Good-Practice Non-Radioactive Assays of Inorganic Pyrophosphatase Activities
Abstract
:1. Introduction
2. Continuous Low-Substrate Assay of PPi-Hydrolyzing Activity
3. Sensitive Fixed-Time Assay of PPi-Hydrolyzing Activity
4. Continuous and Fixed-Time Assays of PPi-Synthesizing Activity
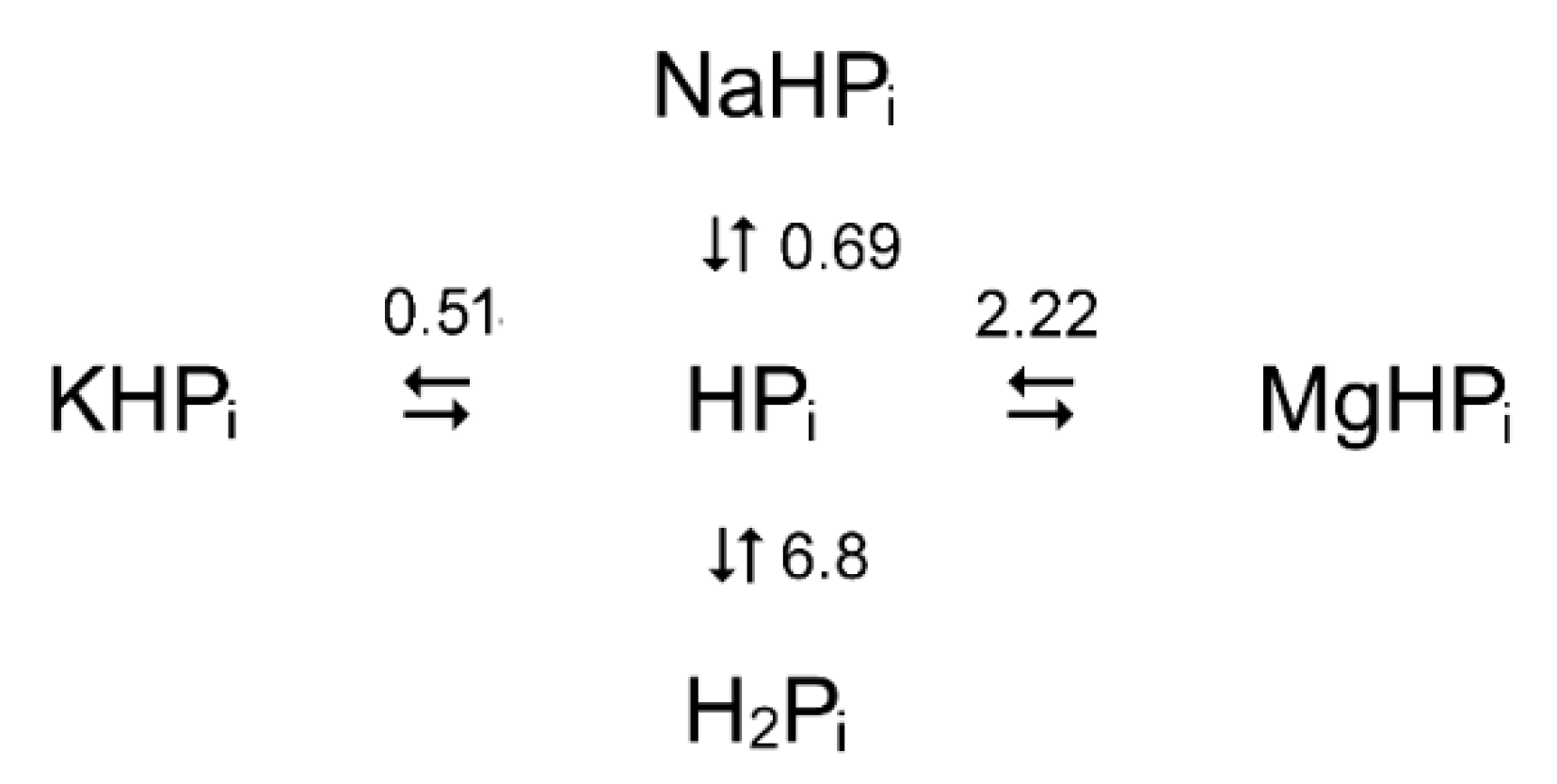
5. Ionic Equilibria in the Assay Media
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A
Estimation of Initial Velocities from Product Formation Curves


References
- Heinonen, J.K. Biological Role of Inorganic Pyrophosphate; Kluwer Academic Publishers: London, UK, 2001. [Google Scholar]
- Baykov, A.A.; Malinen, A.M.; Luoto, H.H.; Lahti, R. Pyrophosphate-fueled Na+ and H+ transport in prokaryotes Microbiol. Mol. Biol. Rev. 2013, 77, 267–276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsai, J.-Y.; Kellosalo, J.; Sun, Y.-J.; Goldman, A. Proton/sodium pumping pyrophosphatases: The last of the primary ion pumps. Curr. Opin. Struct. Biol. 2014, 27, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Gaxiola, R.A.; Regmi, K.; Paez-Valencia, J.; Pizzio, G.; Zhang, S. Plant H+-PPases: Reversible enzymes with contrasting functions dependent on membrane environment. Mol. Plant. 2016, 9, 317–319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scholz-Starke, J.; Primo, C.; Yang, J.; Kandel, R.; Gaxiola, R.A.; Hirschi, K.D. The flip side of the Arabidopsis type I proton-pumping pyrophosphatase (AVP1): Using a transmembrane H+ gradient to synthesize pyrophosphate. J. Biol Chem. 2019, 294, 1290–1299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baykov, A.A.; Cooperman, B.S.; Goldman, A.; Lahti, R. Cytoplasmic inorganic pyrophosphatases. Progr. Mol. Subcell. Biol. 1999, 23, 127–150. [Google Scholar]
- Kajander, T.K.; Kellosalo, J.; Goldman, A. Inorganic pyrophosphatases: One substrate, three mechanisms. FEBS Lett. 2013, 587, 1863–1869. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chappelet-Tordo, D.; Fosset, M.; Iwatsuba, M.; Gache, C.; Lazdunski, M. Intestinal alkaline phosphatase: Catalytic properties and half of the sites reactivity. Biochemistry 1974, 13, 1788–1795. [Google Scholar] [CrossRef]
