Recent Advances and Trends in Chemical CPP–Drug Conjugation Techniques
Abstract
:1. Introduction
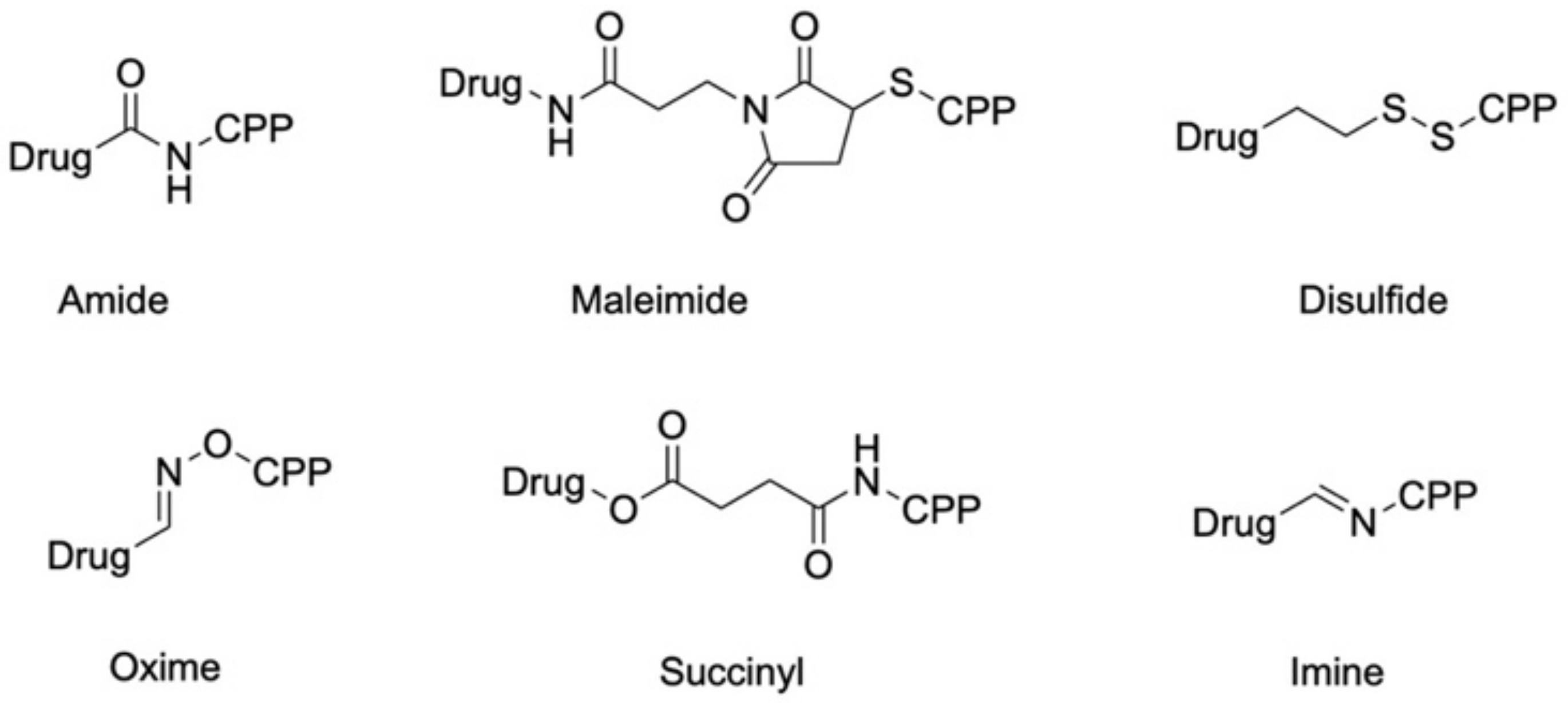
2. Amide Bond Formation and Related Chemistry
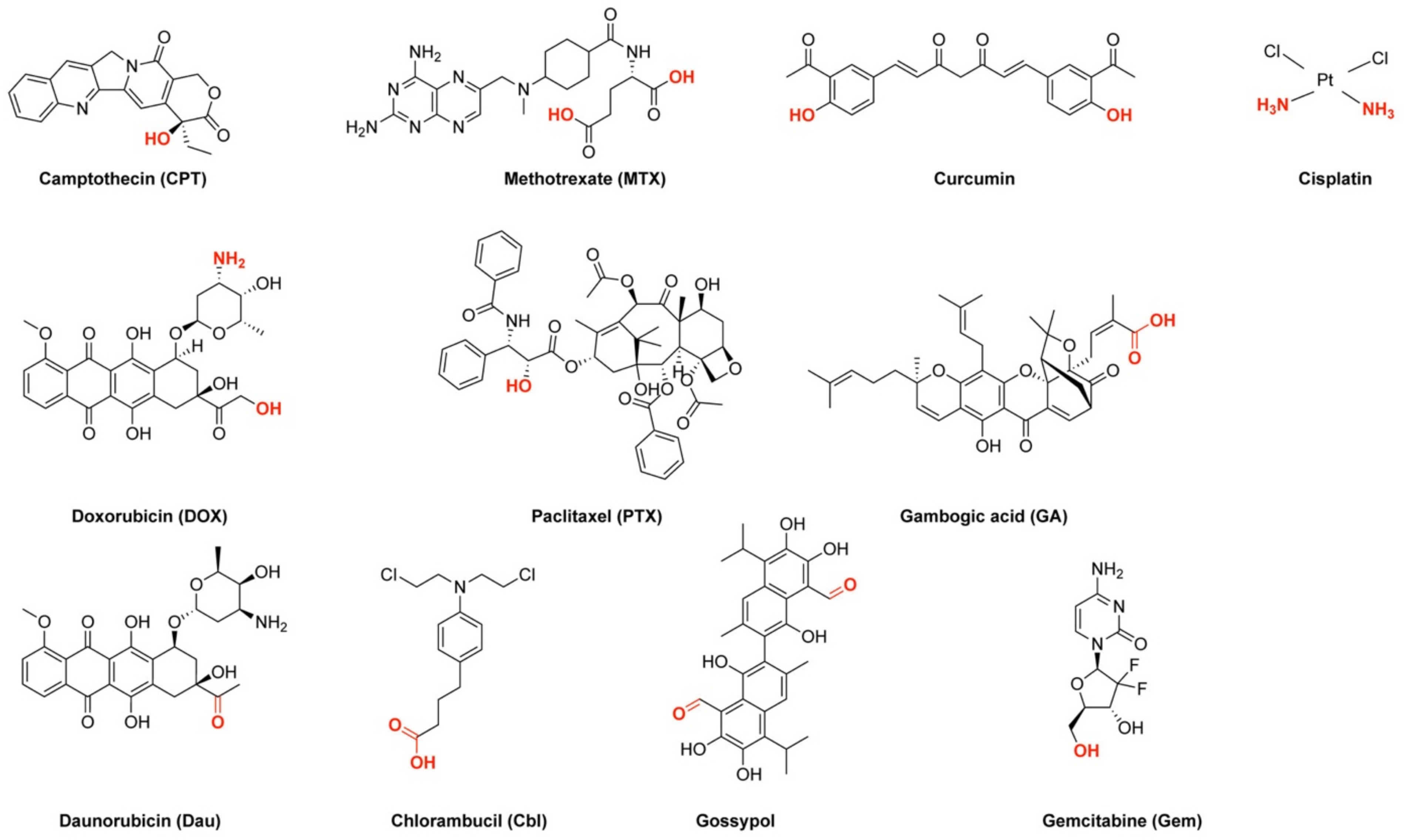
2.1. CPP–Drug Conjugates Comprising a Classical Amide Bond
2.2. CPP–Metal Complex Conjugates
3. CPP–Drug Conjugation Using Functionalized Linkers
3.1. Bifunctional Succinyl Linkers
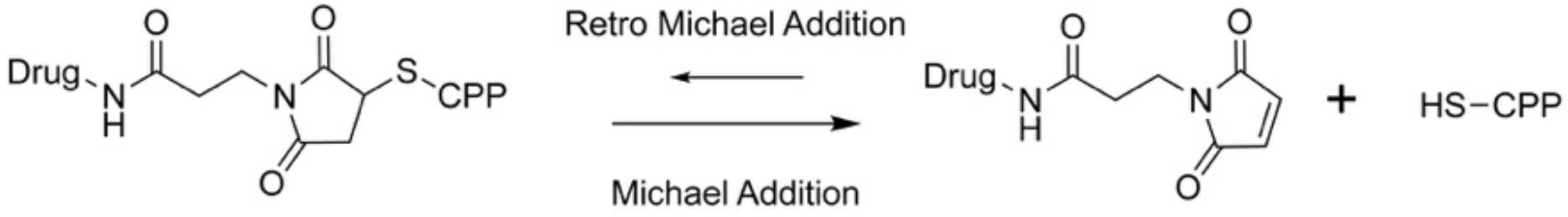
3.2. Redox-Sensitive Disulfide Linkers
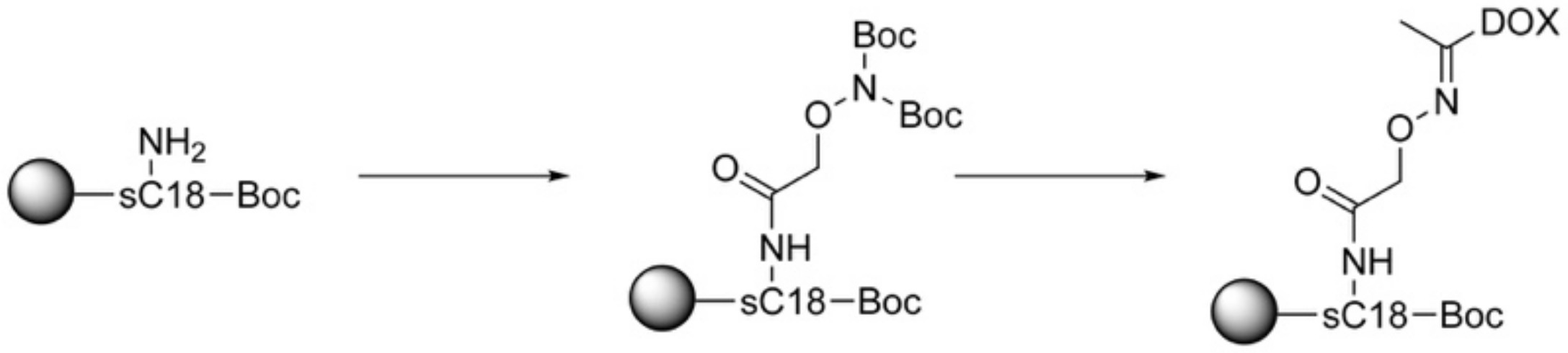
3.3. Acid-Sensitive Linkers
4. Conclusions and Future Perspectives
- What functional group within the drug structure is available for coupling to the CPP?It is important to know which groups define the pharmacophore, what positions are useful for modifications, and which reaction conditions are selectable.
- How does the coupled drug influence the CPP?It should be carefully considered which cargo moieties are allowed to be introduced that do not disturb the activity of the CPP.
- Can the tumor/tumor environment be targeted by the chosen linkage strategy?The microenvironment of the tumor tissue is distinguishable to that from healthy cells, and thus, linker systems should be introduced between CPP and drug that enable the generation of a highly active and cell selective anticancer therapeutic.
