Exogenous Sodium Nitroprusside Mitigates Salt Stress in Lentil (Lens culinaris Medik.) by Affecting the Growth, Yield, and Biochemical Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Arrangements
2.2. NaCl and SNP Treatments
2.3. Determination of Growth and Yield Parameters
2.4. Estimation of Leaf Relative Water Content
2.5. Determination of Leaf Greenness Index
2.6. Estimation of H2O2 and MDA Content
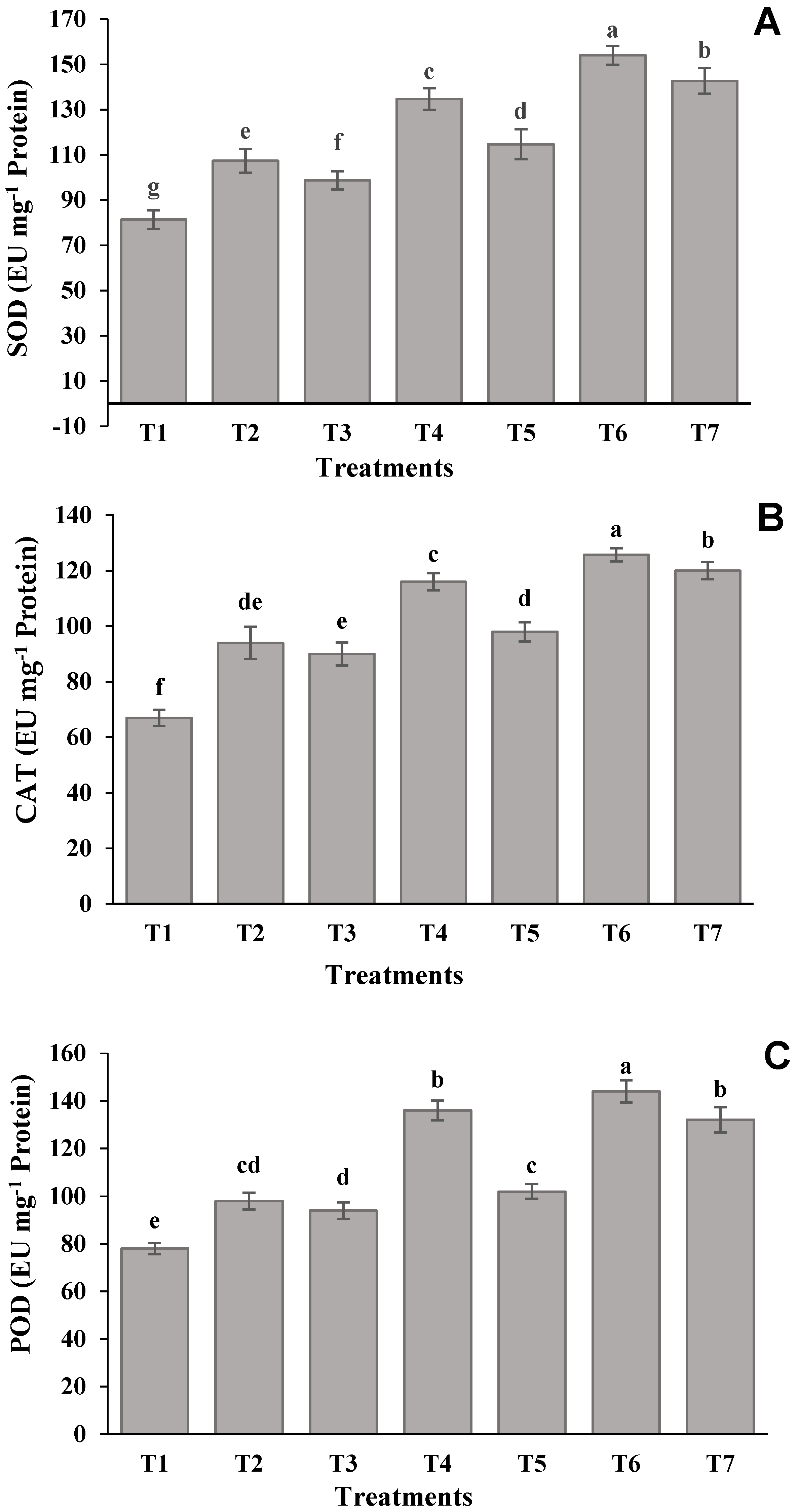
2.7. Antioxidant Enzyme Assays
2.8. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Abdelhamid, M.T.; El-Masry, R.R.; Darwish, D.S.; Abdalla, M.M.; Oba, S.; Ragab, R.; EL-Sabagh, A.; EL-Kholy, M.A.; Omer, E. Mechanisms of seed priming involved in salt stress amelioration. In Priming and Pretreatment of Seeds and Seedlings; Springer: Singapore, 2019; pp. 219–251. [Google Scholar]
- EL-Sabagh, A.; Hossain, A.; Barutçular, C.; Iqbal, M.A.; Islam, M.S.; Fahad, S.; Erman, M. Consequences of salinity stress on the quality of crops and its mitigation strategies for sustainable crop production: An outlook of arid and semi-arid regions. In Environment, Climate, Plant and Vegetation Growth; Springer: Cham, Switzerland, 2020; pp. 503–533. [Google Scholar]
- Islam, M.S.; Akhter, M.M.; El-Sabagh, A.; Liu, L.Y.; Nguyen, N.T.; Ueda, A.; Masaoka, Y.; Saneoka, H. Comparative studies on growth and physiological responses to saline and alkaline stresses of Foxtail millet (Setaria italica L.) and Proso millet (Panicum miliaceum L.). Aust. J. Crop Sci. 2011, 5, 1269–1277. [Google Scholar]
- Ali, Q.; Daud, M.K.; Haider, M.Z.; Ali, S.; Rizwan, M.; Aslam, N.; Noman, A.; Iqbal, N.; Shahzad, F.; Deeba, F.; et al. Seed priming by sodium nitroprusside improves salt tolerance in wheat (Triticum aestivum L.) by enhancing physiological and biochemical parameters. Plant Physiol. Biochem. 2017, 119, 50–59. [Google Scholar] [CrossRef]
- Yang, Y.; Guo, Y. Unravelling salt signaling in plants. J. Integr. Plant Biol. 2018, 60, 796–804. [Google Scholar]
- Morari, F.; Meggio, F.; Lunardon, A.; Scudiero, E.; Forestan, C.; Farinati, S.; Varotto, S. Time course of biochemical, physiological, and molecular responses to field-mimicked conditions of drought, salinity, and recovery in two maize lines. Front. Plant Sci. 2015, 6, 314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Monsur, M.B.; Ivy, N.A.; Haque, M.M.; Hasanuzzaman, M.; EL-Sabagh, A.; Rohman, M.M. Oxidative stress tolerance mechanism in rice under salinity. Phyton Int. J. Exp. Bot. 2020, 89, 497–517. [Google Scholar]
- Ashraf, M.; Shahzad, S.M.; Imtiaz, M.; Rizwan, M.S. Salinity effects on nitrogen metabolism in plants—Focusing on the activities of nitrogen metabolizing enzymes: A review. J. Plant Nutr. 2018, 41, 1065–1081. [Google Scholar] [CrossRef]