- Lee, H.S.; Cho, Y.; Kim, Y.-J.; Lho, T.O.; Cha, S.-S.; Lee, J.-H.; Kang, S.G. A novel inorganic pyrophosphatase in Thermococcus onnurineus NA1. FEMS Microbiol. Lett. 2009, 300, 68–74. [Google Scholar] [CrossRef] [Green Version]
- Huang, H.; Patskovsky, Y.; Toro, R.; Farelli, J.D.; Pandya, C.; Almo, S.C.; Allen, K.N.; Dunaway-Mariano, D. Divergence of structure and function in the haloacid dehalogenase enzyme superfamily: Bacteroides thetaiotaomicron BT2127 is an inorganic pyrophosphatase. Biochemistry 2011, 50, 8937–8949. [Google Scholar] [CrossRef] [Green Version]
- Baykov, A.A.; Anashkin, V.A.; Salminen, A.; Lahti, R. Inorganic pyrophosphatases of Family II—two decades after their discovery. FEBS Lett. 2017, 591, 3225–3234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Belle, H. New and sensitive reaction for automatic determination of inorganic phosphate and its application to serum. Anal. Biochem. 1970, 33, 132–142. [Google Scholar] [CrossRef]
- Baykov, A.A.; Avaeva, S.M. A simple and sensitive apparatus for continuous monitoring of orthophosphate in the presence of acid-labile compounds. Anal. Biochem. 1982, 116, 1–4. [Google Scholar] [CrossRef]
- Baykov, A.A.; Alexandrov, A.P.; Smirnova, I.N. A two-step mechanism of fluoride inhibition of rat liver inorganic pyrophosphatase. Arch. Biochem. Biophys. 1992, 294, 238–243. [Google Scholar] [CrossRef]
- Baykov, A.A.; Evtushenko, O.A.; Avaeva, S.M. A malachite green procedure for orthophosphate determination and its use in alkaline phosphatase-based enzyme immunoassay. Anal. Biochem. 1988, 171, 266–270. [Google Scholar] [CrossRef]
- Baykov, A.A.; Kasho, V.N.; Avaeva, S.M. Inorganic pyrophosphatase as a label in heterogeneous enzyme immunoassay. Anal. Biochem. 1988, 171, 271–276. [Google Scholar] [CrossRef]
- Vidilaseris, K.; Kellosalo, J.; Goldman, A. A high-throughput method for orthophosphate determination of thermostable membrane-bound pyrophosphatase activity. Anal. Methods 2018, 10, 646–651. [Google Scholar] [CrossRef] [Green Version]