Funding
Conflicts of Interest
Abbreviations
| A2780 | human ovarian carcinoma cells |
| ADC | antibody–drug conjugate |
| CCRF-CEM | human leukemia carcinoma cells |
| CLSM | confocal laser scanning microscopy |
| COS7 | primate fibroblast-like kidney cells |
| DCM | dichloromethane |
| DIC | N,N’-diisopropylcarbodiimide |
| DIPEA | N,N’-diisopropylethylamine |
| DMA | 2,3-dimethylmaleic anhydride |
| DMAP | 4-dimethylaminopyridine |
| DMF | dimethylformamide |
| DMSO | dimethyl sulfoxide |
| EJ | human bladder cancer cells |
| GSH | glutathione |
| HBTU | hexafluorophosphate benzotriazole tetramethyl uronium |
| HEK-293 | human embryonic kidney cells |
| HeLa | human cervical cancer cells |
| HOBt | hydroxybenzotriazole |
| HT-1080 | human fibrosarcoma cells |
| HT-29 | human colon cancer cells |
| IC50 | half maximal inhibitory concentration |
| LLCPK | porcine kidney cells |
| MCF-7 | human breast cancer cells |
| MDA-MB-231 | epithelial human breast cancer |
| MG63 | human osteosarcoma |
| MMP-2/9 | metalloproteinase 2 and 9 |
| MRP | multidrug resistance protein |
| mtDNA | mitochondrial DNA |
| NCM460 | normal human colon mucosal epithelial cells |
| NHDF | normal human dermal fibroblast cells |
| PDC | peptide–drug conjugate |
| ROS | reactive oxygen species |
| SKOV-3 | human ovarian cancer cells |
| TEA | triethylamine |
| U87 | human primary glioblastoma |
| Wi-38 | normal human fibroblast-like lung cells |
References
- Birrer, M.J.; Moore, K.N.; Betella, I.; Bates, R.C. Antibody-Drug Conjugate-Based Therapeutics: State of the Science. J. Natl. Cancer Inst. 2019, 111, 538–549. [Google Scholar] [CrossRef]
- Tsuchikama, K.; An, Z. Antibody-drug conjugates: Recent advances in conjugation and linker chemistries. Protein Cell 2018, 9, 33–46. [Google Scholar]
- Iqbal, J.; Anwar, F.; Afridi, S. Targeted Drug Delivery Systems and Their Therapeutic Applications in Cancer and Immune Pathological Conditions. Infect. Disord. Drug Targets 2017, 17, 149–159. [Google Scholar] [CrossRef]
- Yang, M.; Li, J.; Gu, P.; Fan, X. The application of nanoparticles in cancer immunotherapy: Targeting tumor microenvironment. Bioact. Mater. 2021, 6, 1973–1987. [Google Scholar]
- Cooper, B.M.; Iegre, J.; O’Donovan, D.H.; Halvarsson, M.Ö.; Spring, D.R. Peptides as a platform for targeted therapeutics for cancer: Peptide–drug conjugates (PDCs). Chem. Soc. Rev. 2021, 50, 1480–1494. [Google Scholar] [CrossRef]
- Balogh, B.; Ivánczi, M.; Nizami, B.; Beke-Somfai, T.; Mándity, I.M. ConjuPepDB: A database of peptide–drug conjugates. Nucleic Acids Res. 2021, 49, D1102–D1112. [Google Scholar] [CrossRef]
- Mousavizadeh, A.; Jabbari, A.; Akrami, M.; Bardania, H. Cell targeting peptides as smart ligands for targeting of therapeutic or diagnostic agents: A systematic review. Colloids Surf. B Biointerfaces 2017, 158, 507–517. [Google Scholar]
- Silva, S.; Almeida, A.J.; Vale, N. Combination of cell-penetrating peptides with nanoparticles for therapeutic application: A review. Biomolecules 2019, 9, 22. [Google Scholar] [CrossRef] [Green Version]
- Zhang, D.; Wang, J.; Xu, D. Cell-penetrating peptides as noninvasive transmembrane vectors for the development of novel multifunctional drug-delivery systems. J. Control. Release 2016, 229, 130–139. [Google Scholar]
- Durzyńska, J.; Przysiecka, Ł.; Nawrot, R.; Barylski, J.; Nowicki, G.; Warowicka, A.; Musidlak, O.; Goździcka-Józefiak, A. Viral and other cell-penetrating peptides as vectors of therapeutic agents in medicine. J. Pharmacol. Exp. Ther. 2015, 354, 32–42. [Google Scholar]
- Gagat, M.; Zielińska, W.; Grzanka, A. Cell-penetrating peptides and their utility in genome function modifications (Review). Int. J. Mol. Med. 2017, 40, 1615–1623. [Google Scholar]
- Vivès, E.; Schmidt, J.; Pèlegrin, A. Cell-penetrating and cell-targeting peptides in drug delivery. Biochim. Biophys. Acta Rev. Cancer 2008, 1786, 126–138. [Google Scholar]
- Feni, L.; Neundorf, I. The Current Role of Cell-Penetrating Peptides in Cancer Therapy; Springer: Cologne, Germany, 2017; Volume 1030. [Google Scholar]
- Klimpel, A.; Lützenburg, T.; Neundorf, I. Recent advances of anti-cancer therapies including the use of cell-penetrating peptides. Curr. Opin. Pharmacol. 2019, 47, 8–13. [Google Scholar] [CrossRef]
- Joliot, A.; Pernelle, C.; Deagostini-Bazin, H.; Prochiantz, A. Antennapedia homeobox peptide regulates neural morphogenesis. Proc. Natl. Acad. Sci. USA 1991, 88, 1864–1868. [Google Scholar] [CrossRef] [Green Version]
- Green, M.; Loewenstein, P.M. Autonomous functional domains of chemically synthesized human immunodeficiency virus tat trans-activator protein. Cell 1988, 55, 1179–1188. [Google Scholar] [CrossRef]
- Frankel, A.D.; Pabo, C.O. Cellular uptake of the tat protein from human immunodeficiency virus. Cell 1988, 55, 1189–1193. [Google Scholar] [CrossRef]
- Futaki, S.; Suzuki, T.; Ohashi, W.; Yagami, T.; Tanaka, S.; Ueda, K.; Sugiura, Y. Arginine-rich peptides. An abundant source of membrane-permeable peptides having potential as carriers for intracellular protein delivery. J. Biol. Chem. 2001, 276, 5836–5840. [Google Scholar] [CrossRef] [Green Version]