- Khan, H.A.; Siddique, K.H.M.; Munir, R.; Colmer, T.D. Salt sensitivity in chickpea: Growth, photosynthesis, seed yield components and tissue ion regulation in contrasting genotypes. J. Plant Physiol. 2015, 182, 1–12. [Google Scholar] [CrossRef]
- Manchanda, G.; Garg, N. Salinity and its effects on the functional biology of legumes. Acta Physiol. Plant. 2008, 30, 595–618. [Google Scholar] [CrossRef]
- Hasan, M.K.; Islam, M.S.; Islam, M.R.; Ismaan, H.N.; EL-Sabagh, A.; Barutçular, C.; Saneoka, H. Water relations and dry matter accumulation of black gram and mungbean as affected by salinity. Thai J. Agric. Sci. 2019, 52, 54–67. [Google Scholar]
- EL-Sabagh, A.; Hossain, A.; Islam, M.S.; Barutçular, C.; Ratnasekera, D.; Kumar, N.; Meena, R.S.; Gharib, H.S.; Saneoka, H.; Teixeira da Silva, J.A. Sustainable soybean production and abiotic stress management in saline environments: A critical review. Aust. J. Crop Sci. 2019, 13, 228–236. [Google Scholar] [CrossRef]
- Farooq, M.; Hussain, M.; Wakeel, A.; Siddique, K.H.M. Salt stress in maize effects resistance mechanisms and management: A review. Agron. Sustain. Dev. 2015, 35, 461–481. [Google Scholar] [CrossRef] [Green Version]
- Rasool, S.; Ahmad, A.; Siddiqi, T.O.; Ahmad, P. Changes in growth, lipid per oxidation and some key antioxidant enzymes in chickpea genotypes under salt stress. Acta. Physiol. Plant. 2013, 35, 1039–1050. [Google Scholar] [CrossRef]
- Ahmad, P. Growth and antioxidant responses in mustard (Brassica juncea L.) plants subjected to combined effect of gibberellic acid and salinity. Arch. Agron. Soil Sci. 2010, 56, 575–588. [Google Scholar] [CrossRef]
- Liu, L.; Nakamura, Y.; Taliman, N.A.; Sabagh, A.E.; Moghaieb, R.E.; Saneoka, H. Differences in the growth and physiological responses of the leaves of Peucedanum japonicum and Hordeum vulgare exposed to salinity. Agriculture 2020, 10, 317. [Google Scholar] [CrossRef]
- Islam, M.S.; Hasan, M.K.; Islam, B.; Renu, N.A.; Hakim, M.A.; Islam, M.R.; Chowdhury, M.K.; Ueda, A.; Saneoka, H.; Raza, M.A.; et al. Responses of water and pigments status, dry matter partitioning, seed production, and traits of yield and quality to foliar application of GA3 in Mungbean (Vigna radiata L.). Front. Agron. 2021, 2, 596850. [Google Scholar] [CrossRef]
- Patil, G.; Do, T.; Vuong, T.D.; Valliyodan, B.; Lee, J.D.; Chaudhary, J.; Shannon, J.G.; Nguyen, H.T. Genomic-assisted haplotype analysis and the development of high-throughput SNP markers for salinity tolerance in soybean. Sci. Rep. 2016, 6, 19199. [Google Scholar] [CrossRef] [Green Version]
