Influence of Material Properties on the Damage-Reporting and Self-Healing Performance of a Mechanically Active Dynamic Network Polymer in Coating Applications
Abstract
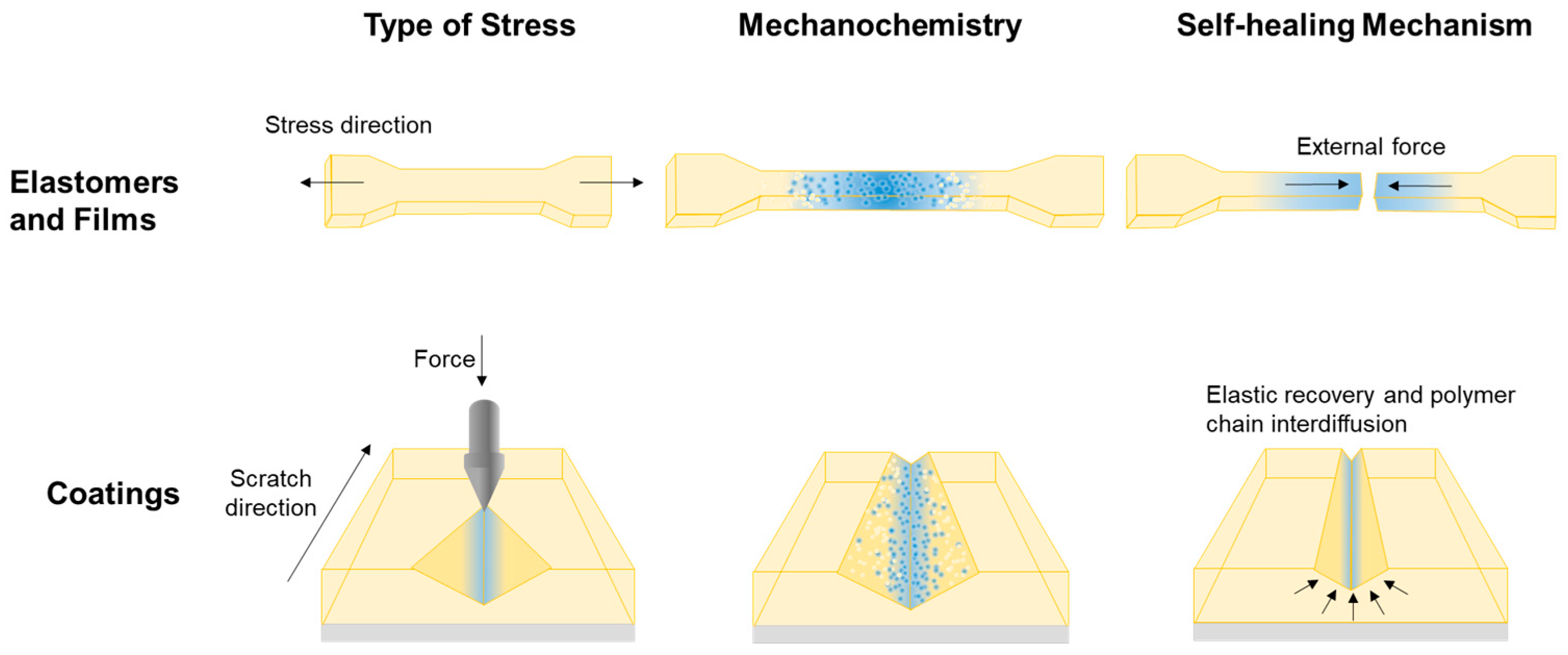
:1. Introduction
2. Experiment
2.1. Materials
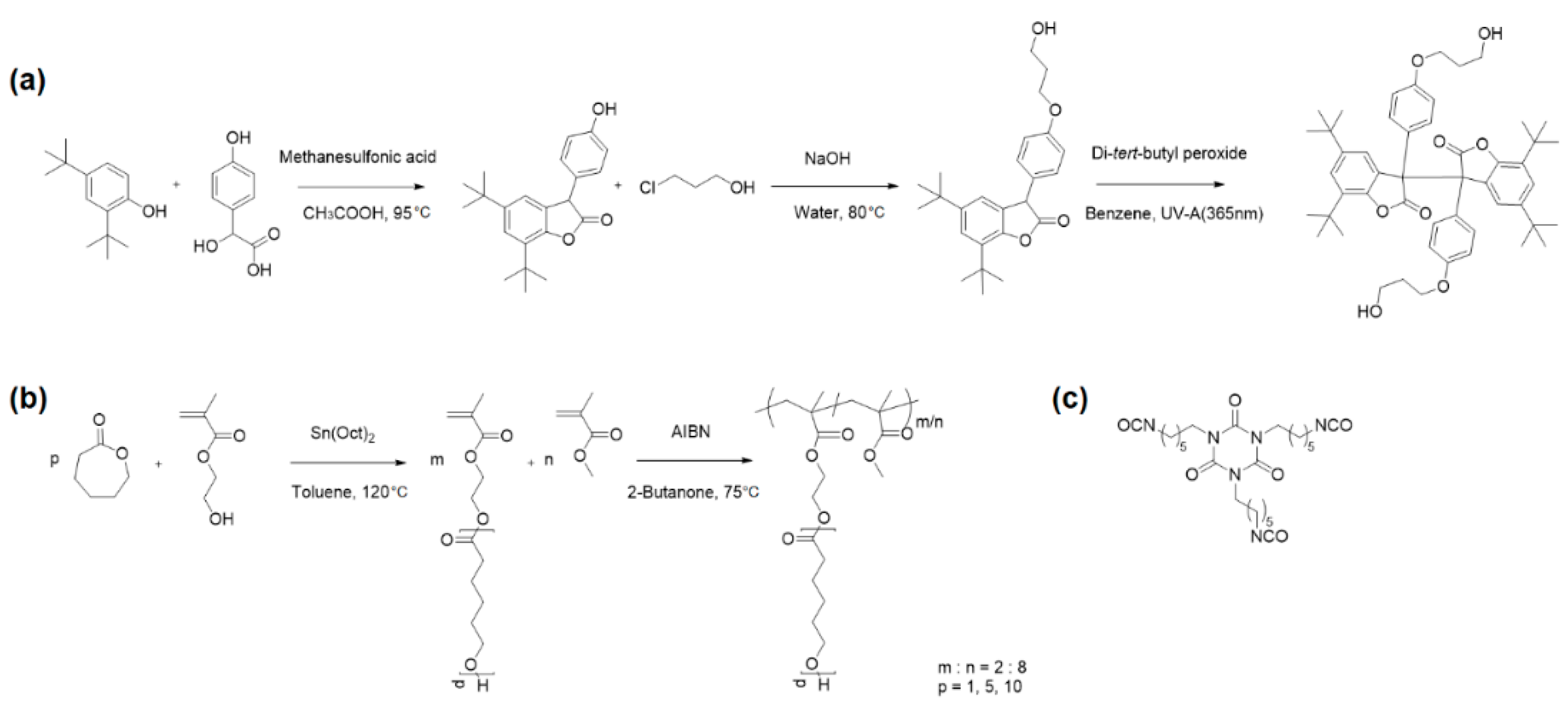
2.2. Synthesis
2.3. Preparation of the Polymer Network Coatings (C-GCLs)
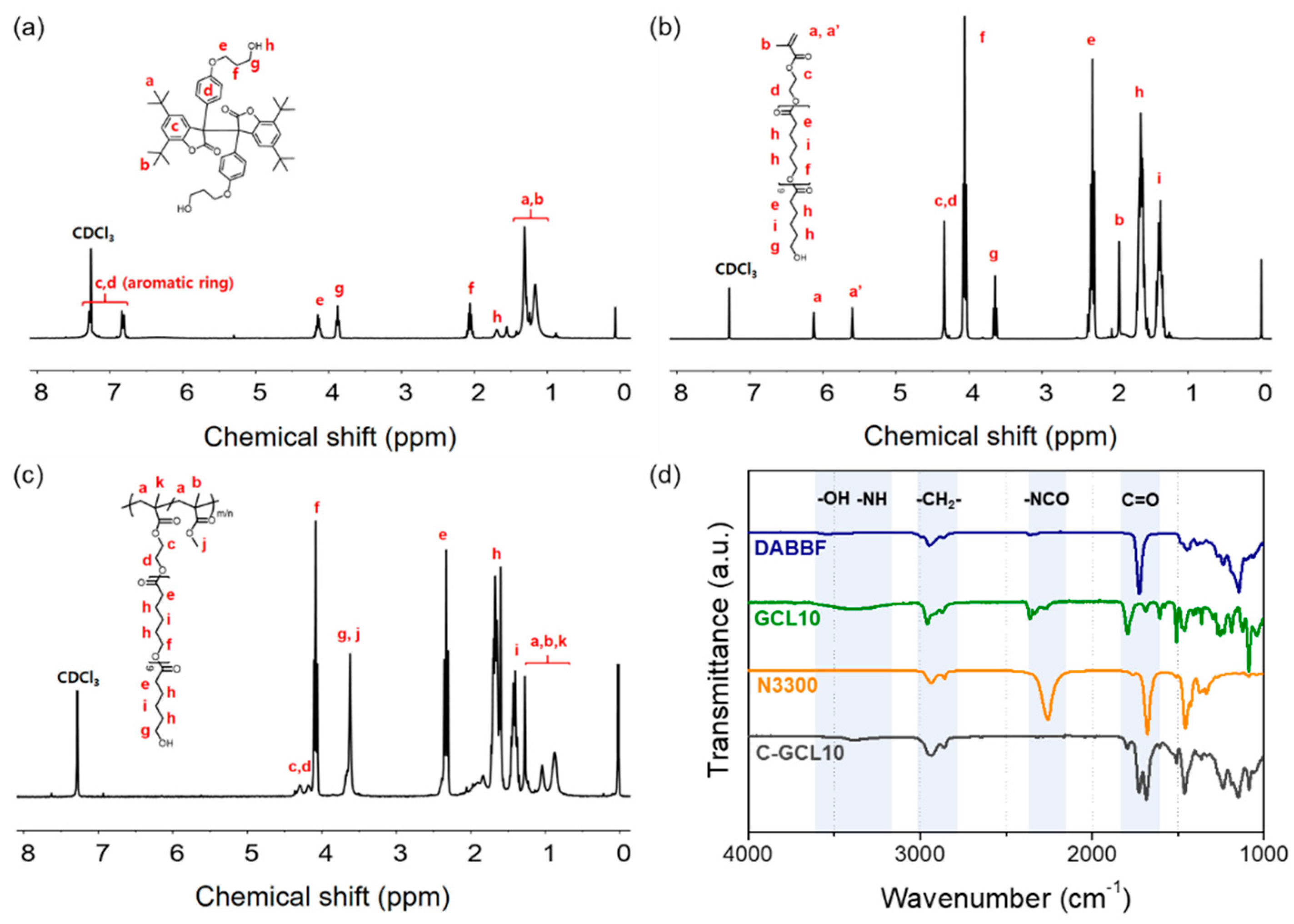
2.4. Chemical Structure Confirmation
2.5. Molecular-Weight Determination
2.6. Thermal Property Determination
2.7. Nanoindentation Test
2.8. Micro-Scratch Test
2.9. Scratch-Healing Efficiency Determination
2.10. Measurement of DABBF Dissociation and Recombination Efficiencies
3. Results and discussion
3.1. Material Design and Preparation
3.2. Results of Nanoindentation Tests Conducted on the C-GCL Coatings
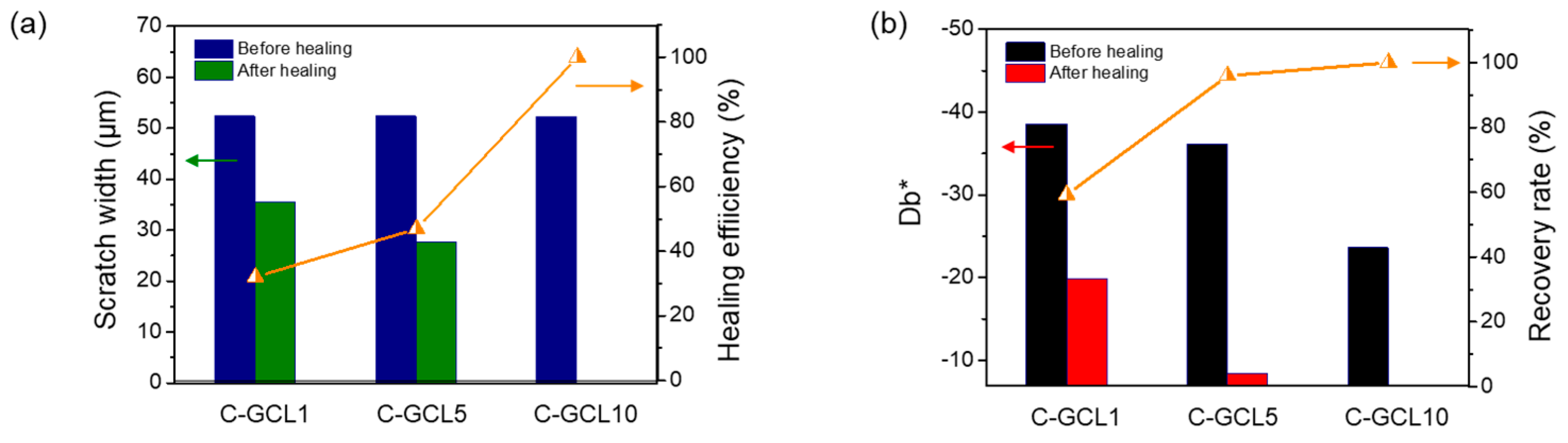
3.3. Results of the Scratch and Damage-Reporting Tests Conducted on the C-GCL Coatings
3.4. Scratch-Healing Performance of the C-GCL Coatings
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- May, P.A.; Moore, J.S. Polymer Mechanochemistry: Techniques to Generate Molecular Force via Elongational Flows. Chem. Soc. Rev. 2013, 42, 7497–7506. [Google Scholar] [CrossRef]
- Caruso, M.M.; Davis, D.A.; Shen, Q.; Odom, S.A.; Sottos, N.R.; White, S.R.; Moore, J.S. Mechanically-Induced Chemical Changes in Polymeric Materials. Chem. Rev. 2009, 109, 5755–5798. [Google Scholar] [CrossRef] [PubMed]
