Highlights on Steroidal Arylidene Derivatives as a Source of Pharmacologically Active Compounds: A Review
Abstract
:1. Introduction
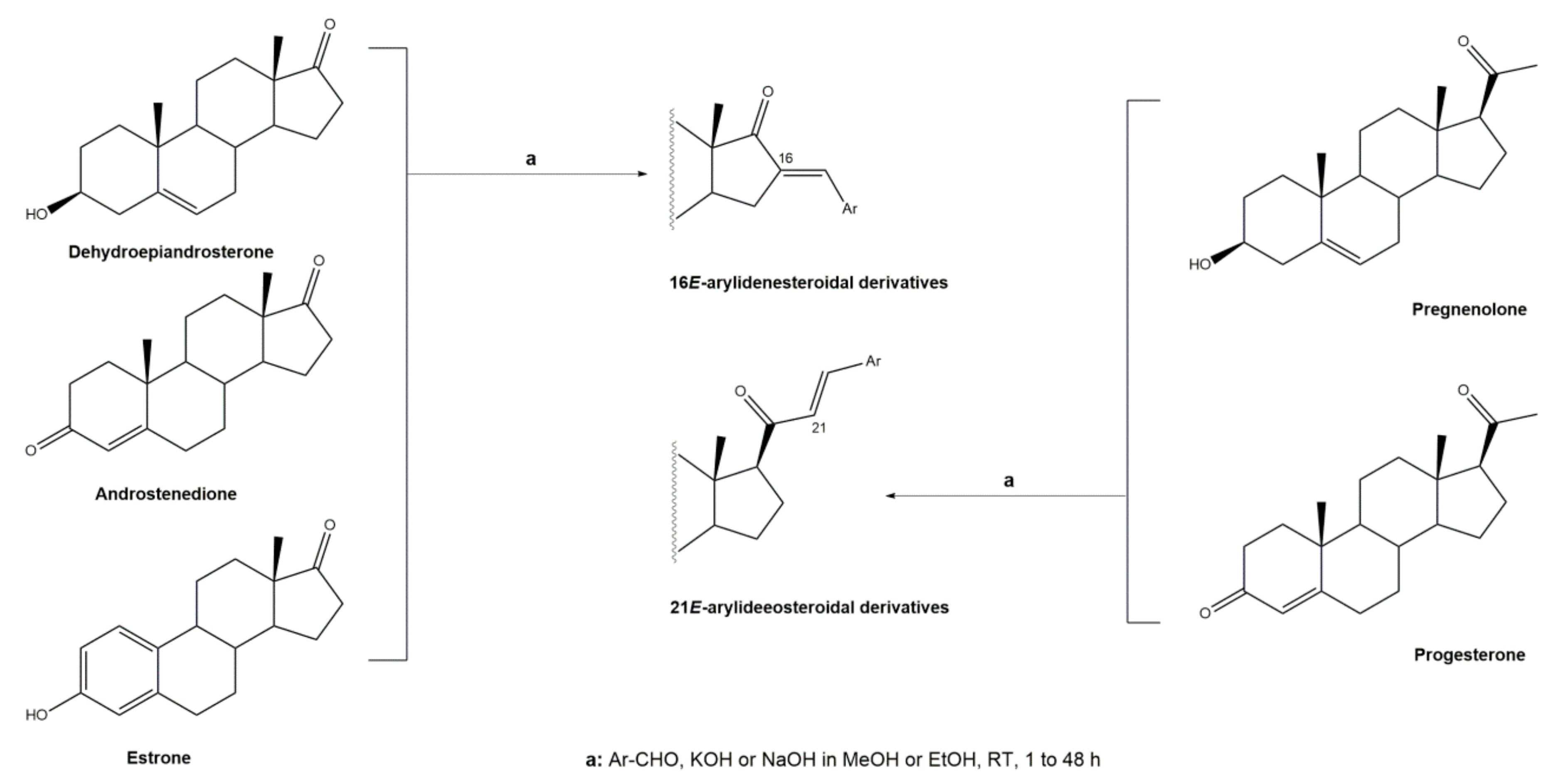
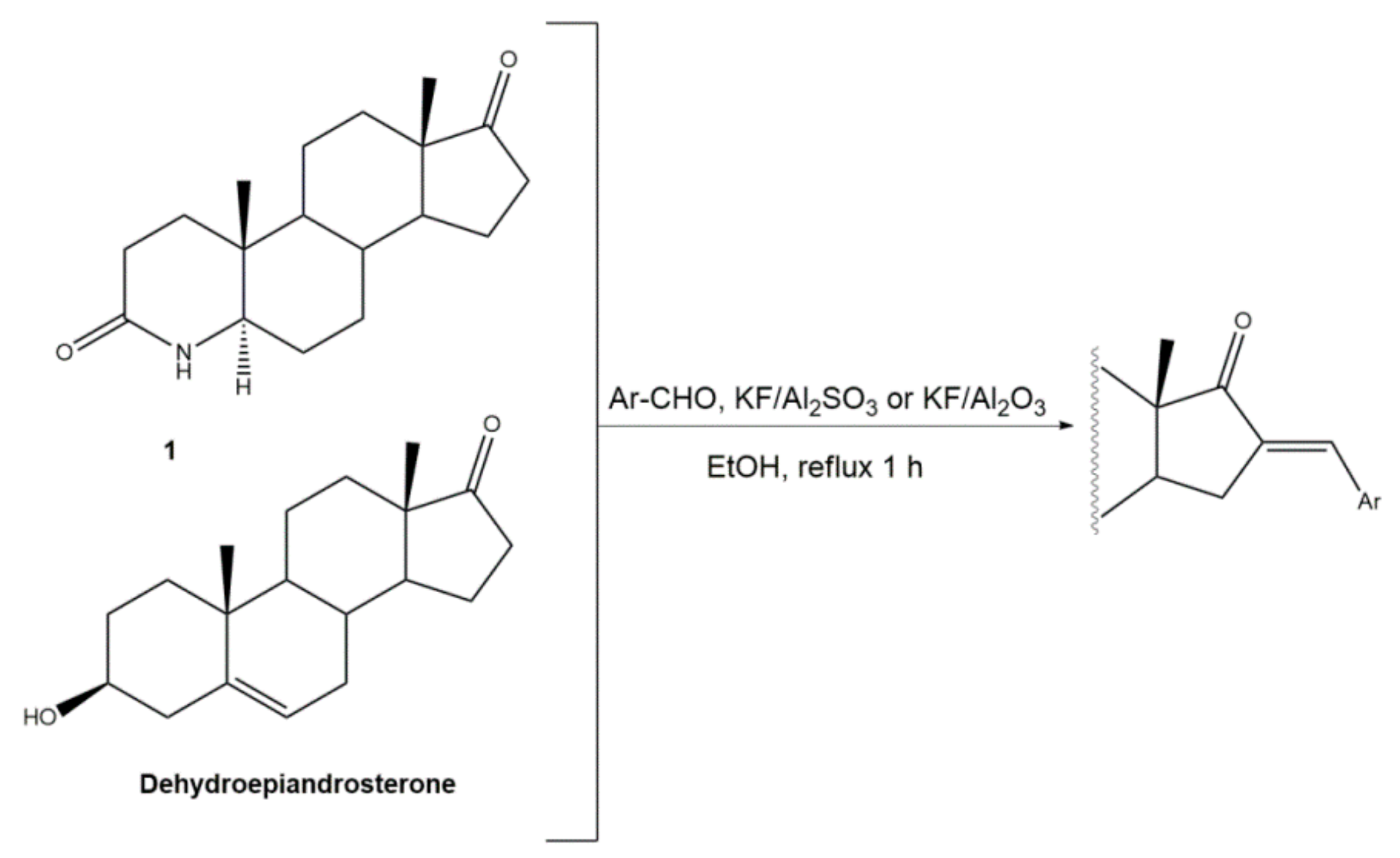
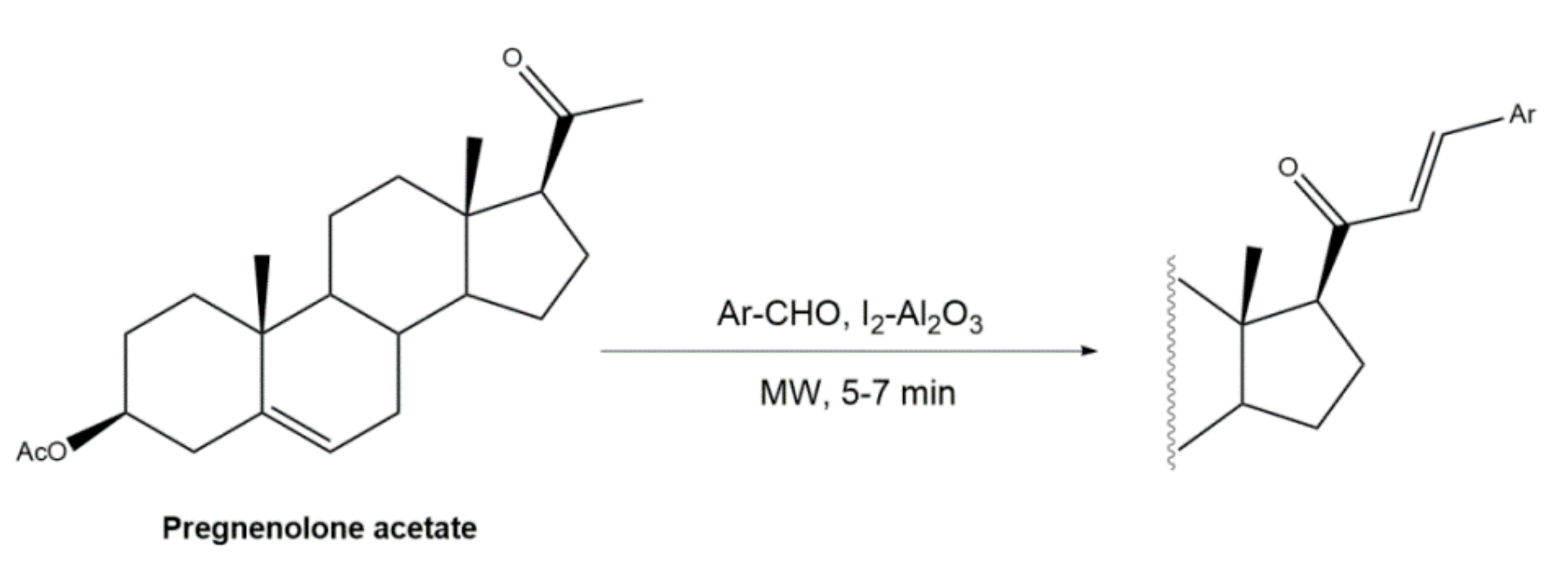
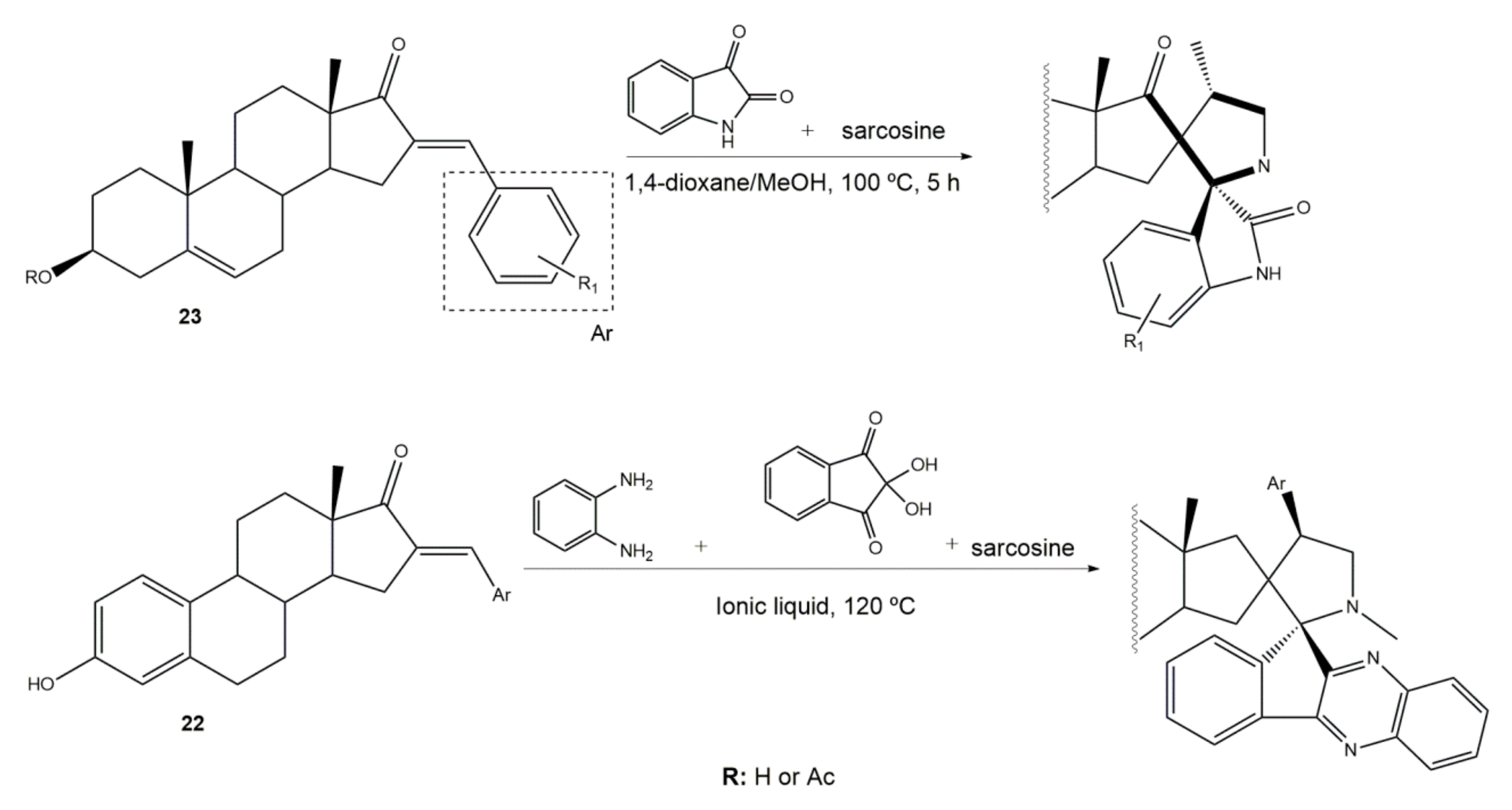
2. Synthetic Approaches to Prepare Arylidene Steroidal Derivatives
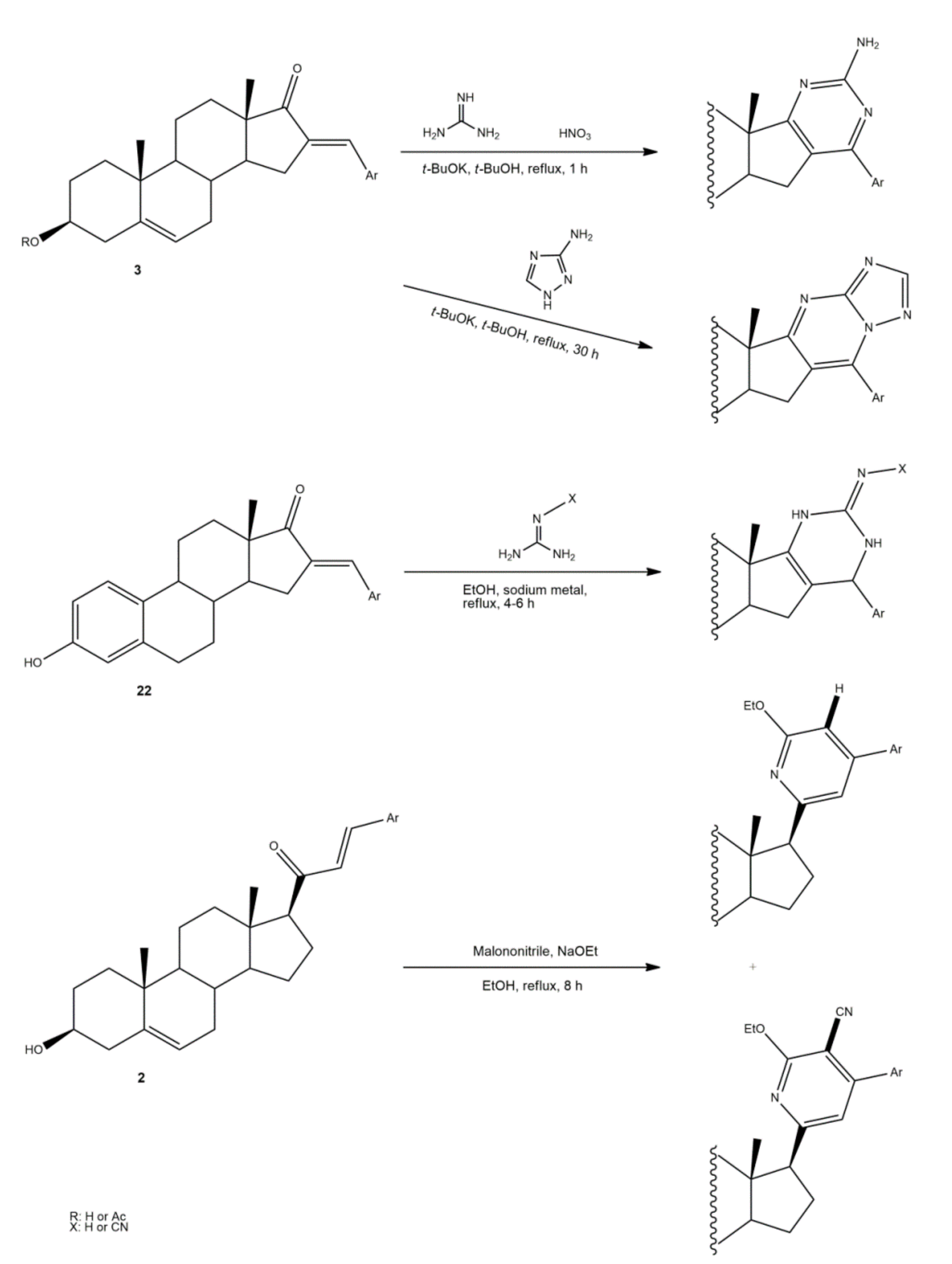
3. Bioactivity of 2- and 16E-arylideneandrostane Derivatives
4. Bioactivity of 21E-arylidenepregnene Derivatives
5. Bioactivity of 16E-arylidenoestrone and 16E-arylidenoestradiol Derivatives
6. Importance of Steroidal Arylidene Derivatives as Synthetic Intermediates of Bioactive Molecules
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lednicer, D. Steroid Chemistry at a Glance; Wiley: Hoboken, NJ, USA, 2011; ISBN 9780470660850. [Google Scholar]
- Burger, A.; Abraham, D.J.; Rotella, D.P. Burger’s Medicinal Chemistry, Drug Discovery and Development; Wiley: Hoboken, NJ, USA, 2010; Volume 7, ISBN 9780470770085. [Google Scholar]
- Latham, K.A.; Zamora, A.; Drought, H.; Matejuk, A.; Offner, H.; Edward, F. Estradiol Treatment Redirects the Isotype of the Autoantibody Response and Prevents the Development of Autoimmune Arthritis. J. Immunol. 2003, 171, 5820–5827. [Google Scholar] [CrossRef] [PubMed]
- Banday, A.H.; Mir, B.P.; Lone, I.H.; Suri, K.A.; Kumar, H.M.S. Studies on Novel D-Ring Substituted Steroidal Pyrazolines as Potential Anticancer Agents. Steroids 2010, 75, 805–809. [Google Scholar] [CrossRef] [PubMed]
- Tantawy, M.A.; Nafie, M.S.; Elmegeed, G.A.; Ali, I.A.I. Auspicious Role of the Steroidal Heterocyclic Derivatives as a Platform for Anti-Cancer Drugs. Bioorg. Chem. 2017, 73, 128–146. [Google Scholar] [CrossRef] [PubMed]
- Vil, V.A.; Terent, A.O.; Savidov, N.; Gloriozova, T.A. Hydroperoxy Steroids and Triterpenoids Derived from Plant and Fungi: Origin, Structures and Biological Activities. J. Steroid Biochem. Mol. Biol. 2019, 190, 76–87. [Google Scholar] [CrossRef]
- Dembitsky, V.M. Progress in Lipid Research Antitumor and Hepatoprotective Activity of Natural and Synthetic Neo Steroids. Prog. Lipid Res. 2020, 79, 101048. [Google Scholar] [CrossRef]
- Xiao, J.; Gao, M.; Fei, B.; Huang, G.; Diao, Q. Fitoterapia Nature-Derived Anticancer Steroids Outside Cardica Glycosides. Fitoterapia 2020, 147, 104757. [Google Scholar] [CrossRef]
- Dubey, R.K.; Oparil, S.; Imthurn, B.; Jackson, E.K. Sex Hormones and Hypertension. Cardiovasc. Res. 2002, 53, 688–708. [Google Scholar] [CrossRef] [Green Version]
- Sheridan, P.J.; Blum, K.; Trachtenberg, M.C. Steroid Receptors and Disease: Cancer, Autoimmune, Bone, and Circulatory Disorders. Trends Pharmacol. Sci. 1988, 10, 122. [Google Scholar] [CrossRef]
- Holst, J.P.; Soldin, S.J.; Tractenberg, R.E.; Guo, T.; Kundra, P.; Verbalis, J.G.; Jonklaas, J.; Clinical, G. Use of Steroid Profiles in Determining the Cause of Adrenal Insufficiency. Steroids 2006, 72, 71–84. [Google Scholar] [CrossRef] [Green Version]
- Jursic, B.S.; Kumar, S.; Creech, C.C.; Neumann, D.M. Novel and Efficient Synthesis and Antifungal Evaluation of 2,3-Functionalized Cholestane and Androstane Derivatives. Bioorg. Med. Chem. Lett. 2020, 20, 7372–7375. [Google Scholar] [CrossRef]
- Banday, A.H.; Zargar, M.I.; Ganaie, B.A. Synthesis and Antimicrobial Studies of Chalconyl Pregnenolones. Steroids 2011, 76, 1358–1362. [Google Scholar] [CrossRef]
- Na, M.S.; Tantawy, M.A.; Elmgeed, G.A. Screening of Different Drug Design Tools to Predict the Mode of Action of Steroidal Derivatives as Anti-Cancer Agents. Steroids 2019, 152. [Google Scholar] [CrossRef]
- Lemke, T.L.; Williams, D.A.; Roche, V.F.; Zito, S.W. Foye’s Principles of Medicinal Chemistry, 7th ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2012. [Google Scholar]
- Huang, X.; Shen, Q.; Zhang, H.; Li, J.; Tian, Y.; Quan, Z. Design and Synthesis of Novel Dehydroepiandrosterone Analogues as Potent Antiproliferative Agents. Molecules 2018, 23, 2243. [Google Scholar] [CrossRef] [Green Version]
- Acharya, P.C.; Bansal, R.; Kharkar, P.S.; Res, D.; Bansal, R. Hybrids of Steroid and Nitrogen Mustard as Antiproliferative Agents: Synthesis, in Vitro Evaluation and in Silico Inverse Screening Authors. Drug Res. (Stuttg). 2017. [Google Scholar] [CrossRef] [Green Version]