- Golezani, K.G.; Yengabad, F.M. Physiological responses of lentil (Lens culinaris Medik.) to salinity. Int. J. Agric. Crop Sci. 2012, 4, 531–535. [Google Scholar]
- Wang, B.; Jiang, H.; Bi, Y.; He, X.; Wang, Y.; Zheng, X.; Prusky, D. Preharvest multiple sprays with sodium nitroprusside promote wound healing of harvested muskmelons by activation of phenylpropanoid metabolism. Postharvest Biol. Technol. 2019, 158, 110988. [Google Scholar] [CrossRef]
- Som, P.R. Nitric oxide induced resistance in tea plants against Glomerella cingulata (stoneman) spauld & schrenk. BIOINFOLET 2020, 17, 336–342. [Google Scholar]
- Li, G.; Zhu, S.; Wu, W.; Zhang, C.; Peng, Y.; Wang, Q.; Shi, J. Exogenous nitric oxide (NO) induces disease against Monilinia fructicola through activating the phenylpropanoid pathway in peach fruit. J. Sci. Food Agric. 2017, 97, 3030–3038. [Google Scholar] [CrossRef]
- Lai, T.F.; Wang, Y.Y.; Li, B.Q.; Qin, G.Z.; Tian, S.P. Defensive responses of tomato fruit to exogenous nitric oxide during postharvest storage. Postharvest Biol. Technol. 2011, 62, 127–132. [Google Scholar] [CrossRef]
- Lu, R.; Liu, Z.; Shao, Y.; Su, J.; Li, X.; Sun, F.; Zhang, Y.; Li, S.; Zhang, Y.; Cui, J.; et al. Nitric oxide enhances rice resistance to rice black-streaked dwarf virus infection. Rice 2020, 13, 24. [Google Scholar] [CrossRef] [Green Version]
- Hameed, A.; Farooq, T.; Hameed, A.; Sheikh, M.A. Sodium nitroprusside mediated priming memory invokes water-deficit stress acclimation in wheat plants through physio-biochemical alterations. Plant Physiol. Biochem. 2021, 160, 329–340. [Google Scholar] [CrossRef]
- Kram, N.A.; Hafeez, N.; Farid-ul-Haq, M.; Ahmad, A.; Sadiq, M.; Ashraf, M. Foliage application and seed priming with nitric oxide causes mitigation of salinity-induced metabolic adversaries in broccoli (Brassica oleracea L.) plants. Acta Physiol. Plant. 2020, 42, 155. [Google Scholar]
- Lee, H.J.; Lee, J.H.; Lee, S.G.; An, S.; Lee, H.S.; Choi, C.K.; Kim, S.K. Foliar application of biostimulants affects physiological responses and improves heat stress tolerance in Kimchi cabbage. Hortic. Environ. Biotechnol. 2019, 60, 841–851. [Google Scholar] [CrossRef]
- Diao, Q.; Cao, Y.; Fan, H.; Zhang, Y. Transcriptome analysis deciphers the mechanisms of exogenous nitric oxide action on the response of melon leaves to chilling stress. Biol. Plant. 2020, 64, 465–472. [Google Scholar] [CrossRef]
- Ahmad, P.; Alam, P.; Balawi, T.H.; Atalayan, F.H.; Ahanger, M.A.; Ashraf, M. Sodium nitroprusside (SNP) improves tolerance to arsenic (As) toxicity in Vicia faba through the modifications of biochemical attributes, antioxidants, ascorbate-glutathione cycle and glyoxalase cycle. Chemosphere 2020, 244, 125480. [Google Scholar] [CrossRef] [PubMed]