- Neundorf, I.; Rennert, R.; Hoyer, J.; Schramm, F.; Löbner, K.; Kitanovic, I.; Wölfl, S. Fusion of a short HA2-derived peptide sequence to cell-penetrating peptides improves cytosolic uptake, but enhances cytotoxic activity. Pharmaceuticals 2009, 2, 49–65. [Google Scholar] [CrossRef] [Green Version]
- Horn, M.; Reichart, F.; Natividad-Tietz, S.; Diaz, D.; Neundorf, I. Tuning the properties of a novel short cell-penetrating peptide by intramolecular cyclization with a triazole bridge. Chem. Commun. 2016, 52, 2261–2264. [Google Scholar] [CrossRef]
- Cheng, H.; Zhu, J.Y.; Xu, X.D.; Qiu, W.X.; Lei, Q.; Han, K.; Cheng, Y.J.; Zhang, X.Z. Activable Cell-Penetrating Peptide Conjugated Prodrug for Tumor Targeted Drug Delivery. ACS Appl. Mater. Interfaces 2015, 7, 16061–16069. [Google Scholar] [CrossRef]
- Parker, J.P.; Devocelle, M.; Morgan, M.P.; Marmion, C.J. Derivatisation of buforin IIb, a cationic henicosapeptide, to afford its complexation to platinum(II) resulting in a novel platinum(II)-buforin IIb conjugate with anti-cancer activity. Dalt. Trans. 2016, 45, 13038–13041. [Google Scholar] [CrossRef]
- Jerath, G.; Goyal, R.; Trivedi, V.; Santhoshkumar, T.R.; Ramakrishnan, V. Conformationally constrained peptides for drug delivery. J. Pept. Sci. 2020, 26, e3244. [Google Scholar] [CrossRef]
- Darwish, S.; Mozaffari, S.; Parang, K.; Tiwari, R. Cyclic peptide conjugate of curcumin and doxorubicin as an anticancer agent. Tetrahedron Lett. 2017, 58, 4617–4622. [Google Scholar] [CrossRef] [Green Version]
- Darwish, S.; Sadeghiani, N.; Fong, S.; Mozaffari, S.; Hamidi, P.; Withana, T.; Yang, S.; Tiwari, R.K.; Parang, K. Synthesis and antiproliferative activities of doxorubicin thiol conjugates and doxorubicin-SS-cyclic peptide. Eur. J. Med. Chem. 2019, 161, 594–606. [Google Scholar] [CrossRef]
- Zang, C.; Wang, H.; Li, T.; Zhang, Y.; Li, J.; Shang, M.; Du, J.; Xi, Z.; Zhou, C. A light-responsive, self-immolative linker for controlled drug delivery: Via peptide- and protein-drug conjugates. Chem. Sci. 2019, 10, 8973–8980. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Li, L.; Chang, L.; Liu, H.; Song, J.; Liu, Y.; Bao, H.; Liu, B.; Wang, R.; Ni, J. Design of a new pH-activatable cell-penetrating peptide for drug delivery into tumor cells. Chem. Biol. Drug Des. 2019, 94, 1884–1893. [Google Scholar] [CrossRef]
- Nam, S.H.; Jang, J.; Cheon, D.H.; Chong, S.E.; Ahn, J.H.; Hyun, S.; Yu, J.; Lee, Y. pH-Activatable cell penetrating peptide dimers for potent delivery of anticancer drug to triple-negative breast cancer. J. Control. Release 2020, 330, 898–906. [Google Scholar] [CrossRef]
- Yu, M.; Li, X.; Huang, X.; Zhang, J.; Zhang, Y.; Wang, H. New Cell-Penetrating Peptide (KRP) with Multiple Physicochemical Properties Endows Doxorubicin with Tumor Targeting and Improves Its Therapeutic Index. ACS Appl. Mater. Interfaces 2019, 11, 2448–2458. [Google Scholar] [CrossRef]
- Duan, Z.; Chen, C.; Qin, J.; Liu, Q.; Wang, Q.; Xu, X.; Wang, J. Cell-penetrating peptide conjugates to enhance the antitumor effect of paclitaxel on drug-resistant lung cancer. Drug Deliv. 2017, 24, 752–764. [Google Scholar] [CrossRef] [Green Version]
- Maity, S.K.; Stahl, P.; Hensel, A.; Knauer, S.; Hirschhäuser, C.; Schmuck, C. Cancer-Cell-Specific Drug Delivery by a Tumor-Homing CPP-Gossypol Conjugate Employing a Tracelessly Cleavable Linker. Chem. Eur. J. 2020, 26, 3010–3015. [Google Scholar] [CrossRef]
- El-Sayed, N.S.; Shirazi, A.N.; Sajid, M.I.; Park, S.E.; Parang, K.; Tiwari, R.K. Synthesis and Antiproliferative Activities of Conjugates of Paclitaxel and Camptothecin with a Cyclic Cell-Penetrating Peptide. Molecules 2019, 24, 1427. [Google Scholar] [CrossRef] [Green Version]
- Szabó, I.; Orbán, E.; Schlosser, G.; Hudecz, F.; Bánóczi, Z. Cell-penetrating conjugates of pentaglutamylated methotrexate as potential anticancer drugs against resistant tumor cells. Eur. J. Med. Chem. 2016, 115, 361–368. [Google Scholar] [CrossRef] [Green Version]
- Song, Q.; Chuan, X.; Chen, B.; He, B.; Zhang, H.; Dai, W.; Wang, X.; Zhang, Q. A smart tumor targeting peptide–drug conjugate, pHLIP-SS-DOX: Synthesis and cellular uptake on MCF-7 and MCF-7/Adr cells. Drug Deliv. 2016, 23, 1734–1746. [Google Scholar] [CrossRef] [Green Version]
- Puckett, C.A.; Barton, J.K. Targeting a ruthenium complex to the nucleus with short peptides. Bioorg. Med. Chem. 2010, 18, 3564–3569. [Google Scholar] [CrossRef] [Green Version]
- Lelle, M.; Freidel, C.; Kaloyanova, S.; Tabujew, I.; Schramm, A.; Musheev, M.; Niehrs, C.; Müllen, K.; Peneva, K. Overcoming drug resistance by cell-penetrating peptide-mediated delivery of a doxorubicin dimer with high DNA-binding affinity. Eur. J. Med. Chem. 2017, 130, 336–345. [Google Scholar] [CrossRef]
- Śmiłowicz, D.; Metzler-Nolte, N. Bioconjugates of Co(III) complexes with Schiff base ligands and cell penetrating peptides: Solid phase synthesis, characterization and antiproliferative activity. J. Inorg. Biochem. 2020, 206, 111041. [Google Scholar] [CrossRef]
- Shi, N.Q.; Qi, X.R. Taming the Wildness of “trojan-Horse” Peptides by Charge-Guided Masking and Protease-Triggered Demasking for the Controlled Delivery of Antitumor Agents. ACS Appl. Mater. Interfaces 2017, 9, 10519–10529. [Google Scholar] [CrossRef]