- Fan, N.; Tang, J.; Li, H.; Li, X.; Luo, B.; Gao, J. Synthesis and Cytotoxic Activity of Some Novel Steroidal C-17 Pyrazolinyl Derivatives. Eur. J. Med. Chem. 2013, 69, 182–190. [Google Scholar] [CrossRef]
- Gogoi, J.; Bezbaruah, P.; Saikia, P.; Goswami, J.; Gogoi, P.; Boruah, R.C. Synthesis of a Novel Class of Steroidal Tetrazolo[1,5-a]Pyridines Via Intramolecular 1,3-Dipolar Cycloadditions. Tetrahedron Lett. 2012, 53, 1497–1500. [Google Scholar] [CrossRef]
- Chowdhury, P.; Borah, J.M.; Goswami, P.; Das, A.M. A Convenient Synthesis of the Side Chain of Loteprednol Etabonate - an Ocular Soft Corticosteroid from 20-Oxopregnanes Using Metal-Mediated Halogenation as a Key Reaction. Steroids 2011, 76, 497–501. [Google Scholar] [CrossRef]
- Delong, W.; Yongling, W.; Lanying, W.; Juntao, F.; Xing, Z. Design, Synthesis and Evaluation of 3-Arylidene Azetidin-2-Ones as Potential Antifungal Agents Against Alternaria Solani Sorauer. Bioorg. Med. Chem. 2017, 25, 6661–6673. [Google Scholar] [CrossRef]
- Szymanska, E.; Kiec-Kononowicz, K. Antimycobacterial Activity of 5-Arylidene Aromatic Derivatives of Hydantoin. Farm. 2002, 57, 355–362. [Google Scholar] [CrossRef]
- Ottanà, R.; Maccari, R.; Giglio, M.; Del, A.; Cappiello, M.; Mura, U.; Cosconati, S.; Marinelli, L.; Novellino, E.; Sartini, S.; et al. Identification of 5-Arylidene-4-Thiazolidinone Derivatives Endowed with Dual Activity as Aldose Reductase Inhibitors and Antioxidant Agents for the Treatment of Diabetic Complications. Eur. J. Med. Chem. 2011, 46, 2797–2806. [Google Scholar] [CrossRef]
- Stadnicka, K.; Broda, A.; Filipek, B.; Kiec, K. Synthesis, Structure–Activity Relationship of Some New Anti-Arrhythmic 5-Arylidene Imidazolidine-2,4-Dione Derivatives. Eur. J. Med. Chem. 2005, 40, 259–269. [Google Scholar] [CrossRef]
- Li, N.; Xin, W.; Yao, B.; Wang, C.; Cong, W.; Zhao, F.; Li, H.; Hou, Y.; Meng, Q.; Hou, G. Antitumor Agents with Biological Evaluation in Vitro and in Vivo. Eur. J. Med. Chem. 2018, 147, 21–33. [Google Scholar] [CrossRef]
- Amr, A.E.; Mohamed, A.M.; Mohamed, S.F.; Abdel-hafez, N.A.; Hammam, A.E.G. Anticancer Activities of Some Newly Synthesized Pyridine, Pyrane and Pyrimidine Derivatives. Bioorg. Med. Chem. 2006, 14, 5481–5488. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Kumar, A.; Saini, D. Experimental Parasitology Novel Arylidene Derivatives of Quinoline Based Thiazolidinones: Synthesis, in Vitro, in Vivo and in Silico Study as Antimalarials. Exp. Parasitol. 2018, 185, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Valla, A.; Valla, B.; Cartier, D.; Le, R.; Labia, R.; Florent, L.; Charneau, S.; Schrevel, J.; Potier, P. New Syntheses and Potential Antimalarial Activities of New ‘Retinoid-like Chalcones. ’ Eur. J. Med. Chem. 2006, 41, 142–146. [Google Scholar] [CrossRef] [PubMed]
- Maccari, R.; Maria, R.; Ottanà, R.; Rocchiccioli, M.; Marrazzo, A.; Cardile, V.; Carol, A.; Graziano, E.; Amodeo, P.; Mura, U.; et al. Structure-Activity Relationships and Molecular Modelling of New 5-Arylidene-4-Thiazolidinone Derivatives as Aldose Reductase Inhibitors and Potential Anti-Inflammatory Agents. Eur. J. Med. Chem. 2014, 81, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Hee, S.; Seog, G.; Yeong, J.; Yu, X.; Kim, H.; Hwan, D. Heme Oxygenase 1 Mediates Anti-Inflammatory Effects of 2′,4′,6′-Tris(Methoxymethoxy) Chalcone. Eur. J. Pharmacol. 2006, 532, 178–186. [Google Scholar] [CrossRef]
- Taylor, P.; Schultz, T.W.; Yarbrough, J.W. Trends in Structure-Toxicity Relationships for Carbonyl-Containing α,β-Unsaturated Compounds. SAR QSAR Environ. Res. 2007, 2, 37–41. [Google Scholar] [CrossRef]
- Eder, E.; Scheckenbach, S.; Deininger, C.; Hoffman, C. The Possible Role of α,β-Unsaturated Carbonyl Compounds in Mutagenesis and Carcinogenesis. Toxicol. Lett. 1993, 67, 87–103. [Google Scholar] [CrossRef]
- Aguilera, E.; Perdomo, C.; Espindola, A.; Corvo, I.; Faral-tello, P.; Robello, C.; Serna, E.; Torres, S.; De Bilbao, N.I.V.; Yalu, G. A Nature-Inspired Design Yields a New Class of Steroids Against Trypanosomatids. Molecules 2019, 8, 3800. [Google Scholar] [CrossRef] [Green Version]
- Al-masoudi, N.A.; Abdul-rida, N.A.; Kadhim, R.A.; Krug, S.J.; Engel, M.; Saeed, B.A. Synthesis and CYP17 a Hydroxylase Inhibition Activity of New 3α- and 3β-Ester Derivatives of Pregnenolone and Related Ether Analogues. Med. Chem. Res. 2016, 25, 310–321. [Google Scholar] [CrossRef]