- Karthik, S.; Pavan, G.; Krishnan, V.; Sathish, S.; Manickavasagam, M. Sodium nitroprusside enhances regeneration and alleviates salinity stress in soybean (Glycine max Merrill). Biocatal. Agric. Biotechnol. 2019. [Google Scholar] [CrossRef]
- Campos, F.V.; Oliveria, J.A.; Pereira, M.G.; Farnese, F.S. Nitric oxide and phytohormone interactions in the response of Lactuca sativa to salinity stress. Planta 2019, 250, 1475–1479. [Google Scholar] [CrossRef]
- Kausar, F.; Shahbaz, M. Interactive effect of foliar application of nitric oxide (NO) and salinity on wheat (Triticum aestivum L.). Pak. J. Bot. 2013, 45, 67–73. [Google Scholar]
- Ahmad, P.; Abdel-Latif, A.A.; Hashem, A.; Abd-Allah, E.F.; Gucel, S.; Tran, L.S.P. Nitric oxide mitigates salt stress by regulating levels of osmolytes and antioxidant enzymes in chickpea. Front. Plant Sci. 2016, 7, 347. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.Y.; Liu, J.; Liu, Y.L. Nitric oxide alleviates growth inhibition of maize seedlings under salt stress. J. Plant Physiol. Mol. Biol. 2004, 30, 455–459. [Google Scholar]
- Manai, J.; Kalai, T.; Gouia, H.; Corpas, F.J. Exogenous nitric oxide (NO) ameliorates salinity-induced oxidative stress in tomato (Solanum lycopersicum) plants. J. Soil Sci. Plant Nutr. 2014, 14, 433–446. [Google Scholar] [CrossRef]
- Habib, N.; Ashraf, M.; Shahbaz, M. Effect of exogenously applied nitric oxide on some key physiological attributes of rice (Oryza sativa L.) plants under salt stress. Pak. J. Bot. 2013, 45, 1563–1569. [Google Scholar]
- Liu, S.; Dong, Y.; Xu, L.; Kong, J. Effects of foliar applications of nitric oxide and salicylic acid on salt-induced changes in photosynthesis and antioxidative metabolism of cotton seedlings. Plant Growth Regul. 2013, 73, 67–78. [Google Scholar] [CrossRef]
- Sahay, S.; Gupta, M. An update on nitric oxide and its benign role in plant responses under metal stress. Nitric Oxide 2017, 67, 39–52. [Google Scholar] [CrossRef] [PubMed]
- Mohasseli, V.; Sadeghi, S. Exogenously applied sodium nitroprusside improves physiological attributes and essential oil yield of two drought susceptible and resistant specie of Thymus under reduced irrigation. Ind. Crop. Prod. 2019, 130, 130–136. [Google Scholar] [CrossRef]
- Yamasaki, S.; Dillenburg, L.C. Measurements of leaf relative water content in Araucaria angustifolia. Rev. Bras. Fisiol. Veg. 1999, 11, 69–75. [Google Scholar]
- Heath, R.L.; Packer, L. Photoperoxidation in isolated chloroplasts. I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef]
