
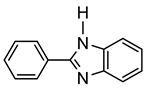 L27 | 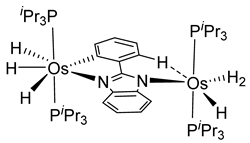 77 | 60% | [63] | ||
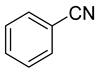 L28 |  78 | 73% (R = iPr3) 74% (R = Ph) R = Ph prepared from [OsH6(PiPr3)(PPh3)] | [64] | ||
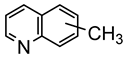 L29 | 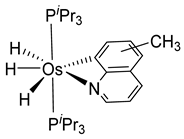 79 | 60–75% | [65] | ||
 L12 | 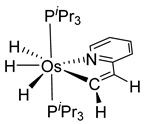 80 | 83% | [56] | ||
| Complex | E° (mV) | k2 (M−1s−1) |
|---|---|---|
| 162 | −51 | 0.67 × 106 |
| 163 | 13 | 4.80 × 106 |
| 161 | 32 | 2.00 × 106 |
| 94 | 84 | 2.90 × 106 |
| 95 | 31 | 1.80 × 106 |
| 159 | 109 | 2.90 × 106 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cerón-Camacho, R.; Roque-Ramires, M.A.; Ryabov, A.D.; Le Lagadec, R. Cyclometalated Osmium Compounds and beyond: Synthesis, Properties, Applications. Molecules 2021, 26, 1563. https://doi.org/10.3390/molecules26061563
Cerón-Camacho R, Roque-Ramires MA, Ryabov AD, Le Lagadec R. Cyclometalated Osmium Compounds and beyond: Synthesis, Properties, Applications. Molecules. 2021; 26(6):1563. https://doi.org/10.3390/molecules26061563
Chicago/Turabian StyleCerón-Camacho, Ricardo, Manuel A. Roque-Ramires, Alexander D. Ryabov, and Ronan Le Lagadec. 2021. "Cyclometalated Osmium Compounds and beyond: Synthesis, Properties, Applications" Molecules 26, no. 6: 1563. https://doi.org/10.3390/molecules26061563