- Bowser, B.H.; Craig, S.L. Empowering Mechanochemistry with Multi-Mechanophore Polymer Architectures. Polym. Chem. 2018, 9, 3583–3593. [Google Scholar] [CrossRef]
- Do, J.-L.; Friščić, T. Mechanochemistry: A Force of Synthesis. ACS Cent. Sci. 2017, 3, 13–19. [Google Scholar] [CrossRef] [Green Version]
- Stolar, T.; Užarević, K. Mechanochemistry: An Efficient and Versatile Toolbox for Synthesis, Transformation, and Functionalization of Porous Metal–Organic Frameworks. Cryst. Eng. Comm. 2020, 22, 4511–4525. [Google Scholar] [CrossRef]
- Gomes, C.; Vinagreiro, C.S.; Damas, L.; Aquino, G.; Quaresma, J.; Chaves, C.; Pimenta, J.; Campos, J.; Pereira, M.; Pineiro, M. Advanced Mechanochemistry Device for Sustainable Synthetic Processes. ACS Omega 2020, 5, 10868–10877. [Google Scholar] [CrossRef] [PubMed]
- Willis-Fox, N.; Rognin, E.; Aljohani, T.A.; Daly, R. Polymer Mechanochemistry: Manufacturing Is Now a Force to Be Reckoned With. Chem 2018, 4, 2499–2537. [Google Scholar] [CrossRef] [Green Version]
- Akbulatov, S.; Boulatov, R. Experimental Polymer Mechanochemistry and Its Interpretational Frameworks. Chem. Phys. Chem. 2017, 18, 1422–1450. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Nagamani, C.; Moore, J.S. Polymer Mechanochemistry: From Destructive to Productive. Acc. Chem. Res. 2015, 48, 2181–2190. [Google Scholar] [CrossRef] [PubMed]