- Poirier, D.; Chang, H.; Azzi, A.; Boivin, R.P.; Lin, S. Estrone and Estradiol C-16 Derivatives as Inhibitors of Type 1 17b-Hydroxysteroid Dehydrogenase. Mol. Cell. Endocrinol. 2006, 248, 236–238. [Google Scholar] [CrossRef]
- Bansal, R.; Guleria, S.; Young, L.C.; Harvey, A.L. Synthesis of Quaternary Ammonium Salts of 16E-[4-(2-Alkylaminoethoxy)-3-Methoxybenzylidene]Androstene Derivatives as Skeletal Muscle Relaxants. Steroids 2011, 76, 254–260. [Google Scholar] [CrossRef]
- Guo, H.; Wu, H.; Yang, J.; Xiao, Y.; Altenbach, H.; Qiu, G. Synthesis, Characterization and Biological Evaluation of Some 16E-Arylidene Androstane Derivatives as Potential Anticancer Agents. Steroids 2011, 76, 709–723. [Google Scholar] [CrossRef]
- Fan, N.; Han, Y.; Li, Y.; Gao, J.; Tang, J. Synthesis of Novel 4′-Acylamino Modified 21E-Benzylidene Steroidal Derivatives and Their Cytotoxic Activities. Steroids 2017, 123, 20–26. [Google Scholar] [CrossRef]
- Dubey, S.; Kaur, P.; Jindal, D.P.; Satyanarayan, Y.D.; Piplani, P. Synthesis, Evaluation and QSAR Studies of 16-(4 & 3,4-Substituted) Benzylidene Androstene Derivatives as Anticancer Agents. Med Chem 2008, 4, 229–236. [Google Scholar] [CrossRef]
- Jiang, C.; Guo, X.; Gong, J.; Zhu, T.; Zhang, H.; Guo, Y. Synthesis and Biological Evaluation of 21-Arylidenepregnenolone Derivatives as Neuroprotective Agents. Bioorg. Med. Chem. Lett. 2012, 22, 2226–2229. [Google Scholar] [CrossRef]
- Huang, L.H.; Zheng, Y.F.; Song, C.J.; Wang, Y.G.; Xie, Z.Y.; Lai, Y.W.; Lu, Y.Z.; Liu, H.M. Synthesis of Novel D-Ring Fused 7′-Aryl-Androstano[17,16-d][1,2,4]Triazolo[1,5-a]Pyrimidines. Steroids 2012, 77, 367–374. [Google Scholar] [CrossRef]
- Huang, L.-H.; Zheng, Y.-F.; Lu, Y.-Z.; Song, C.-J.; Wang, Y.-G.; Yu, B.; Liu, H.-M. Synthesis and Biological Evaluation of Novel Steroidal[17,16-d][1,2,4]Triazolo[1,5-a]Pyrimidines. Steroids 2012, 77, 710–715. [Google Scholar] [CrossRef]
- Ke, S. Recent Progress of Novel Steroid Derivatives and Their Potential Biological Properties. Mini-Reviews Med. Chem. 2018, 18, 745–775. [Google Scholar] [CrossRef]
- Hanson, J.R. Steroids: Partial Synthesis in Medicinal Chemistry. Nat. Prod. Rep. 2010, 27, 887–899. [Google Scholar] [CrossRef] [PubMed]
- Frank, É.; Schneider, G. Synthesis of Sex Hormone-Derived Modified Steroids Possessing Antiproliferative Activity. J. Steroid Biochem. Mol. Biol. 2013, 137, 301–315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Furniss, B.S.; Hannaford, A.J.; Smith, P.W.G.; Tatchell, A.R. Vogel’s Textboom of Practical Organic Chemistry, 5th ed.; Pearson: Delhi, India, 1989. [Google Scholar]
- Karatas, S.; Çapan, İ.; Servi, S. Synthesis of Indole and Benzimidazole Substituted Novel 16-Arylidene Steroid Derivatives. Lett. Org. Chem. 2019, 16, 884–890. [Google Scholar] [CrossRef]
- Brito, V.; Santos, A.O.; Almeida, P.; Silvestre, S. Novel 4-Azaandrostenes as Prostate Cancer Cell Growth Inhibitors: Synthesis, Antiproliferative Effects and Molecular Docking Studies. Comptes Rendus Chim. 2018, 22, 73–83. [Google Scholar] [CrossRef]
- Ke, S.; Shi, L.; Zhang, Z.; Yang, Z. Pyrimidines Derived from Dehydroepiandrosterone: A Convenient Synthesis, Antiproliferation Activity, Structure-Activity Relationships, and Role of Heterocyclic Moiety. Nat. Publ. Gr. 2017, 7, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dubey, S.; Kaur, P.; Paul, D. Synthesis and QSAR Studies of 16-(3-Methoxy-4-Substitued Benzylidene) Androstene Derivatives as Anticancer Agents. Indian J. Chem. 2010, 49B, 948–955. [Google Scholar] [CrossRef]
- Dubey, S.; Piplani, P.; Jindal, D.P. Synthesis and in Vitro Antineoplastic Evaluation of Certain 16-(4-Substituted Benzylidene) Derivatives of Androst-5-Ene. Chem. Biodivers. 2004, 1, 1529–1536. [Google Scholar] [CrossRef]
- Kakati, D.; Sarma, R.K.; Saikia, R.; Barua, N.C.; Sarma, J.C. Rapid Microwave Assisted Synthesis and Antimicrobial Bioevaluation of Novel Steroidal Chalcones. Steroids 2013, 78, 321–326. [Google Scholar] [CrossRef]
- Mótyána, G.; Molnár, B.; Wölfling, J.; Frank, É. Microwave-Assisted Stereoselective Heterocyclization to Novel Ring D-Fused Arylpyrazolines in the Estrone Series. Molecules 2019, 24, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Riebe, S.; Jopp, S.; Ehlers, P.; Frank, E.; Schneider, G.; Wölfling, J.; Villinger, A.; Langer, P. Synthesis of 16-E-([Aryl]Idene)-3-Methoxy-Estrones by a Palladium Catalysed Mizoroki-Heck Reaction. Tetrahedron Lett. 2017, 58, 2801–2803. [Google Scholar] [CrossRef]