- Himeno, S.; Ueda, T.; Shiomi, M.; Hori, T. Raman studies on the formation of 12-molybdopyrophosphate. Inorg. Chim. Acta 1997, 262, 219–223. [Google Scholar] [CrossRef]
- Cooperman, B.S.; Chiu, N.Y.; Bruckmann, R.H.; Bunick, G.J.; McKenna, G.P. Yeast inorganic pyrophosphatase. I. New methods of purification, assay, and crystallization. Biochemistry 1973, 12, 1665–1669. [Google Scholar] [CrossRef]
- Shakhov, Y.A.; Nyrén, P. A sensitive and rapid method for determination of pyrophosphatase activity. Acta Chem. Scand. 1982, B36, 689–694. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.K.; Lee, D.H.; Hong, J.-I.; Yoon, J. Chemosensors for pyrophosphate. Acc. Chem. Res. 2009, 42, 23–31. [Google Scholar] [CrossRef]
- Zeng, Z.; Torriero, A.A.J.; Bond, A.M.; Spiccia, L. Fluorescent and electrochemical sensing of polyphosphate nucleotides by ferrocene functionalised with two Zn(II)(TACN)(pyrene) complexes. Chem. Eur. J. 2010, 16, 9154–9163. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.J.; Credo, G.M.; Su, X.; Wu, K.; Lim, H.C.; Elibol, O.H.; Bashir, R.; Varma, M. Surface immobilizable chelator for label-free electrical detection of pyrophosphate. Chem. Commun. 2011, 47, 8310–8312. [Google Scholar] [CrossRef] [PubMed]
- Terenteva, E.A.; Apyari, V.V.; Dmitrienko, S.G.; Garshev, A.V.; Volkov, P.A.; Zolotov, Y.A. Determination of pyrophosphate and sulfate using polyhexamethylene guanidine hydrochloride-stabilized silver nanoparticles. Talanta 2018, 180, 346–351. [Google Scholar] [CrossRef]
- Lin, Y.; Hu, L.; Li, L.; Wang, K.; Ji, Y.; Zou, H. Electrochemical determination of pyrophosphate at nanomolar levels using a gold electrode covered with a cysteine nanofilm and based on competitive coordination of Cu(II) ion to cysteine and pyrophosphate. Microchim. Acta 2015, 182, 2069–2075. [Google Scholar] [CrossRef]
- Baykov, A.A.; Hyytia, T.; Turkina, M.V.; Efimova, I.S.; Kasho, V.N.; Goldman, A.; Cooperman, B.S.; Lahti, R. Functional characterization of Escherichia coli inorganic pyrophosphatase in zwitterionic buffers. Eur. J. Biochem. 1999, 260, 308–317. [Google Scholar] [CrossRef]