- Diesendruck, C.E.; Moore, J.S. Mechanophores for Self-Healing Applications. In Self-Healing Polymers; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2013; pp. 193–214. ISBN 978-3-527-67018-5. [Google Scholar]
- Hu, H.; Ma, Z.; Jia, X. Reaction Cascades in Polymer Mechanochemistry. Mater. Chem. Front. 2020, 4, 3115–3129. [Google Scholar] [CrossRef]
- White, S.R.; Sottos, N.R.; Geubelle, P.H.; Moore, J.S.; Kessler, M.R.; Sriram, S.R.; Brown, E.N.; Viswanathan, S. Autonomic Healing of Polymer Composites. Nature 2001, 409, 794–797. [Google Scholar] [CrossRef]
- Robb, M.J.; Li, W.; Gergely, R.C.R.; Matthews, C.C.; White, S.R.; Sottos, N.R.; Moore, J.S. A Robust Damage-Reporting Strategy for Polymeric Materials Enabled by Aggregation-Induced Emission. Acs Cent. Sci. 2016, 2, 598–603. [Google Scholar] [CrossRef]
- Li, W.; Matthews, C.C.; Yang, K.; Odarczenko, M.T.; White, S.R.; Sottos, N.R. Autonomous Indication of Mechanical Damage in Polymeric Coatings. Adv. Mater. 2016, 28, 2189–2194. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.H.; Song, Y.K.; Park, S.H.; Park, Y.I.; Noh, S.M.; Kim, J.C. Dual Stimuli Responsive Self-Reporting Material for Chemical Reservoir Coating. Appl. Surf. Sci. 2018, 434, 1327–1335. [Google Scholar] [CrossRef]