- Zakeri-Milani, P.; Farkhani, S.M.; Shirani, A.; Mohammadi, S.; Mojarrad, J.S.; Akbari, J.; Valizadeh, H. Cellular uptake and anti-tumor activity of gemcitabine conjugated with new amphiphilic cell penetrating peptides. EXCLI J. 2017, 16, 650–662. [Google Scholar] [CrossRef]
- Klimpel, A.; Neundorf, I. Bifunctional peptide hybrids targeting the matrix of mitochondria. J. Control. Release 2018, 291, 147–156. [Google Scholar] [CrossRef]
- Feni, L.; Parente, S.; Robert, C.; Gazzola, S.; Arosio, D.; Piarulli, U.; Neundorf, I. Kiss and Run: Promoting Effective and Targeted Cellular Uptake of a Drug Delivery Vehicle Composed of an Integrin-Targeting Diketopiperazine Peptidomimetic and a Cell-Penetrating Peptide. Bioconjug. Chem. 2019, 30, 2011–2022. [Google Scholar] [CrossRef] [Green Version]
- Klein, A.; Haseloer, A.; Lützenburg, T.; Strache, J.P.; Neudörfl, J.; Neundorf, I. Building up Pt(II)-thiosemicarbazone-lysine-sC18 conjugates. ChemBioChem 2020, 22, 694–704. [Google Scholar] [CrossRef]
- Feni, L.; Jütten, L.; Parente, S.; Piarulli, U.; Neundorf, I.; Diaz, D. Cell-penetrating peptides containing 2,5-diketopiperazine (DKP) scaffolds as shuttles for anti-cancer drugs: Conformational studies and biological activity. Chem. Commun. 2020, 56, 5685–5688. [Google Scholar] [CrossRef]
- Deng, X.; Mai, R.; Zhang, C.; Yu, D.; Ren, Y.; Li, G.; Cheng, B.; Li, L.; Yu, Z.; Chen, J. Discovery of Novel Cell-penetrating and Tumor-targeting Peptide-Drug Conjugate (PDC) for Programmable Delivery of Paclitaxel and Cancer Treatment. Eur. J. Med. Chem. 2020, 213, 113050. [Google Scholar] [CrossRef]
- Lyu, L.; Huang, L.; Huang, T.; Xiang, W.; Yuan, J.-D.; Zhang, C. Cell-penetrating peptide conjugates of gambogic acid enhance the antitumor effect on human bladder cancer EJ cells through ROS-mediated apoptosis. Drug Des. Devel. Ther. 2018, 12, 743–756. [Google Scholar] [CrossRef] [Green Version]
- Yao, J.; Ma, Y.; Zhang, W.; Li, L.; Zhang, Y.; Zhang, L.; Liu, H.; Ni, J.; Wang, R. Design of new acid-activated cellpenetrating peptides for tumor drug delivery. PeerJ 2017, 5, e3429. [Google Scholar] [CrossRef] [Green Version]
- Carvalho, C.; Santos, R.; Cardoso, S.; Correia, S.; Oliveira, P.; Santos, M.; Moreira, P. Doxorubicin: The Good, the Bad and the Ugly Effect. Curr. Med. Chem. 2009, 16, 3267–3285. [Google Scholar] [CrossRef]
- Wall, M.E.; Wani, M.C.; Cook, C.E.; Palmer, K.H.; McPhail, A.T.; Sim, G.A. Plant Antitumor Agents. I. The Isolation and Structure of Camptothecin, a Novel Alkaloidal Leukemia and Tumor Inhibitor from Camptotheca acuminata. J. Am. Chem. Soc. 1966, 88, 3888–3890. [Google Scholar]
- Liu, L.F.; Desai, S.D.; Li, T.K.; Mao, Y.; Sun, M.; Sim, S.P. Mechanism of action of camptothecin. In Proceedings of the Annals of the New York Academy of Sciences; New York Academy of Sciences: New York, NY, USA, 2000; Volume 922, pp. 1–10. [Google Scholar]
- Zeng, H.; Chen, Z.S.; Belinsky, M.G.; Rea, P.A.; Kruh, G.D. Transport of methotrexate (MTX) and folates by multidrug resistance protein (MRP) 3 and MRP1: Effect of polyglutamylation on MTX transport. Cancer Res. 2001, 61, 7225–7232. [Google Scholar]
- Jolivet, J.; Chabner, B.A. Intracellular pharmacokinetics of methotrexate polyglutamates in human breast cancer cells. Selective retention and less dissociable binding of 4-NH2-10-CH3-pteroylglutamate4 and 4-NH2-10-CH3-pteroylglutamate5 to dihydrofolate reductase. J. Clin. Investig. 1983, 72, 773–778. [Google Scholar] [CrossRef]
- Ruan, H.; Hao, S.; Young, P.; Zhang, H. Targeting cathepsin B for cancer therapies. In Horizons in Cancer Research; Nova Science Publishers, Inc.: Philadelphia, PA, USA, 2015; Volume 56, pp. 23–39. ISBN 9781634822480. [Google Scholar]
- Marshall, G.R.; Hodgkin, E.E.; Langs, D.A.; Smith, G.D.; Zabrocki, J.; Leplawy, M.T. Factors governing helical preference of peptides containing multiple α,α-dialkyl amino acids. Proc. Natl. Acad. Sci. USA 1990, 87, 487–491. [Google Scholar] [CrossRef] [Green Version]
- Conlon, J.M.; Al-Kharrge, R.; Ahmed, E.; Raza, H.; Galadari, S.; Condamine, E. Effect of aminoisobutyric acid (Aib) substitutions on the antimicrobial and cytolytic activities of the frog skin peptide, temporin-1DRa. Peptides 2007, 28, 2075–2080. [Google Scholar] [CrossRef]
- Basu, G.; Kuki, A. Conformational preferences of oligopeptides rich in?-aminoisobutyric acid. II. A model for the 310/?-helix transition with composition and sequence sensitivity. Biopolymers 1992, 32, 61–71. [Google Scholar] [CrossRef]
- Jean, S.R.; Ahmed, M.; Lei, E.K.; Wisnovsky, S.P.; Kelley, S.O. Peptide-Mediated Delivery of Chemical Probes and Therapeutics to Mitochondria. Acc. Chem. Res. 2016, 49, 1893–1902. [Google Scholar] [CrossRef]
- Kazak, L.; Reyes, A.; Holt, I.J. Minimizing the damage: Repair pathways keep mitochondrial DNA intact. Nat. Rev. Mol. Cell Biol. 2012, 13, 659–671. [Google Scholar] [CrossRef]
- Lee, M.H.; Yang, Z.; Lim, C.W.; Lee, Y.H.; Dongbang, S.; Kang, C.; Kim, J.S. Disulfide-cleavage-triggered chemosensors and their biological applications. Chem. Rev. 2013, 113, 5071–5109. [Google Scholar]
- Ouellette, R.J.; Rawn, J.D. Carboxylic Acid Derivatives. In Organic Chemistry; Elsevier: Amsterdam, The Netherlands, 2018; pp. 665–710. [Google Scholar]
- Ndagi, U.; Mhlongo, N.; Soliman, M.E. Metal complexes in cancer therapy–An update from drug design perspective. Drug Des. Devel. Ther. 2017, 11, 599–616. [Google Scholar]