- Baykov, A.A.; Sergina, N.V.; Evtushenko, O.A.; Dubnova, E.B. Kinetic characterization of Rhodospirillum rubrum H+-pyrophosphatase in membrane-bound and isolated states. Eur. J. Biochem. 1996, 236, 121–127. [Google Scholar] [CrossRef]
- Gordon-Weeks, R.; Korenkov, V.D.; Steele, S.H.; Leigh, R.A. Tris is a competitive inhibitor of K+ activation of the vacuolar H+-pumping pyrophosphatase. Plant. Physiol. 1997, 114, 901–905. [Google Scholar] [CrossRef] [Green Version]
- Nagul, E.A.; McKelviea, I.D.; Worsfold, P.; Kolev, S.D. The molybdenum blue reaction for the determination of orthophosphate revisited: Opening the black box. Anal. Chim. Acta 2015, 890, 60–82. [Google Scholar] [CrossRef] [Green Version]
- Itaya, K.; Ui, M. A new micromethod for the colorimetric determination of inorganic phosphate. Clin. Chim. Acta 1966, 14, 361–366. [Google Scholar] [CrossRef]
- Flodgaard, H.; Fleron, P. Thermodynamic parameters for the hydrolysis of inorganic pyrophosphate at pH 7.4 as a function of [Mg2+], [K+], and ionic strength determined from equilibrium studies of the reaction. J. Biol. Chem. 1974, 249, 3465–3474. [Google Scholar] [CrossRef]
- Nyrén, P.; Lundin, A. Enzymatic method for continuous monitoring of inorganic pyrophosphate synthesis. Anal. Biochem. 1985, 151, 504–509. [Google Scholar] [CrossRef]
- Ronaghi, M.; Uhlén, M.; Nyrén, P. A sequencing method based on real-time pyrophosphate. Science 1998, 281, 363–365. [Google Scholar] [CrossRef]
- Baykov, A.A.; Fabrichniy, I.P.; Pohjanjoki, P.; Zyryanov, A.B.; Lahti, R. Fluoride effects along the reaction pathway of pyrophosphatase. Evidence for a second enzyme⋅pyrophosphate intermediate. Biochemistry 2000, 39, 11939–11947. [Google Scholar] [CrossRef]
- Belogurov, G.A.; Malinen, A.M.; Turkina, M.V.; Jalonen, U.; Rytkönen, K.; Baykov, A.A.; Lahti, R. Membrane-bound pyrophosphatase of Thermotoga maritima requires sodium for activity. Biochemistry 2005, 44, 4004–4010. [Google Scholar] [CrossRef]
- Fabrichniy, I.P.; Kasho, V.N.; Hyytia, T.; Salminen, T.; Halonen, P.; Dudarenkov, V.Y.; Heikinheimo, P.; Chernyak, V.Y.; Goldman, A.; Lahti, R.; et al. Structural and functional consequenses of substitutions at the tyrosine 55-lysine 104 hydrogen bond in Escherichia coli inorganic pyrophosphatase. Biochemistry 1997, 36, 7746–7753. [Google Scholar] [CrossRef]