- Peña-López, M.; Ayán-Varela, M.; Sarandeses, L.A.; Pérez Sestelo, J. Palladium-Catalyzed Cross-Coupling Reactions of Organogold(I) Reagents with Organic Electrophiles. Chemistry 2010, 16, 9905–9909. [Google Scholar] [CrossRef]
- Yu, B.; Shi, X.; Qi, P.; Yu, D.; Liu, H. Design, Synthesis and Biological Evaluation of Novel Steroidal Spiro-Oxindoles as Potent Antiproliferative Agents. J. Steroid Biochem. Mol. Biol. 2014, 141, 121–134. [Google Scholar] [CrossRef]
- Chattopadhaya, R.; Jindal, D.P.; Minu, M.; Gupta, R. Synthesis and Cytotoxic Studies of Hydroximino Derivatives of Some 16E-Arylidenosteroids. Arzneim. Forshung Drug Res. 2004, 556, 551–556. [Google Scholar] [CrossRef]
- Longley, D.B.; Harkin, D.P.; Johnston, P.G. 5-Fluorouracil: Mechanisms of Action and Clinical Strategies. Nat. Rev. Cancer 2003, 3, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Narváez-Pita, X.; Rheingold, A.L.; Meléndez, E. Ferrocene-Steroid Conjugates: Synthesis, Structure and Biological Activity. J. Organomet. Chem. 2017, 846, 113–120. [Google Scholar] [CrossRef]
- Vosooghi, M.; Yahyavi, H.; Divsalar, K.; Shamsa, H.; Kheirollahi, A.; Safavi, M.; Ardestani, S.K.; Sadeghi-Neshat, S.; Mohammadhosseini, N.; Edraki, N.; et al. Synthesis and in Vitro Cytotoxic Activity Evaluation of (E)-16-(Substituted Benzylidene) Derivatives of Dehydroepiandrosterone. Daru 2013, 21, 34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bansal, R.; Acharya, P.C. Synthesis and Antileukemic Activity of 16E-[4-(2-Carboxy)Ethoxybenzylidene]-Androstene Amides. Steroids 2012, 77, 552–557. [Google Scholar] [CrossRef]
- Bansal, R.; Guleria, S. Synthesis of 16E-[3-Methoxy-4-(2-Aminoethoxy)Benzylidene]Androstene Derivatives as Potent Cytotoxic Agents. Steroids 2008, 73, 1391–1399. [Google Scholar] [CrossRef]
- Chumsri, S.; Howes, T.; Bao, T.; Sabnis, G.; Brodie, A. Aromatase, Aromatase Inhibitors, and Breast Cancer. J. Steroid Biochem. Mol. Biol. 2011, 125, 13–22. [Google Scholar] [CrossRef] [Green Version]
- Lonning, P.E.; Eikesdal, H.P. Aromatase Inhibition 2013: Clinical State of the Art and Questions That Remain to Be Solved. Endocr. Relat. Cancer 2013, 20, 183–201. [Google Scholar] [CrossRef] [Green Version]
- Coates, A.S.; Keshaviah, A.; Thu, B.; Del Mastro, L.; Smith, I.; Chirgwin, J.; Nogaret, J.; Pienkowski, T.; Wardley, A. Five Years of Letrozole Compared With Tamoxifen As Initial Adjuvant Therapy for Postmenopausal Women With Endocrine-Responsive Early Breast Cancer: Update of Study BIG 1-98. J. Clin. Oncol. 2007, 25, 486–492. [Google Scholar] [CrossRef] [PubMed]
- Amir, E.; Seruga, B.; Niraula, S.; Carlsson, L.; Ocaña, A. Toxicity of Adjuvant Endocrine Therapy in Postmenopausal Breast Cancer Patients: A Systematic Review and Meta-Analysis. J. Natl. Cancer Inst. 2011, 103, 1299–1309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sobral, A.F.; Amaral, C.; Correia-da-silva, G.; Teixeira, N. Unravelling Exemestane: From Biology to Clinical Prospects. J. Steroid Biochem. Mol. Biol. 2016, 163, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Gasi, K.M.P.; Stankovic, S.M.; Sakac, M.N.; Stojanovic, S.Z.; Andric, S.; Molnar, D.; Kovac, R. New D-Modified Androstane Derivatives as Aromatase Inhibitors. Steroids 2001, 66, 645–653. [Google Scholar] [CrossRef]
- Banday, A.H.; Akram, S.M.M.; Shameem, S.A. Benzylidine Pregnenolones and Their Oximes as Potential Anticancer Agents: Synthesis and Biological Evaluation. Steroids 2014, 84, 64–69. [Google Scholar] [CrossRef]
- Bansal, R.; Thota, S.; Karkra, N.; Minu, M.; Zimmer, C.; Hartmann, R.W. Synthesis and Aromatase Inhibitory Activity of Some New 16E-Arylidenosteroids. Bioorg. Chem. 2012, 45, 36–40. [Google Scholar] [CrossRef]
- Bansal, R.; Guleria, S.; Thota, S.; Hartmann, R.W.; Zimmer, C. Synthesis of Imidazole-Derived Steroidal Hybrids as Potent Aromatase Inhibitors. Med. Chem. Res. 2013, 22, 692–698. [Google Scholar] [CrossRef]
- Singh, R.; Bansal, R. Investigations on 16-Arylideno Steroids as a New Class of Neuroprotective Agents for the Treatment of Alzheimer’s and Parkinson’s Diseases. ACS Chem. Neurosci. 2016. [Google Scholar] [CrossRef]