- Rao, K.V.M.; Sresty, T.V.S. Antioxidative parameters in the seedlings of pigeon pea (Cajanus cajan L. Millspaugh) in response to Zn and Ni stresses. Plant Sci. 2000, 157, 113–128. [Google Scholar]
- Giannopolitis, C.N.; Ries, S.K. Superoxide dismutase I. Occurrence in higher plants. J. Plant Physiol. 1977, 59, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Aebi, H. Catalase in vitro. Method Enzymol. 1984, 105, 121–126. [Google Scholar]
- Chance, B.; Maehly, A.C. Assay of catalases and peroxidases. Method Enzymol. 1995, 2, 764–775. [Google Scholar]
- Bandeoglu, E.; Eyidogan, F.; Yucel, M.; Oktem, H.A. Antioxidant responses of shoots and roots of lentil to NaCl-salinity stress. Plant Growth Regul. 2004, 42, 69–77. [Google Scholar] [CrossRef]
- Ibrahim, E.A. Seed priming to alleviate salinity stress in germinating seeds. J. Plant Physiol. 2016, 192, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Garg, N.; Bharti, A. Salicylic acid improves arbuscular mycorrhizal symbiosis, and chickpea growth and yield by modulating carbohydrate metabolism under salt stress. Mycorrhiza 2018, 28, 727–746. [Google Scholar] [CrossRef] [PubMed]
- Garg, N.; Manchanda, G. ROS generation in plants: Boon or bane? Plant Biosyst. 2009, 143, 81–96. [Google Scholar] [CrossRef]
- Mostofa, M.G.; Fujita, M.; Tran, L.S.P. Nitric oxide mediates hydrogen peroxide- and salicylic acid-induced salt tolerance in rice (Oryza sativa L.) seedlings. Plant Growth Regul. 2015, 77, 265–277. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Hossain, M.A.; Fujita, M. Nitric oxide modulates antioxidant defense and the methylglyoxal detoxification system and reduces salinity-induced damage of wheat seedlings. Plant Biotechnol. Rep. 2011, 5, 353–365. [Google Scholar] [CrossRef]
- Demir, M.; Arif, I. Effects of different soil salinity levels on germination and seedling growth of safflower (Carthamus tinctorius L.). Turk. J. Agric. 2003, 27, 221–227. [Google Scholar]
- Leshem, Y.Y.; Haramaty, E. The characterization and contrasting effects of the nitric oxide free radical in vegetative stress and senescence of Pisum sativum Linn. foliage. J. Plant Physiol. 1996, 148, 258–263. [Google Scholar] [CrossRef]
- Li, Y.; He, N.; Hou, J.; Xu, L.; Liu, C.; Zhang, J.; Wang, Q.; Zhang, X.; Wu, X. Factors influencing leaf chlorophyll content in natural forests at the biome scale. Front. Ecol. Evol. 2018, 6, 64. [Google Scholar] [CrossRef] [Green Version]
- Zeng, C.L.; Liu, L.; Wang, B.R.; Wu, X.M.; Zhou, Y. Physiological effects of exogenous nitric oxide on Brassica juncea seedlings under NaCl stress. Biol. Plant. 2011, 55, 345–348. [Google Scholar] [CrossRef]