- Lee, H.M.; Perumal, S.; Kim, G.Y.; Kim, J.C.; Kim, Y.-R.; Kim, M.P.; Ko, H.; Rho, Y.; Cheong, I.W. Enhanced Thermomechanical Property of a Self-Healing Polymer via Self-Assembly of a Reversibly Cross-Linkable Block Copolymer. Polym. Chem. 2020, 11, 3701–3708. [Google Scholar] [CrossRef]
- Kim, S.Y.; Lee, T.H.; Park, Y.I.; Nam, J.H.; Noh, S.M.; Cheong, I.W.; Kim, J.C. Influence of Material Properties on Scratch-Healing Performance of Polyacrylate-Graft-Polyurethane Network That Undergo Thermally Reversible Crosslinking. Polymer 2017, 128, 135–146. [Google Scholar] [CrossRef]
- Kim, G.Y.; Sung, S.; Kim, M.P.; Kim, S.C.; Lee, S.-H.; Park, Y.I.; Noh, S.M.; Cheong, I.W.; Kim, J.C. Reversible Polymer Networks Based on the Dynamic Hindered Urea Bond for Scratch Healing in Automotive Clearcoats. Appl. Surf. Sci. 2020, 505, 144546. [Google Scholar] [CrossRef]
- Park, J.I.; Choe, A.; Kim, M.P.; Ko, H.; Lee, T.H.; Noh, S.M.; Kim, J.C.; Cheong, I.W. Water-Adaptive and Repeatable Self-Healing Polymers Bearing Bulky Urea Bonds. Polym. Chem. 2018, 9, 11–19. [Google Scholar] [CrossRef]
- Sung, S.; Kim, S.Y.; Lee, T.H.; Favaro, G.; Park, Y.I.; Lee, S.-H.; Ahn, J.B.; Noh, S.M.; Kim, J.C. Thermally Reversible Polymer Networks for Scratch Resistance and Scratch Healing in Automotive Clear Coats. Prog. Org. Coat. 2019, 127, 37–44. [Google Scholar] [CrossRef]