- Zhu, L.; Yang, Y.; Gao, P.; An, X.; Sun, Y.; Sun, X.; Hou, Y.; Shan, L. Synthesis and Anti-Inflammatory Activity Evaluation of 2-Dehydroepiandrosterone Benzene Methyl Derivatives. Chinese J. Org. Chem. 2019, 39, 2625–2631. [Google Scholar] [CrossRef]
- Semenenko, A.N.; Babak, N.L.; Popov, S.S.; Svitlana, V.; Mazepa, A.V.; Lipson, V.V.; Semenenko, A.N.; Babak, N.L.; Popov, S.S.; Svitlana, V. New Ylidene and Spirocyclopropyl Derivatives of Cholestanone and Dehydroepiandrosterone Series and Their Ability to Induce Cholesteric Mesophase in Nematic Solvent. Synth. Commun. 2018, 48, 1008–1015. [Google Scholar] [CrossRef]
- Boyd, M.R. The NCI Human Tumor Cell Line (60-Cell) Screen. In Cancer Drug Discovery and Development: Anticancer Drugs Development Guide: Preclinical Screening, Clinical Trials, and Approval—Part I; Teicher, B.A., Andrews, P.A., Eds.; Humana Press Inc.: Totowa, NJ, USA, 2004; pp. 41–61. [Google Scholar]
- Abood, N.K.; Ibraheem, H.H. Synthesis, Characterize and Antimicrobial Study of New Chalcones and Pyrazole Derivatives from Progesterone. Int. J. Sci. Res. 2016, 5, 2319–7064. [Google Scholar] [CrossRef]
- Bernstein, L.; Ross, R.K. Endogenous Hormones and Breast Cancer Risk. Epidemiol. Rev. 1993, 15, 48–65. [Google Scholar] [CrossRef]
- Smuc, T.; Hevir, N.; Ribiˇ, M.; Husen, B.; Thole, H.; Laniˇ, T. Disturbed Estrogen and Progesterone Action in Ovarian Endometriosis. Mol. Cell. Endocrinol. 2009, 301, 59–64. [Google Scholar] [CrossRef] [Green Version]
- Sawetawan, C.; Milewich, L.; Word, R.A.; Carr, B.R.; Rainey, W.E. Compartmentalization of Type I 17β-Hydroxysteroid Oxidoreductase in the Human Ovary. Mol. Cell. Endocrinol. 1994, 99, 161–168. [Google Scholar] [CrossRef]
- Fournet-Dulguerov, N.; Maclusky, N.J.; Leranth, C.Z.; Todd, R.; Mendelson, C.R.; Simpson, E.R.; Naftolin, F. Immunohistochemical Localization of Aromatase Cytochrome P-450 and Estradiol Dehydrogenase in the Syncytiotrophoblast of the Human Placenta*. J. Clin. Endocrinol. Metab. 1987, 65, 757–764. [Google Scholar] [CrossRef]
- Vihko, P.; Herrala, A. Control of Cell Proliferation by Steroids: The Role of 17HSDs. Mol. Cell. Endocrinol. 2006, 248, 141–148. [Google Scholar] [CrossRef]
- Kruchten, P.; Werth, R.; Marchais-oberwinkler, S.; Frotscher, M.; Hartmann, R.W. Development of a Biological Screening System for the Evaluation of Highly Active and Selective 17β-HSD1-Inhibitors as Potential Therapeutic Agents. Mol. Cell Endocrinol. 2009, 301, 154–157. [Google Scholar] [CrossRef]
- Allan, G.M.; Lawrence, H.R.; Cornet, J.; Bubert, C.; Fischer, D.S.; Vicker, N.; Smith, A.; Tutill, H.J.; Purohit, A.; Day, J.M.; et al. Modification of Estrone at the 6, 16, and 17 Positions: Novel Potent Inhibitors of 17 -Hydroxysteroid Dehydrogenase Type 1. J. Med. Chem. 2006, 49, 1325–1345. [Google Scholar] [CrossRef]
- Mueck, A.O.; Seeger, H. 2-Methoxyestradiol- Biology and Mechanism of Action. Steroids 2010, 75, 625–631. [Google Scholar] [CrossRef]
- Wang, C.; Li, L.; Fu, D.; Qin, T.; Ran, Y.; Xu, F.; Du, X. Discovery of Chalcone-Modified Estradiol Analogs as Antitumour Agents That Inhibit Tumour Angiogenesis and Epithelial to Mesenchymal Transition. Eur. J. Med. Chem. 2019, 176, 135–148. [Google Scholar] [CrossRef]
- Minu, M.; Jindal, D.P. Synthesis and Biological Activity of 16-Arylidene Derivatives of Estrone and Estrone Methyl Ether. Indian J. Chem. 2003, 42, 166–172. [Google Scholar] [CrossRef]
- Fan, N.-J.; Bai, Y.-B.; Zhang, F.-Y.; Luo, B.; Tang, J.-J.; Zhang, Q.-Z.; Gao, J.-M. Synthesis and Cytotoxicity of Some Novel 21E-Benzylidene Steroidal Derivatives. Steroids 2013, 78, 874–879. [Google Scholar] [CrossRef] [PubMed]
- Shan, L.; Liu, H.; Huang, K.; Dai, G.; Cao, C.; Dong, R. Synthesis of 3b, 7a, 11a-Trihydroxy-Pregn-21-Benzylidene-5-En-20-One Derivatives and Their Cytotoxic Activities. Bioorg. Med. Chem. Lett. 2009, 19, 6637–6639. [Google Scholar] [CrossRef] [PubMed]
- Canário, C.; Matias, M.; De Brito, V.; Adriana, O.; Falcão, A.; Silvestre, S.; Alves, G. ∆9,11-Estrone Derivatives as Potential Antiproliferative Agents: Synthesis, in Vitro Biological Evaluation and Docking Studies. Comptes Rendus Chim. 2020, 23, 201–217. [Google Scholar] [CrossRef]
- Kolo, A.M.; İpek, E.; Çapan, İ.; Servi, S. Synthesis of Heterocyclic-Substituted Novel Hydroxysteroids with Regioselective and Stereoselective Reactions. J. Heterocycl. Chem. 2018, 55, 492–497. [Google Scholar] [CrossRef]