- Springs, B.; Welsh, K.M.; Cooperman, B.S. Thermodynamics, kinetics, and mechanism in yeast inorganic pyrophosphatase catalysis of inorganic pyrophosphate: Inorganic phosphate equilibration. Biochemistry 1981, 20, 6384–6391. [Google Scholar] [CrossRef]
- Baykov, A.A.; Shestakov, A.S.; Kasho, V.N.; Vener, A.V.; Ivanov, A.H. Kinetics and thermodynamics of catalysis by the inorganic pyrophosphatase of Escherichia coli in both directions. Eur. J. Biochem. 1990, 194, 879–887. [Google Scholar] [CrossRef]
- Smirnova, I.N.; Kasho, V.N.; Volk, S.E.; Ivanov, A.H.; Baykov, A.A. Rates of elementary steps catalyzed by rat liver cytosolic and mitochondrial inorganic pyrophosphatases in both directions. Arch. Biochem. Biophys. 1995, 318, 340–348. [Google Scholar] [CrossRef] [PubMed]
- Arakawa, H.; Karasawa, K.; Igarashi, T.; Suzuki, S.; Goto, N.; Maeda, M. Detection of cariogenic bacteria genes by a combination of allele-specific polymerase chain reactions and a novel bioluminescent pyrophosphate assay. Anal. Biochem. 2004, 333, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Baykov, A.A.; Bakuleva, N.P.; Rea, P.A. Steady-state kinetics of substrate hydrolysis by vacuolar H+-pyrophosphatase. A simple three-state model. Eur. J. Biochem. 1993, 217, 755–762. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.M.; Alberty, R.A. The apparent stability constants of ionic complexes of various adenosine phosphates with divalent cations. J. Am. Chem. Soc. 1956, 78, 2376–2380. [Google Scholar] [CrossRef]
- Childs, C.W. Potentiometric study of equilibriums in aqueous divalent metal orthophosphate solutions. Inorg. Chem. 1970, 9, 2465–2469. [Google Scholar] [CrossRef]
- Smirnova, I.N.; Shestakov, A.S.; Dubnova, E.B.; Baykov, A.A. Spectral and kinetic studies of phosphate and magnesium ion binding to yeast inorganic pyrophosphatase. Eur. J. Biochem. 1989, 182, 451–456. [Google Scholar] [CrossRef] [PubMed]