- Rosenberg, B.; VanCamp, L.; Trosko, J.E.; Mansour, V.H. Platinum compounds: A new class of potent antitumour agents. Nature 1969, 222, 385–386. [Google Scholar]
- Jungwirth, U.; Kowol, C.R.; Keppler, B.K.; Hartinger, C.G.; Berger, W.; Heffeter, P. Anticancer activity of metal complexes: Involvement of redox processes. Antioxid. Redox Signal. 2011, 15, 1085–1127. [Google Scholar] [CrossRef] [Green Version]
- Kelland, L. The resurgence of platinum-based cancer chemotherapy. Nat. Rev. Cancer 2007, 7, 573–584. [Google Scholar]
- Neundorf, I. Metal Complex-Peptide Conjugates: How to Modulate Bioactivity of Metal-Containing Compounds by the Attachment to Peptides. Curr. Med. Chem. 2017, 24, 1853–1861. [Google Scholar] [CrossRef]
- Brunner, J.; Barton, J.K. Targeting DNA Mismatches with Rhodium Intercalators Functionalized with a Cell-Penetrating Peptide. Biochemistry 2006, 45, 12295–12302. [Google Scholar] [CrossRef] [Green Version]
- Mahon, K.P.; Potocky, T.B.; Blair, D.; Roy, M.D.; Stewart, K.M.; Chiles, T.C.; Kelley, S.O. Deconvolution of the Cellular Oxidative Stress Response with Organelle-Specific Peptide Conjugates. Chem. Biol. 2007, 14, 923–930. [Google Scholar] [CrossRef] [Green Version]
- Gross, A.; Alborzinia, H.; Piantavigna, S.; Martin, L.L.; Wölfl, S.; Metzler-Nolte, N. Vesicular disruption of lysosomal targeting organometallic polyarginine bioconjugates. Metallomics 2015, 7, 371–384. [Google Scholar] [CrossRef] [Green Version]
- Albada, B.; Metzler-Nolte, N. Organometallic–Peptide Bioconjugates: Synthetic Strategies and Medicinal Applications. Chem. Rev. 2016, 116, 11797–11839. [Google Scholar] [CrossRef]
- Battistin, F.; Siegmund, D.; Balducci, G.; Alessio, E.; Metzler-Nolte, N. Ru(ii)-Peptide bioconjugates with the cppH linker (cppH = 2-(2′-pyridyl)pyrimidine-4-carboxylic acid): Synthesis, structural characterization, and different stereochemical features between organic and aqueous solvents. Dalt. Trans. 2019, 48, 400–414. [Google Scholar] [CrossRef] [Green Version]
- Slootweg, J.C.; Albada, H.B.; Siegmund, D.; Metzler-Nolte, N. Efficient reagent-saving method for the N-terminal labeling of bioactive peptides with organometallic carboxylic acids by solid-phase synthesis. Organometallics 2016, 35, 3192–3196. [Google Scholar] [CrossRef]
- Slootweg, J.C.; Prochnow, P.; Bobersky, S.; Bandow, J.E.; Metzler-Nolte, N. Exploring Structure-Activity Relationships in Synthetic Antimicrobial Peptides (synAMPs) by a Ferrocene Scan. Eur. J. Inorg. Chem. 2017, 2017, 360–367. [Google Scholar] [CrossRef]
- Albada, B.; Metzler-Nolte, N. Highly Potent Antibacterial Organometallic Peptide Conjugates. Acc. Chem. Res. 2017, 50, 2510–2518. [Google Scholar] [CrossRef]
- Śmiłowicz, D.; Metzler-Nolte, N. Synthesis of monofunctional platinum(iv) carboxylate precursors for use in Pt(iv)-peptide bioconjugates. Dalt. Trans. 2018, 47, 15465–15476. [Google Scholar] [CrossRef]
- Böttger, R.; Hoffmann, R.; Knappe, D. Differential stability of therapeutic peptides with different proteolytic cleavage sites in blood, plasma and serum. PLoS ONE 2017, 12, e0178943. [Google Scholar] [CrossRef]
- Vrettos, E.I.; Mező, G.; Tzakos, A.G. On the design principles of peptide–drug conjugates for targeted drug delivery to the malignant tumor site. Beilstein J. Org. Chem. 2018, 14, 930–954. [Google Scholar]
- Chang, M.; Zhang, F.; Wei, T.; Zuo, T.; Guan, Y.; Lin, G.; Shao, W. Smart linkers in polymer-drug conjugates for tumor-targeted delivery. J. Drug Target. 2016, 24, 475–491. [Google Scholar]
- Worm, D.J.; Els-Heindl, S.; Beck-Sickinger, A.G. Targeting of peptide-binding receptors on cancer cells with peptide-drug conjugates. Pept. Sci. 2020, 112, e24171. [Google Scholar] [CrossRef]
- Kiew, L.-V.; Cheong, S.-K.; Sidik, K.; Chung, L.-Y. Improved plasma stability and sustained release profile of gemcitabine via polypeptide conjugation. Int. J. Pharm. 2010, 391, 212–220. [Google Scholar] [CrossRef] [Green Version]
- Cavallaro, G.; Mariano, L.; Salmaso, S.; Caliceti, P.; Gaetano, G. Folate-mediated targeting of polymeric conjugates of gemcitabine. Int. J. Pharm. 2006, 307, 258–269. [Google Scholar] [CrossRef]
- Gamcsik, M.P.; Kasibhatla, M.S.; Teeter, S.D.; Colvin, O.M. Glutathione levels in human tumors. Biomarkers 2012, 17, 671–691. [Google Scholar] [CrossRef]
- Deponte, M. Glutathione catalysis and the reaction mechanisms of glutathione-dependent enzymes. Biochim. Biophys. Acta Gen. Subj. 2013, 1830, 3217–3266. [Google Scholar]
- Mandal, D.; Shirazi, A.N.; Parang, K. Cell-penetrating homochiral cyclic peptides as nuclear-targeting molecular transporters. Angew. Chem. Int. Ed. 2011, 50, 9633–9637. [Google Scholar] [CrossRef]
- Shirazi, A.N.; Tiwari, R.; Chhikara, B.S.; Mandal, D.; Parang, K. Design and biological evaluation of cell-penetrating peptide-doxorubicin conjugates as prodrugs. Mol. Pharm. 2013, 10, 488–499. [Google Scholar] [CrossRef]
- Shirazi, A.N.; El-Sayed, N.S.; Tiwari, R.K.; Tavakoli, K.; Parang, K. Cyclic Peptide Containing Hydrophobic and Positively Charged Residues as a Drug Delivery System for Curcumin. Curr. Drug Deliv. 2016, 13, 409–417. [Google Scholar] [CrossRef]