- Thamotharan, S.; Parthasarathi, V.; Gupta, R. Two Androst-5-Ene Derivatives: 16-[4-(3-Chloropropoxy)-3-Methoxybenzylidene]-17-Oxoandrost-5-En-3β-Ol and 16-[3-Methoxy-4-(2-Pyrrolidin-1-Ylethoxy)Benzylidene]-3β-Pyrrolidinoandrost-5-En-17β-Ol Monohydrate. Acta Crystallogr. Sect. C Cryst. Struct. Commun. 2004, 60, 75–78. [Google Scholar] [CrossRef] [Green Version]
- Dubey, S.; Piplani, P.; Jindal, D.P. Synthesis and Evaluation of Some 16-Benzylidene Substituted 3,17-Dioximino Androstene Derivatives as Anticancer Agents. Lett. Drug Des. Discov. 2005, 2, 537–545. [Google Scholar] [CrossRef]
- Choudhary, M.I.; Alam, M.S.; Yousuf, S.; Wu, Y.; Lin, A.; Shaheen, F. Pregnenolone Derivatives as Potential Anticancer Agents. Steroids 2011, 76, 1554–1559. [Google Scholar] [CrossRef]
- Bryce, A.; Ryan, C.J. Development and Clinical Utility of Abiraterone Acetate as an Androgen Synthesis Inhibitor. Clin. Pharmacol. Ther. 2009, 91, 101–108. [Google Scholar] [CrossRef]
- Bastos, D.A.; Antonarakis, E.S. Galeterone for the Treatment of Advanced Prostate Cancer: The Evidence to Date. Drug Des. Devel. Ther. 2016, 10, 2289–2297. [Google Scholar] [CrossRef] [Green Version]
- Banday, A.H.; Shameem, S.A.; Jeelani, S. Steroidal Pyrazolines and Pyrazoles as Potential 5α-Reductase Inhibitors: Synthesis and Biological Evaluation. Steroids 2014, 92, 13–19. [Google Scholar] [CrossRef]
- Singh, R.; Thota, S.; Bansal, R. Studies on 16,17-Pyrazoline Substituted Heterosteroids as Anti-Alzheimer and Anti-Parkinsonian Agents Using LPS Induced Neuroinflammation Models of Mice and Rats. ACS Chem. Neurosci. 2017. [Google Scholar] [CrossRef]
- Banday, A.H.; Shameem, S.A.; Banday, J.A.; Ganaie, B.A. Synthesis, 17α-Hydroxylase-C 17,20-Lyase Inhibitory and 5AR Reductase Activity of Novel Pregnenolone Derivatives. Anticancer. Agents Med. Chem. 2018, 18, 1919–1926. [Google Scholar] [CrossRef]
- El-Naggar, M.; Amr, A.E.E.; Fayed, A.A.; Elsayed, E.A. Potent Anti-Ovarian Cancer with Inhibitor Activities on Both Topoisomerase II and V600E BRAF of Synthesized Substituted Estrone Candidates. Molecules 2019, 24, 2054. [Google Scholar] [CrossRef] [Green Version]
- Amr, A.E.E.; Abdel-latif, N.A.; Abdalla, M.M. Synthesis and Antiandrogenic Activity of Some New 3-Substituted Androstano [17,16-c]-5′-Aryl-Pyrazoline and Their Derivatives. Bioorg. Med. Chem. 2006, 14, 373–384. [Google Scholar] [CrossRef]
- Shi, Y.; Wang, B.; Shi, X.; Zhao, Y. Synthesis and Biological Evaluation of New Steroidal Pyridines as Potential Anti-Prostate Cancer Agents. Eur. J. Med. Chem. 2018, 145, 11–22. [Google Scholar] [CrossRef]
- Amr, A.E.E.; Elsayed, E.A.; Al-omar, M.A.; Eldin, H.O.B.; Nossier, E.S.; Abdallah, M.M. Design, Synthesis, Anticancer Evaluation and Molecular Modeling of Novel Estrogen Derivatives. Molecules 2019, 24, 416. [Google Scholar] [CrossRef] [Green Version]
- Babu, A.R.S.; Raghunathan, R. An Easy Access to Novel Steroidal Dispiropyrrolidines through 1, 3-Dipolar Cycloaddition of Azomethine Ylides. Tetrahedron Lett. 2008, 49, 4618–4620. [Google Scholar] [CrossRef]
- Gavaskar, D.; Babu, A.R.S.; Raghunathan, R.; Dharani, M.; Balasubramanian, S. An Expedient Sequential One-Pot Four Component Synthesis of Novel Steroidal Spiro-Pyrrolidine Heterocycles in Ionic Liquid. Steroids 2016, 109, 1–6. [Google Scholar] [CrossRef]
- Mótyána, G.; Gopisetty, M.K.; Kiss-Faludy, R.E.; Kulmány, Á.; Zupkó, I.; Frank, É.; Kiricsi, M. Anti-Cancer Activity of Novel Dihydrotestosterone-Derived Ring A-Condensed Pyrazoles on Androgen Non-Responsive Prostate Cancer Cell Lines. Int. J. Mol. Sci. 2019, 20, 2170. [Google Scholar] [CrossRef] [Green Version]
- Iványi, Z.; Szabó, N.; Wölfling, J.; Szécsi, M.; Julesz, J.; Schneider, G. Novel Series of 17β-Pyrazolylandrosta-5,16-Diene Derivatives and Their Inhibitory Effect on 17α-Hydroxylase/C17,20-Lyase. Steroids 2012, 77, 1152–1159. [Google Scholar] [CrossRef] [PubMed]
- Russell, D.W.; Wilson, J.D. Steroid 5α-Reductase: Two Genes/Two Enzymes. Annu. Rev. Biochem. 1994, 63, 25–61. [Google Scholar] [CrossRef] [PubMed]
- Thigpen, A.E.; Silver, R.; Guileyardo, J.M.; Casey, I.M.L.; Mcconnell, J.D.; Russell, D.W. Tissue Distribution and Ontogeny of Steroid 5α-Reductase Isozyme. J. Clin. Invest. 1993, 92, 903–910. [Google Scholar] [CrossRef] [PubMed]