- Geitner, R.; Legesse, F.-B.; Kuhl, N.; Bocklitz, T.W.; Zechel, S.; Vitz, J.; Hager, M.; Schubert, U.S.; Dietzek, B.; Schmitt, M.; et al. Do You Get What You See? Understanding Molecular Self-Healing. Chem. Eur. J. 2018, 24, 2493–2502. [Google Scholar] [CrossRef]
- Imato, K.; Kanehara, T.; Nojima, S.; Ohishi, T.; Higaki, Y.; Takahara, A.; Otsuka, J. Repeatable Mechanochemical Activation of Dynamic Covalent Bonds in Thermoplastic Elastomers. Chem. Commun. 2016, 52, 10482–10485. [Google Scholar] [CrossRef] [Green Version]
- Imato, K.; Natterodt, J.C.; Sapkota, J.; Goseki, R.; Weder, C.; Takahara, A.; Otsuka, H. Dynamic Covalent Diarylbibenzofuranone- Modified Nanocellulose: Mechanochromic Behaviour and Application in Self-Healing Polymer Composites. Polym. Chem. 2017, 8, 2115–2122. [Google Scholar] [CrossRef]
- Kosuge, T.; Aoki, D.; Otsuka, H. Network reorganization in cross-linked polymer/silica composites based on exchangeable dynamic covalent carbon–carbon bonds. Polymer 2019, 177, 10–18. [Google Scholar] [CrossRef]
- Ishizuki, K.; Aoki, D.; Goseki, R.; Otsuka, H. Multicolor Mechanochromic Polymer Blends That Can Discriminate between Stretching and Grinding. ACS Macro Lett. 2018, 7, 556–560. [Google Scholar] [CrossRef]
- Kosuge, T.; Imato, K.; Goseki, R.; Otsuka, H. Polymer–Inorganic Composites with Dynamic Covalent Mechanochromophore. Macromolecules 2016, 49, 5903–5911. [Google Scholar] [CrossRef]




| Monomer Code | ÐMc | ||||
|---|---|---|---|---|---|