- Daniele, P.G.; De Robertis, A.; De Stefano, C.; Gianguzza, A.; Sammartano, S. Salt effects on the protonation of orthophosphate between 10 and 50 degrees Celsius in aqueous solution. A complex formation model. J. Solut. Chem. 1991, 20, 495–515. [Google Scholar] [CrossRef]
- Bisswanger, H. Enzyme Kinetics. Principles and Methods, 2nd ed.; Wiley-VCH Verlag: Weinheim, Germany, 2008; pp. 78–85. [Google Scholar]
- Cornish-Bowden, A. The use of the direct linear plot for determining initial velocities. Biochem. J. 1975, 149, 305–312. [Google Scholar] [CrossRef] [PubMed]
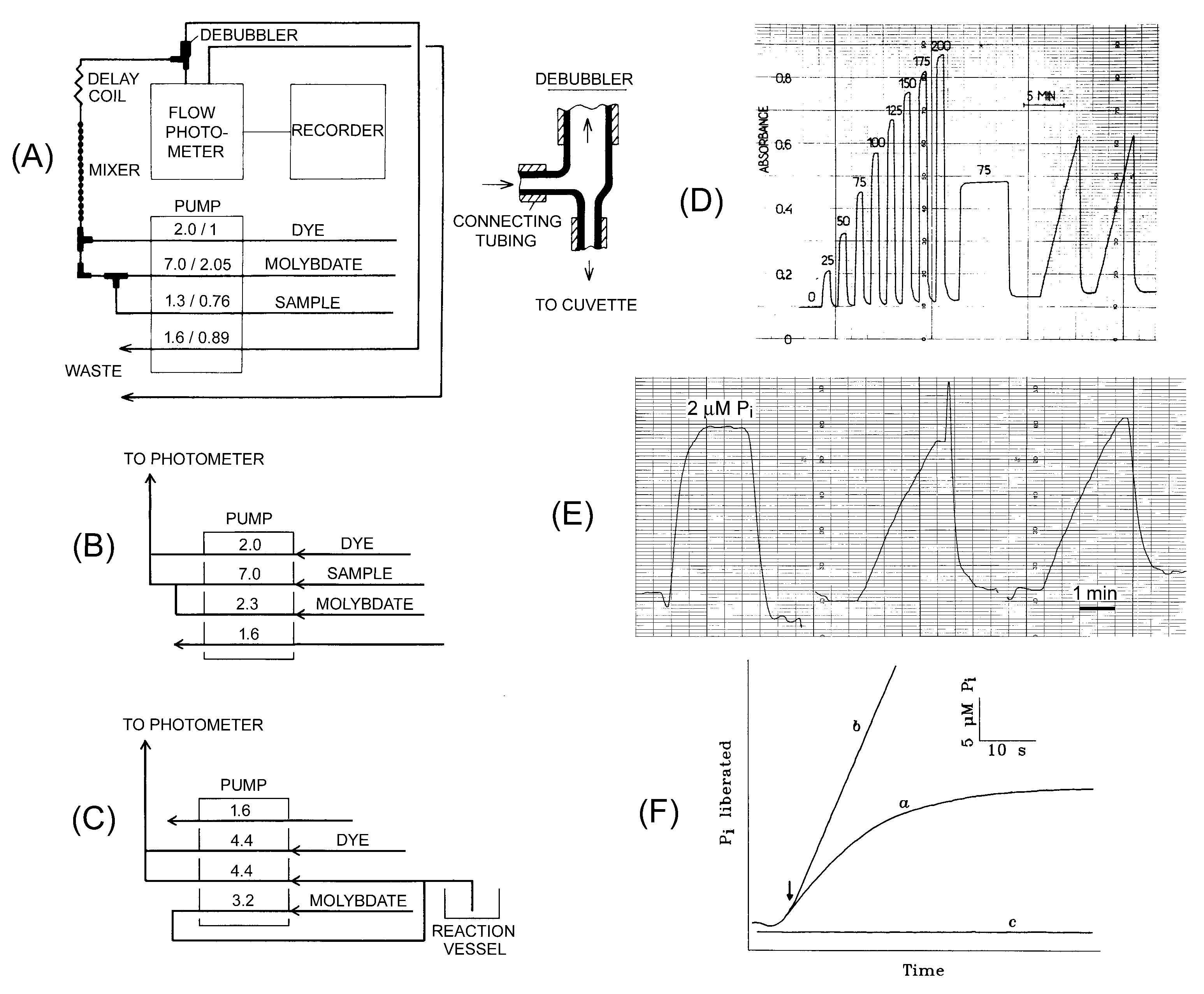

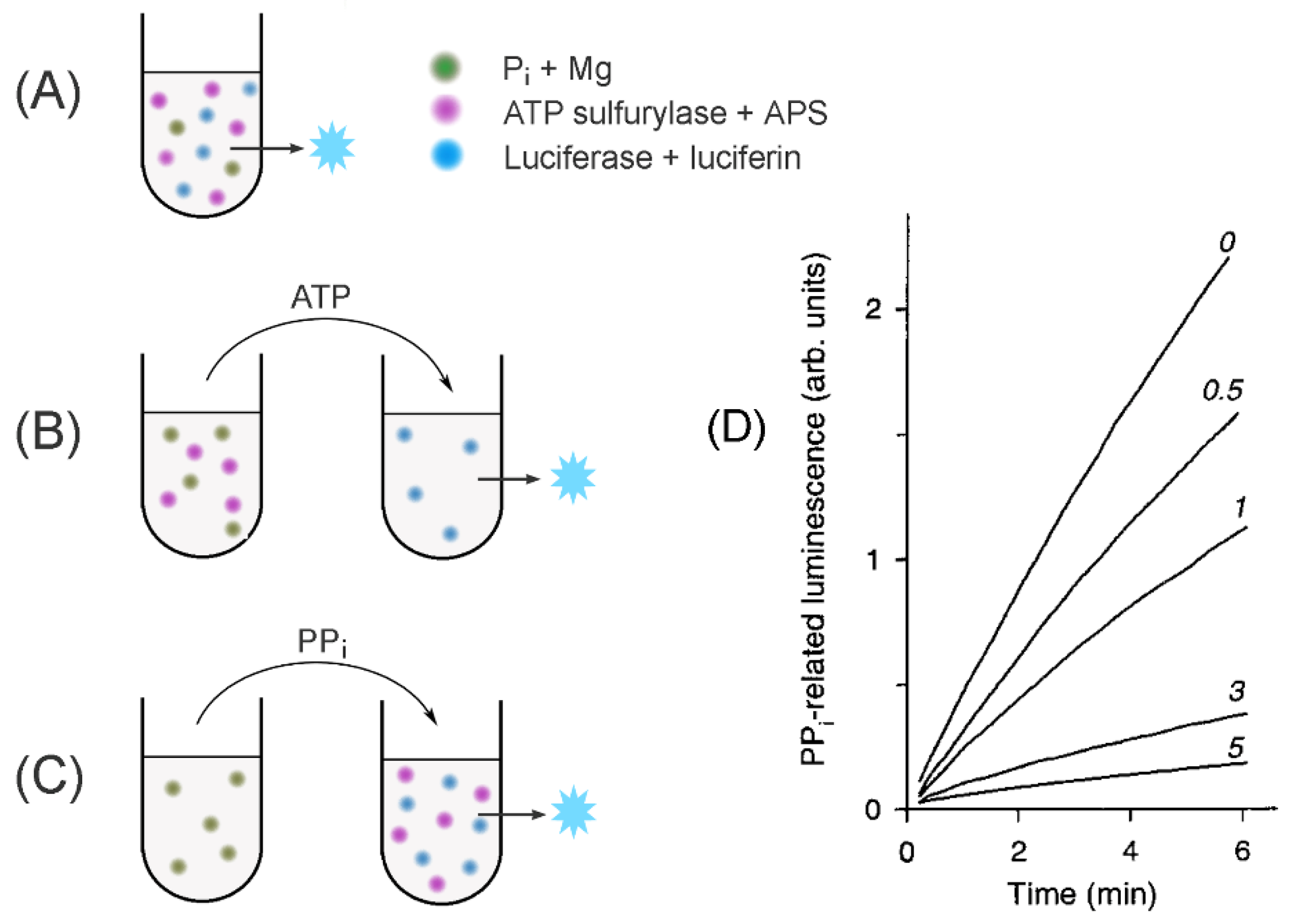

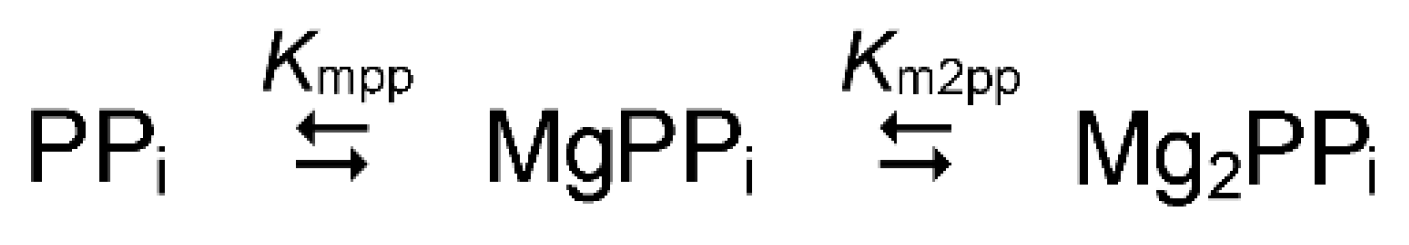
| Assay Type and Procedure | Comments |
|---|---|
| Continuous, version A [13] Stock dye solution contains 80 mg/L methyl green and 3.4 g/L Triton X-305. Stock acid/molybdate solution contains 0.55 M H2SO4 (32 mL concentrated H2SO4 per 1 L final solution 1), 12 g/L ammonium molybdate, and 2 µM Pi. The mixing ratio sample: acid/molybdate:dye is 1.5:7:2. Washing solution: 0.1 M NaOH and 2 g/L Triton X-305. The enzymatic reaction is performed in a thermostated vessel with magnetic stirring at 10–60 °C; the reaction volume is typically 5 mL. | Methyl green and Triton X-305 are from Sigma-Aldrich, ammonium molybdate from Fisher Scientific. Phosphate-free sulfuric acid (1.83 g/cm3) is used. When switching to a new batch of Triton X-305, its concentration may need adjustment to ensure linearity of the calibration plot and low baseline drift because of dye deposition on the photometer cuvette. |
| Continuous, version B [13] Stock dye solution contains 86 mg/L methyl green and 3.7 g/L Triton X-305. Stock acid/molybdate solution contains 1.8 M H2SO4 (105 mL concentrated H2SO4 per 1 L final solution), 39 g/L ammonium molybdate, and 7 µM Pi. The mixing ratio sample:acid/molybdate:dye is 7:2.3:2. The PPase reaction volume is typically 25–40 mL. | Acid-resistant Iso-Versinic tubing is preferable on the pump for the acid/molybdate solution in this case. |