- Habib, N.; Ashraf, M. Effect of exogenously applied nitric oxide on water relations and ionic composition of rice (Oryza sativa L.) plants under salt stress. Pak. J. Bot. 2014, 46, 111–116. [Google Scholar]
- Farag, M.; Najeeb, U.; Yang, J.; Hu, Z.; Fang, Z.M. Nitric oxide protects carbon assimilation process of watermelon from boron-induced oxidative injury. Plant Physiol. Biochem. 2017, 111, 166–173. [Google Scholar] [CrossRef]
- Farooq, M.; Gogoi, N.; Barthakur, S.; Baroowa, B.; Bharadwaj, N.; Alghamdi, S.S.; Siddique, K.H.M. Drought stress in grain legumes during reproduction and grain filling. J. Agron. Crop Sci. 2017, 203, 81–102. [Google Scholar] [CrossRef]
- Khan, H.A.; Siddique, K.H.M.; Colmer, T.D. Salt sensitivity in chickpea is determined by sodium toxicity. Planta 2016, 244, 623–637. [Google Scholar] [CrossRef]
- Vadez, V.; Krishnamurthy, L.; Serraj, R.; Gaur, P.M.; Upadhyaya, H.D.; Hoisington, D.A.; Varshney, R.K.; Turner, N.C.; Siddique, K.H.M. Large variation in salinity tolerance in chickpea is explained by differences in sensitivity at the reproductive stage. Field Crop. Res. 2007, 104, 123–129. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, S. Effect of soil salinity on the yield and yield components of mungbean. Pak. J. Bot. 2009, 41, 63–68. [Google Scholar]
- Zheng, C.F.; Dong, J.G.; Liu, F.L.; Dai, T.B.; Liu, W.C.; Jing, Q.; Cao, W. Exogenous nitric oxide improves seed germination in wheat against mitochondrial oxidative damage induced by high salinity. Environ. Exp. Bot. 2009, 67, 222–227. [Google Scholar] [CrossRef]
- Begara-Morales, J.C.; Sanchez-Calvo, B.; Chaki, M.; Valderrama, R.; MataPerez, C.; Lopez-Jaramillo, J.; Padilla, M.N.; Carreras, A.; Corpas, F.J.; Barroso, J.B. Dual regulation of cytosolic ascorbate peroxidase (APX) by tyrosine nitration and S-nitro sylation. J. Exp. Bot. 2014, 65, 527–538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, M.N.; Siddiqui, M.H.; Mohammad, F.; Naeem, M. Interactive role of nitric oxide and calcium chloride in enhancing tolerance to salt stress. Nitric Oxide 2012, 27, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.J.; Jinc, S.S.; Liu, S.; Xu, L.L.; Kong, J. Effects of exogenous nitric oxide on growth of cotton seedlings under NaCl stress. J. Soil Sci. Plant Nutr. 2014, 14, 1–13. [Google Scholar] [CrossRef] [Green Version]

| Treatments | Shoot Length (cm) | Root Length (cm) | Leaf Relative Water Content (%) | Leaf Greenness Index |
|---|---|---|---|---|
| T1 = 0 mM of NaCl + 0 µM of SNP (Control) | 40.12 ± 1.18 a | 20.82 ± 0.62 a | 81.58 ± 1.88 a | 12.51 ± 0.97 ab |
| T2 = 50 mM of NaCl + 0 µM of SNP | 32.18 ± 0.83 c | 12.55 ± 0.95 de | 64.48 ± 2.05 d | 9.42 ± 1.22 cd |
| T3 = 100 mM of NaCl + 0 µM of SNP | 23.41 ± 1.24 e | 7.62 ± 0.76 f | 42.86 ± 1.06 e | 7.28 ± 1.05 d |