| MCL1 | 1 | 1.8 | 340 | 450 | 1.3 |
| MCL5 | 5 | 5.5 | 1080 | 1310 | 1.2 |
| MCL10 | 10 | 10.3 | 1500 | 1710 | 1.1 |
| Polymer Code | MCL a Contents (mol%) b | ÐMc | |||
|---|---|---|---|---|---|
| Theoretical | Calculated | ||||
| GCL1 | 20 | 20 | 17.0 | 25.6 | 1.5 |
| GCL5 | 20 | 21 | 25.0 | 32.5 | 1.3 |
| GCL10 | 20 | 21 | 34.0 | 44.2 | 1.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Son, D.H.; Kim, G.Y.; Jeong, J.-E.; Lee, S.-H.; Park, Y.I.; Kong, H.; Cheong, I.W.; Kim, J.C. Influence of Material Properties on the Damage-Reporting and Self-Healing Performance of a Mechanically Active Dynamic Network Polymer in Coating Applications. Molecules 2021, 26, 2468. https://doi.org/10.3390/molecules26092468
Son DH, Kim GY, Jeong J-E, Lee S-H, Park YI, Kong H, Cheong IW, Kim JC. Influence of Material Properties on the Damage-Reporting and Self-Healing Performance of a Mechanically Active Dynamic Network Polymer in Coating Applications. Molecules. 2021; 26(9):2468. https://doi.org/10.3390/molecules26092468
Chicago/Turabian StyleSon, Da Hae, Gi Young Kim, Ji-Eun Jeong, Sang-Ho Lee, Young Il Park, Hoyoul Kong, In Woo Cheong, and Jin Chul Kim. 2021. "Influence of Material Properties on the Damage-Reporting and Self-Healing Performance of a Mechanically Active Dynamic Network Polymer in Coating Applications" Molecules 26, no. 9: 2468. https://doi.org/10.3390/molecules26092468