| Continuous, version C [14] Stock dye solution contains 30 mg/L methyl green and 1.3 g/L Triton X-305. Stock acid/molybdate solution contains 1.0 M H2SO4 (59 mL concentrated H2SO4 per 1 L final solution), 22 g/L ammonium molybdate, and 4 µM Pi. The mixing ratio sample: acid/molybdate:dye is 1.2:3.2:4.4. | |
| Fixed-time [15,16] Stock dye/molybdate solution (stable for months in a refrigerator): 115 mg of malachite green are dissolved in 100 mL of 2.5 M H2SO4, followed by 1.4 g of ammonium molybdate. On the day of use, 0.25 mL of 10% Tween-20 (w/w) is added per 10 mL of the dye/molybdate solution. The PPase reaction is performed in a total volume of 0.2 mL in 96-well plates and terminated by adding 0.05 mL of the color reagent. Absorbance at 630 nm is measured after 10 min. | Malachite green is from Sigma-Aldrich, Tween-20 from Ferak Berlin. Tween-20 (0.05%, w/w) can be added instead to the PPase assay mixture, which typically contains 0.05 M Tris-HCl or another buffer, 0.05 mM PPi, and 5 mM MgCl2. |
| Assay Type and Procedure | Comments |
|---|---|
| PPi synthesis (continuous) [34] Assay mixture contains calculated amounts of potassium phosphate and MgCl2, 0.7 U/mL ATP-sulfurylase, 10 µM APS, 5 µL of luciferin/luciferase solution (Sigma ATP assay mix, catalog no. FLAAM, reconstituted with 5 mL of water), 1 mM dithiothreitol, 0.8 mg/mL bovine serum albumin and 0.1 M MOPS–KOH buffer in a total volume of 0.2 mL. The reaction is initiated by adding PPase, and the time-course of luminescence is followed with a luminometer (e.g., LKB model 1250). After the PPi-generated signal stabilizes, 2 µL of 10 µM ATP are added for internal calibration. | ATP sulfurylase is from BioLabs (UK). Its concentration should be sufficiently high so that its doubling does not change the measured rate of ATP accumulation. The luminescence-versus-time dependencies are slightly curved because of the slow inactivation of luciferase in the reaction medium. |