- Lall, S.I.; Mancheno, D.; Castro, S.; Behaj, V.; Cohen, J.I.; Engel, R. Polycations. Part X. LIPs, a New Category of Room Temperature Ionic Liquid Based on Polyammonium Salts. R. Soc. Chem. 2000, 2413–2414. [Google Scholar] [CrossRef]
- Azzouni, F.; Godoy, A.; Li, Y.; Mohler, J. The 5α-Reductase Isozyme Family: A Review of Basic Biology and Their Role in Human Diseases. Adv. Urol. 2012, 2012. [Google Scholar] [CrossRef] [Green Version]
- Sudduth, S.L.; Koronkowski, M.J. Finasteride: The First 5α-Reductase Inhibitor. Pharmacotherapy 1993, 13, 309. [Google Scholar] [CrossRef]
- Kurup, A.; Garg, R.; Hansch, C. Comparative QSAR Analysis of 5α-Reductase Inhibitors. Chem. Rev. 2000, 100, 909–924. [Google Scholar] [CrossRef]
- Mitchell, H.J.; Dankulich, W.P.; Hartman, G.D.; Prueksaritanont, T.; Schmidt, A.; Vogel, R.L.; Bai, C.; Mcelwee-witmer, S.; Zhang, H.Z.; Chen, F.; et al. Design, Synthesis, and Biological Evaluation of 16-Substituted 4-Azasteroids as Tissue-Selective Androgen Receptor Modulators (SARMs). J. Med. Chem. 2009, 52, 4578–4581. [Google Scholar] [CrossRef]
- Dubey, S.; Jindal, D.P.; Piplani, P. Synthesis and Antineoplastic Activity of Some 16-Benzylidene Substituted Steroidal Oximes. Indian J. Chem. 2005, 44, 2126–2137. [Google Scholar] [CrossRef]


| Compound | Bioactivity Data | Ref. | |||||
|---|---|---|---|---|---|---|---|
 2 | Antiproliferative activity (IC50 μM) | [16] | |||||
| Cell line | 2 | 5-FU (+) | |||||
| HepG2 | 9.10 | 10.59 | |||||
| MCF-7 | 9.18 | 28.11 | |||||
| Cell cycle arrest at G2 phase in HepG2 | |||||||
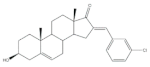 3 | Antiproliferative activity (IC50 ± SEM μM) | [60] | |||||
| Cell line | 3 | Etoposide (+) | |||||
| KB | 0.6 ± 2.0 | 2.8 ± 16.8 | |||||
| T47-D | 1.7 ± 14.8 | 1.2 ± 8 | |||||
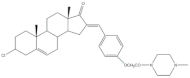 4 | Antiproliferative activity (IC50 μM) | [61] | |||||
| Cell line | 4 | ||||||
| CCRF-CEM | 3.94 | ||||||
| K-562 | 2.61 | ||||||
| RPMI-8226 | 6.90 | ||||||
| SR | 1.79 | ||||||
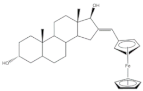 5 | Antiproliferative activity (IC50 μM ± SD μM) | [59] | |||||
| Cell line | 5 | Cis (+) | |||||
| HT-29 | 1.2 ± 0.4 | 66 ± 2 | |||||
 6 | Cytotoxic activity–in vivo hollow fiber assay (score) | [57] | |||||
| 6 | Taxol (+) | ||||||
| I.P. | 2 | Data not shown | |||||
| S.C. | 8 | ||||||
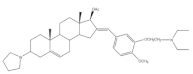 7 | Cytotoxic activity–in vivo hollow fiber assay (score) | [62] | |||||
| 7 | Taxol (+) | ||||||
| I.P. | 12 | Data not shown | |||||
| S.C. | 8 | ||||||
 8 | Aromatase inhibitory activity (IC50 μM) | [70] | |||||
| 8 | Aminoglutethimide (+) | ||||||
| 5.2 | 28.5 | ||||||
 9 | Aromatase inhibitory activity (IC50 μM) | [70] | |||||
| 9 | Aminoglutethimide (+) | ||||||
| 6.4 | 28.5 | ||||||
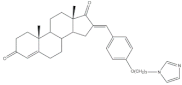 10 | Aromatase inhibitory activity (IC50 μM) | [71] | |||||
| 10 | Aminoglutethimide (+) | ||||||
| 4.4 | 28.5 | ||||||
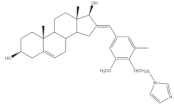 11 | Aromatase inhibitory activity (IC50 μM) | [71] | |||||
| 11 | Aminoglutethimide (+) | ||||||
| 2.4 | 28.5 | ||||||
 12 | Anti-inflammatory activity TNF-α levels (pg.mg−1protein ± SD) | [72] | |||||
| 12 | CEL (+) | DEX (+) | |||||
| 88.6 ± 1.8 | 68.2 ± 1.1 | 89.6 ± 2.0 | |||||
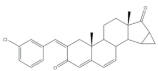 13 | Anti-inflammatory activity (IC50 μM) (NO release of LPS-activated mouse microglial cell line BV2) | [73] | |||||
| 13 | Minocycline (+) | ||||||
| 2.69 | 5.97 | ||||||
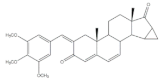 14 | Anti-inflammatory activity (IC50 μM) (NO release of LPS-activated mouse microglial cell line BV2) | [73] | |||||
| 14 | Minocycline (+) | ||||||
| 3.28 | 5.97 | ||||||
| Compound | Bioactivity Data | Ref. | ||
|---|---|---|---|---|
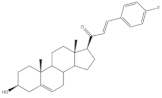 15 | Antiproliferative activity (IC50 μM) | [69] | ||
| Cell line | 15 | |||
| HCT-15 | 0.81 | |||
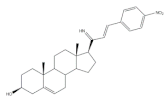 16 | Antiproliferative activity (IC50 μM) | [69] | ||
| Cell line | 16 | |||
| MCF-7 | 0.60 | |||
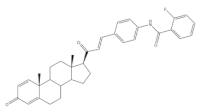 17 | Antiproliferative activity Growth inhibition (%) | [38] | ||
| Cell line | 17 | Cis (+) | ||
| HeLa | 58 | 99 | ||
| MCF-7 | 64 | 88 | ||
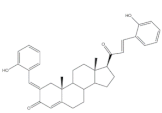 18 | Antimicrobial activity Zone of inhibition (mm) | [76] | ||
| Gram positive | 18 | AMP (+) | ||