| T4 = 50 mM of NaCl + 50 µM of SNP | 36.66 ± 1.14 b | 16.23 ± 0.96 bc | 74.27 ± 1.71 b | 12.27 ± 1.07 ab |
| T5 = 100 mM of NaCl + 50 µM of SNP | 30.55 ± 0.85 c | 13.71 ± 0.83 cd | 69.11 ± 1.68 c | 10.24 ± 0.87 bc |
| T6 = 50 mM of NaCl + 100 µM of SNP | 38.72 ± 1.02 ab | 18.42 ± 0.73 ab | 84.32 ± 2.46 a | 14.80 ± 0.97 a |
| T7 = 100 mM of NaCl + 100 µM of SNP | 27.18 ± 0.82 d | 10.22 ± 0.87 ef | 65.33 ± 1.99 d | 11.71 ± 0.99 bc |
| Treatments | Number of Branches Plant−1 | Number of Pods Plant−1 | Number of Seeds Plant−1 | Seed Yield (g Plant−1) | Biomass (g Plant−1) |
|---|---|---|---|---|---|
| T1 = 0 mM of NaCl + 0 µM of SNP | 10.50 ± 0.53 b | 36.52 ± 2.50 b | 54.50 ± 3.18 b | 1.32 ± 0.18 ab | 9.17 ± 0.73 b |
| T2 = 50 mM of NaCl + 0 µM of SNP | 7.25 ± 0.80 c | 27.51 ± 2.11 d | 34.52 ± 1.97 e | 0.86 ± 0.04 ab | 7.82 ± 0.58 b |
| T3 = 100 mM of NaCl + 0 µM of SNP | 4.91 ± 0.69 d | 20.04 ± 1.73 e | 21.54 ± 1.06 f | 0.53 ± 0.06 b | 4.22 ± 0.71 c |
| T4 = 50 mM of NaCl + 50 µM of SNP | 8.66 ± 0.82 c | 34.54 ± 1.52 bc | 49.48 ± 2.71 c | 1.23 ± 0.08 ab | 8.95 ± 0.84 b |
| T5 = 100 mM of NaCl + 50 µM of SNP | 7.33 ± 0.35 c | 32.20 ± 2.09 c | 39.50 ± 2.44 d | 0.93 ± 0.12 ab | 5.42 ± 0.62 c |
| T6 = 50 mM of NaCl + 100 µM of SNP | 14.58 ± 0.72 a | 48.75 ± 2.81 a | 66.25 ± 2.70 a | 1.65 ± 0.21 a | 14.15 ± 1.06 a |
| T7 = 100 mM of NaCl + 100 µM of SNP | 8.16 ± 1.01 c | 33.75 ± 2.13 bc | 48.52 ± 2.32 c | 1.21 ± 0.13 ab | 8.38 ± 0.74 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yasir, T.A.; Khan, A.; Skalicky, M.; Wasaya, A.; Rehmani, M.I.A.; Sarwar, N.; Mubeen, K.; Aziz, M.; Hassan, M.M.; Hassan, F.A.S.; et al. Exogenous Sodium Nitroprusside Mitigates Salt Stress in Lentil (Lens culinaris Medik.) by Affecting the Growth, Yield, and Biochemical Properties. Molecules 2021, 26, 2576. https://doi.org/10.3390/molecules26092576
Yasir TA, Khan A, Skalicky M, Wasaya A, Rehmani MIA, Sarwar N, Mubeen K, Aziz M, Hassan MM, Hassan FAS, et al. Exogenous Sodium Nitroprusside Mitigates Salt Stress in Lentil (Lens culinaris Medik.) by Affecting the Growth, Yield, and Biochemical Properties. Molecules. 2021; 26(9):2576. https://doi.org/10.3390/molecules26092576
Chicago/Turabian StyleYasir, Tauqeer Ahmad, Ayesha Khan, Milan Skalicky, Allah Wasaya, Muhammad Ishaq Asif Rehmani, Naeem Sarwar, Khuram Mubeen, Mudassir Aziz, Mohamed M. Hassan, Fahmy A. S. Hassan, and et al. 2021. "Exogenous Sodium Nitroprusside Mitigates Salt Stress in Lentil (Lens culinaris Medik.) by Affecting the Growth, Yield, and Biochemical Properties" Molecules 26, no. 9: 2576. https://doi.org/10.3390/molecules26092576