- Cox, J.A.; Comte, M.; Fitton, J.E.; DeGrado, W.F. The interaction of calmodulin with amphiphilic peptides. J. Biol. Chem. 1985, 260, 2527–2534. [Google Scholar] [CrossRef]
- Usui, K.; Kikuchi, T.; Mie, M.; Kobatake, E.; Mihara, H. Systematic screening of the cellular uptake of designed alpha-helix peptides. Bioorg. Med. Chem. 2013, 21, 2560–2567. [Google Scholar] [CrossRef]
- Cardone, R.A.; Casavola, V.; Reshkin, S.J. The role of disturbed pH dynamics and the NA+/H+ exchanger in metastasis. Nat. Rev. Cancer 2005, 5, 786–795. [Google Scholar]
- Jähde, E.; Rajewsky, M.F.; Baumgärtl, H. pH Distributions in Transplanted Neural Tumors and Normal Tissues of BDIX Rats as Measured with pH Microelectrodes. Cancer Res. 1982, 42, 1498–1504. [Google Scholar]
- Tannock, I.F.; Rotin, D. Acid pH in Tumors and Its Potential for Therapeutic Exploitation. Cancer Res. 1989, 49, 4373–4384. [Google Scholar]
- Vaupel, P.; Kallinowski, F.; Okunieff, P. Blood Flow, Oxygen and Nutrient Supply, and Metabolic Microenvironment of Human Tumors: A Review. Cancer Res. 1989, 49, 6449–6465. [Google Scholar]
- Zaro, J.L.; Fei, L.; Shen, W.C. Recombinant peptide constructs for targeted cell penetrating peptide-mediated delivery. J. Control. Release 2012, 158, 357–361. [Google Scholar] [CrossRef]
- Henne, W.A.; Doorneweerd, D.D.; Hilgenbrink, A.R.; Kularatne, S.A.; Low, P.S. Synthesis and activity of a folate peptide camptothecin prodrug. Bioorg. Med. Chem. Lett. 2006, 16, 5350–5355. [Google Scholar] [CrossRef]
- Zhang, W.; Song, J.; Mu, L.; Zhang, B.; Liu, L.; Xing, Y.; Wang, K.; Li, Z.; Wang, R. Improving anticancer activity and selectivity of camptothecin through conjugation with releasable substance P. Bioorg. Med. Chem. Lett. 2011, 21, 1452–1455. [Google Scholar] [CrossRef]
- Zhang, W.; Song, J.; Zhang, B.; Liu, L.; Wang, K.; Wang, R. Design of acid-activated cell penetrating peptide for delivery of active molecules into cancer cells. Bioconjug. Chem. 2011, 22, 1410–1415. [Google Scholar] [CrossRef]
- Lelle, M.; Peneva, K. An amino acid-based heterofunctional cross-linking reagent. Amino Acids 2014, 46, 1243–1251. [Google Scholar] [CrossRef]
- Conn, P.M.; Smith, E.; Spicer, T.; Chase, P.; Scampavia, L.; Janovick, J.A. A phenotypic high throughput screening assay for the identification of pharmacoperones for the gonadotropin releasing hormone receptor. Assay Drug Dev. Technol. 2014, 12, 238–246. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Taratula, O.; Taratula, O.; Schumann, C.; Minko, T. LHRH-Targeted Drug Delivery Systems for Cancer Therapy. Mini-Rev. Med. Chem. 2016, 17, 258–267. [Google Scholar] [CrossRef]
- Fang, X.; Zhang, X.; Wang, X.; Zhong, W.; Ren, X.; Sha, X. Matrix metalloproteinases-2/9-sensitive peptide-conjugated polymer micelles for site-specific release of drugs and enhancing tumor accumulation: Preparation and in vitro and in vivo evaluation. Int. J. Nanomed. 2016, 11, 1643. [Google Scholar] [CrossRef] [Green Version]
- Dong, W.; Li, H.; Zhang, Y.; Yang, H.; Guo, M.; Li, L.; Liu, T. Matrix metalloproteinase 2 promotes cell growth and invasion in colorectal cancer. Acta Biochim. Biophys. Sin. 2011, 43, 840–848. [Google Scholar] [CrossRef] [Green Version]
- Kato, Y.; Ozawa, S.; Miyamoto, C.; Maehata, Y.; Suzuki, A.; Maeda, T.; Baba, Y. Acidic extracellular microenvironment and cancer. Cancer Cell Int. 2013, 13, 89. [Google Scholar]
- Andreucci, E.; Peppicelli, S.; Ruzzolini, J.; Bianchini, F.; Biagioni, A.; Papucci, L.; Magnelli, L.; Mazzanti, B.; Stecca, B.; Calorini, L. The acidic tumor microenvironment drives a stem-like phenotype in melanoma cells. J. Mol. Med. 2020, 98, 1431–1446. [Google Scholar] [CrossRef]
- Ravasco, J.M.J.M.; Faustino, H.; Trindade, A.; Gois, P.M.P. Bioconjugation with Maleimides: A Useful Tool for Chemical Biology. Chem. A Eur. J. 2019, 25, 43–59. [Google Scholar] [CrossRef]
- Yu, M.; Li, X.; Liang, R.; Yang, J.; Zhang, Y.; Wang, H. A new ligand of CD105 screened out by phage display technology provides a reliable identification of recurrent or metastasizing pleomorphic adenoma from pleomorphic adenoma. Int. Immunopharmacol. 2018, 65, 37–43. [Google Scholar] [CrossRef]
- Maeda, H. Toward a full understanding of the EPR effect in primary and metastatic tumors as well as issues related to its heterogeneity. Adv. Drug Deliv. Rev. 2015, 91, 3–6. [Google Scholar]
- Maeda, H.; Wu, J.; Sawa, T.; Matsumura, Y.; Hori, K. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: A review. J. Control. Release 2000, 65, 271–284. [Google Scholar] [CrossRef]
- Matsumura, Y.; Maeda, H. A New Concept for Macromolecular Therapeutics in Cancer Chemotherapy: Mechanism of Tumoritropic Accumulation of Proteins and the Antitumor Agent Smancs. Cancer Res. 1986, 46, 6387–6392. [Google Scholar]
- Dixon, M.J.; Bourré, L.; MacRobert, A.J.; Eggleston, I.M. Novel prodrug approach to photodynamic therapy: Fmoc solid-phase synthesis of a cell permeable peptide incorporating 5-aminolaevulinic acid. Bioorg. Med. Chem. Lett. 2007, 17, 4518–4522. [Google Scholar] [CrossRef]
- Ren, F.; Tang, R.; Zhang, X.; Madushi, W.M.; Luo, D.; Dang, Y.; Li, Z.; Wei, K.; Chen, G. Overexpression of MMP Family Members Functions as Prognostic Biomarker for Breast Cancer Patients: A Systematic Review and Meta-Analysis. PLoS ONE 2015, 10, e0135544. [Google Scholar] [CrossRef] [Green Version]