| Streptococcus pneumoniae | 30 | 20 | ||
| Staphylococcus aureus | 24 | 22 | ||
| Compound | Bioactivity Data | Ref. | |
|---|---|---|---|
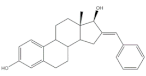 19 | 17β-HSD1 inhibitory activity (IC50 μM) | [35] | |
| 19 | Estradiol (+) | ||
| 3.4 | 7.3 | ||
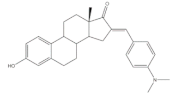 20 | 17β-HSD inhibitory activity (% at 10 μM) | [83] | |
| 20 | |||
| Type 1 | Type 2 | ||
| 72 | 13 | ||
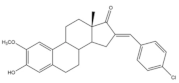 21 | Tumor weight reduction (%) Human breast cancer (MCF-7) xenografts models | [85] | |
| 21 (45 mg/kg/day) | 2ME (+) (45 mg/kg/day) | ||
| About 50% Significative reduction compared with negative control | About 50% Significative reduction compared with negative control | ||
| Compound | Bioactivity Data | Ref. | |||
|---|---|---|---|---|---|
 24 | Antiproliferative activity (IC50 μM) | [4] | |||
| Cell line | 24 | ||||
| HT-29 | 0.24 | ||||
| HCT-15 | 0.25 | ||||
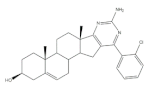 25 | Antiproliferative activity (IC50 μM) | [49] | |||
| Cell line | 25 | 5-FU (+) | |||
| HepG-2 | 5.41 | >100 | |||
| Huh-7 | 5.65 | >95 | |||
| SGC-790 | 10.64 | >100 | |||
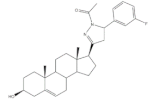 26 | 5AR-1 Inhibition (IC50 ± SEM μM) | [96] | |||
| 26 | Finasteride (+) | ||||
| 14.50 ± 0.48 | 21.6 ± 0.62 | ||||
 27 | 5AR-2 Inhibition (IC50 ± SEM μM) | [96] | |||
| 27 | Finasteride (+) | ||||
| 13.90 ± 0.75 | 15.4 ± 0.58 | ||||
 28 | 5AR-2 Inhibition (IC50 ± SEM μM) | [96] | |||
| 28 | Finasteride (+) | ||||
| 14.20 ± 0.75 | 15.4 ± 0.58 | ||||
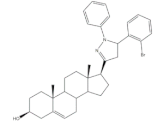 29 | 5AR-2 Inhibition (IC50 ± SEM nM) | [98] | |||
| 29 | Finasteride (+) | ||||
| 7.30 ± 0.62 | 2.4 ± 0.15 | ||||
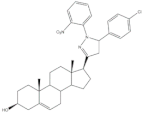 30 | 5AR-2 Inhibition (IC50 ± SEM nM) | [98] | |||
| 30 | Finasteride (+) | ||||
| 8.20 ± 0.55 | 2.4 ± 0.58 | ||||
 31 | Antiproliferative activity (IC50 μg.mL−1) | [18] | |||
| Cell line | 31 | 5-FU (+) | Cis (+) | ||
| NCI-H460 | 10.30 | 2.48 | 0.699 | ||
| HeLa | 12.50 | 0.887 | 2.03 | ||
| Cell cycle arrest at S phase in HeLa cells | |||||
 32 | Antiproliferative activity (IC50 μM) | [56] | |||
| Cell line | 32 | 5-FU (+) | |||
| SMMC-7721 | 4.30 | 9.78 | |||
| MCF-7 | 2.06 | 7.54 | |||
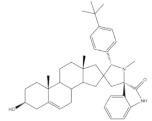 33 | Antiproliferative activity (IC50 ± SEM μM) | [56] | |||
| Cell line | 33 | 5-FU (+) | |||
| SMMC-7721 | 6.05 ± 0.48 | 9.78 ± 0.99 | |||
| MGC-803 | 5.79 ± 0.76 | 6.92 ± 0.35 | |||
| Cell cycle arrest at G2/M phase in MGC cells | |||||
 34 | Antiproliferative activity (IC50 ± SEM μM) | [56] | |||
| Cell line | 34 | 5-FU (+) | |||
| SMMC-7721 | 0.71 ± 0.11 | 9.78 ± 0.99 | |||
 35 | Antiproliferative activity (IC50 μM) | [93] | |||
| Cell line | 35 | DOX (+) | |||
| MDA-MB 231 | 0.91 | 1.23 | |||
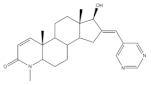 36 | Osteoanabolic activity and tissue-selectivity (% of the effect of DHT) | [113] | |||
| OVX | 36 | DHT | |||
| BRF | 120 | 100 | |||
| ORX | |||||
| VP | 3 | 100 | |||
| SV | 21 | 100 | |||
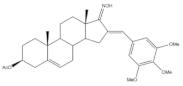 37 | Cytotoxic activity-in vivo hollow fiber assay (score) | [57] | |||
| 37 | Taxol (+) | ||||
| I.P. | 4 | Data not shown | |||
| S.C. | 6 | ||||
 38 | Antiproliferative activity Cell growth (%) | [114] | |||
| Cell line | 38 | ||||
| NCI-H460 | −44 | ||||
| MFC-7 | −44 | ||||
| SF-268 | −79 | ||||
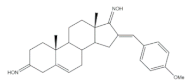 39 | Antiproliferative activity Cell growth (%) | [92] | |||
| Cell line | 39 | ||||
| NCI-H460 | −11 | ||||
| MCF-7 | 5 | ||||
| SF-268 | −8 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brito, V.; Alves, G.; Almeida, P.; Silvestre, S. Highlights on Steroidal Arylidene Derivatives as a Source of Pharmacologically Active Compounds: A Review. Molecules 2021, 26, 2032. https://doi.org/10.3390/molecules26072032
Brito V, Alves G, Almeida P, Silvestre S. Highlights on Steroidal Arylidene Derivatives as a Source of Pharmacologically Active Compounds: A Review. Molecules. 2021; 26(7):2032. https://doi.org/10.3390/molecules26072032
Chicago/Turabian StyleBrito, Vanessa, Gilberto Alves, Paulo Almeida, and Samuel Silvestre. 2021. "Highlights on Steroidal Arylidene Derivatives as a Source of Pharmacologically Active Compounds: A Review" Molecules 26, no. 7: 2032. https://doi.org/10.3390/molecules26072032