| PPi synthesis (fixed-time) [35] Assay mixture contains calculated amounts of potassium phosphate and MgCl2, 0.7 U/mL ATP-sulfurylase, 10 µM APS, and 0.1 M MOPS–KOH buffer in a total volume of 100 µL. The reaction is initiated by adding PPase and carried out for 10 min. Aliquots (15 µL) are withdrawn at various times, quenched with 15 µL of 1 M trifluoroacetic acid, incubated for 4 min at room temperature, and neutralized with 15 µL of 1.5 M Tris. The ATP formed is quantitated by adding 10 µL of the mixture to 200 µL of 0.2 M Tris-HCl buffer, pH 8.0 containing 6 µL of the luciferin/luciferase solution (Sigma ATP assay mix, catalog No. FLAAAM, reconstituted with 5 mL of water) and measuring the luminescence. After the signal stabilizes, 2 µL of 10 µM ATP are added for internal calibration. | Crystalline phosphoric acid (Fluka) is freed from PPi contamination as described in the text. |
| Enzyme-bound PPi formation [36] PPase (typically 20–100 µM) is preequilibrated with Pi and Mg2+ under appropriate conditions in a 50 µL volume and quenched with 10 µL of 5 M trifluoroacetic acid. After several minutes, precipitated protein is removed by centrifugation, a 10 µL aliquot of the supernatant is added to 0.2 mL of the PPi assay cocktail (0.2 M Tris-HCl, pH 8.0, 0.4 U/mL ATP sulfurylase, 10 µM APS, 6 µL of the luciferase/luciferin solution, 1 mM dithiothreitol, 0.8 mg bovine serum albumin, 30 µM EGTA), and the luminescence is recorded. After the signal stabilizes, 2 µL of 10 µM PPi are added for internal calibration. | Determined PPi content refers to the sum of enzyme-bound and medium PPi. The latter is measured similarly but using a 100 times lower enzyme concentration and subtracted. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baykov, A.A.; Anashkin, V.A.; Malinen, A.M. Good-Practice Non-Radioactive Assays of Inorganic Pyrophosphatase Activities. Molecules 2021, 26, 2356. https://doi.org/10.3390/molecules26082356
Baykov AA, Anashkin VA, Malinen AM. Good-Practice Non-Radioactive Assays of Inorganic Pyrophosphatase Activities. Molecules. 2021; 26(8):2356. https://doi.org/10.3390/molecules26082356
Chicago/Turabian StyleBaykov, Alexander A., Viktor A. Anashkin, and Anssi M. Malinen. 2021. "Good-Practice Non-Radioactive Assays of Inorganic Pyrophosphatase Activities" Molecules 26, no. 8: 2356. https://doi.org/10.3390/molecules26082356