- Xia, H.; Gu, G.; Hu, Q.; Liu, Z.; Jiang, M.; Kang, T.; Miao, D.; Song, Q.; Yao, L.; Tu, Y.; et al. Activatable cell penetrating peptide-conjugated nanoparticles with enhanced permeability for site-specific targeting delivery of anticancer drug. Bioconjug. Chem. 2013, 24, 419–430. [Google Scholar] [CrossRef]
- Xia, H.; Gao, X.; Gu, G.; Liu, Z.; Zeng, N.; Hu, Q.; Song, Q.; Yao, L.; Pang, Z.; Jiang, X.; et al. Low molecular weight protamine-functionalized nanoparticles for drug delivery to the brain after intranasal administration. Biomaterials 2011, 32, 9888–9898. [Google Scholar] [CrossRef]
- Park, J.; Ryu, J.; Kim, K.A.; Lee, H.J.; Bahn, J.H.; Han, K.; Choi, E.Y.; Lee, K.S.; Kwon, H.Y.; Choi, S.Y. Mutational analysis of a human immunodeficiency virus type 1 Tat protein transduction domain which is required for delivery of an exogenous protein into mammalian cells. J. Gen. Virol. 2002, 83, 1173–1181. [Google Scholar] [CrossRef]
- Kalia, J.; Raines, R.T. Hydrolytic Stability of Hydrazones and Oximes. Angew. Chem. Int. Ed. 2008, 47, 7523–7526. [Google Scholar] [CrossRef] [Green Version]
- Jin, Y.; Song, L.; Su, Y.; Zhu, L.; Pang, Y.; Qiu, F.; Tong, G.; Yan, D.; Zhu, B.; Zhu, X. Oxime linkage: A robust tool for the design of pH-sensitive polymeric drug carriers. Biomacromolecules 2011, 12, 3460–3468. [Google Scholar] [CrossRef]
- Xu, W.; Ding, J.; Xiao, C.; Li, L.; Zhuang, X.; Chen, X. Versatile preparation of intracellular-acidity-sensitive oxime-linked polysaccharide-doxorubicin conjugate for malignancy therapeutic. Biomaterials 2015, 54, 72–86. [Google Scholar] [CrossRef]
- Kapp, T.G.; Rechenmacher, F.; Neubauer, S.; Maltsev, O.V.; Cavalcanti-Adam, E.A.; Zarka, R.; Reuning, U.; Notni, J.; Wester, H.J.; Mas-Moruno, C.; et al. A comprehensive evaluation of the activity and selectivity profile of ligands for RGD-binding integrins. Sci. Rep. 2017, 7, 39805. [Google Scholar] [CrossRef] [Green Version]
- Hatley, R.J.D.; Macdonald, S.J.F.; Slack, R.J.; Le, J.; Ludbrook, S.B.; Lukey, P.T. An αv-RGD Integrin Inhibitor Toolbox: Drug Discovery Insight, Challenges and Opportunities. Angew. Chem. Int. Ed. 2018, 57, 3298–3321. [Google Scholar] [CrossRef]
- Hamidi, H.; Ivaska, J. Every step of the way: Integrins in cancer progression and metastasis. Nat. Rev. Cancer 2018, 18, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Nieberler, M.; Reuning, U.; Reichart, F.; Notni, J.; Wester, H.J.; Schwaiger, M.; Weinmüller, M.; Räder, A.; Steiger, K.; Kessler, H. Exploring the role of RGD-recognizing integrins in cancer. Cancers 2017, 9, 116. [Google Scholar] [CrossRef]
- Goodman, S.L.; Grote, H.J.; Wilm, C. Matched rabbit monoclonal antibodies against αv-series integrins reveal a novel αvβ3-LIBS epitope, and permit routine staining of archival paraffin samples of human tumors. Biol. Open 2012, 1, 329–340. [Google Scholar] [CrossRef] [Green Version]
- He, Q.; Chen, J.; Yan, J.; Cai, S.; Xiong, H.; Liu, Y.; Peng, D.; Mo, M.; Liu, Z. Tumor microenvironment responsive drug delivery systems. Asian J. Pharm. Sci. 2020, 15, 416–448. [Google Scholar]
- Kondo, E.; Saito, K.; Tashiro, Y.; Kamide, K.; Uno, S.; Furuya, T.; Mashita, M.; Nakajima, K.; Tsumuraya, T.; Kobayashi, N.; et al. Tumour lineage-homing cell-penetrating peptides as anticancer molecular delivery systems. Nat. Commun. 2012, 3, 951. [Google Scholar] [CrossRef]










| Name | Sequence | Citation |
|---|---|---|
| ACPP | RRRRRRRRGGGPKKKKKK | [21] |
| Buforin IIb | RAGLQFPVGRLLRRLLRRLLR-NH2 | [22] |
| CHAP-1 CHAP-2 CHAP-3 | KR-Aib-IRLFTK-Aib-LK KR-Aib-IRLFTK-Aib-FK KR-Aib-FRLFTK-Aib-FK | [23] |
| [C(WR)4K2(β-A)] | RCKβ-ARWRWKWRW | [24] |
| [C(WR)4K] | RCRWRWKβ-AWRW | [25] |
| H3-V35C | ARTKQTARKSTGGKAPRKQLATKAARKSAPATGGCKK | [26] |
| LH | LHHLLHHLHHLLHH | [27] |
| LH2 (monomer) | Acetyl-LHHLCHLLHHLCHLAG-NH2 | [28] |
| KRP | CSSKEKKKGKKRKKKREREGQKEGGRRKEKRKEKKRKEGGREGRKEGRKSADHPS | [29] |
| LMWP | VSRRRRRRGGRRRR | [30] |
| MCF-7 targeting CPP | RLYMRYYSPTTRRYG | [31] |
| P1 | Kβ-AWRWRWRWRW | [32] |
| Penetratin(desMet) | RQIKIWFQNRRKWKK | [33] |
| pHLIP | AEQNPIYWARYADWLFTTPLLLLDLALLVDADEGTCG | [34] |
| Nucleus targeting CPP | RrRK | [35] |
| l-R8 | RRRRRRRR | [33,36] |
| d-R8 | rrrrrrrr | [35] |
| R9GAL FFFF FFFFR9GAL | RRRRRRRRRGAL FFFF FFFRRRRRRRRRGAL | [37] |
| LGA-d-R9C | LGA-rrrrrrrrrC | [38] |
| [RW]6 [RW]3 R5W3R3 | RWRWRWRWRWRW RWRWRW RRRRRWWWRRR | [39] |
| sC18 | GLRKRLRKFRNKIKEK-NH2 | [40,41,42] |
| sC18* | GLRKRLRKFRNK | [43] |
| T2 | FKKFFRKLL | [44] |
| TAT | CGGGYGRKKRRQRRR | [30,45] |
| TH | AGYLLGHINLHHLAHL-Aib-HHIL-NH2 | [46] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gayraud, F.; Klußmann, M.; Neundorf, I. Recent Advances and Trends in Chemical CPP–Drug Conjugation Techniques. Molecules 2021, 26, 1591. https://doi.org/10.3390/molecules26061591
Gayraud F, Klußmann M, Neundorf I. Recent Advances and Trends in Chemical CPP–Drug Conjugation Techniques. Molecules. 2021; 26(6):1591. https://doi.org/10.3390/molecules26061591
Chicago/Turabian StyleGayraud, Félix, Merlin Klußmann, and Ines Neundorf. 2021. "Recent Advances and Trends in Chemical CPP–Drug Conjugation Techniques" Molecules 26, no. 6: 1591. https://doi.org/10.3390/molecules26061591








